Introduction
The onset of cancer may appear insidiously, and
clinical symptoms of duodenal papilla tumors are not typical at the
start. In digestive tract tumors, the incidence rate of small bowel
tumors is <2% (1). Duodenal
neoplasms, including primary and secondary tumors, occur rarely.
The incidence of duodenal malignant tumors accounted for 33–45% of
all small intestine tumors (2), and
the most common duodenal lesions were identified as being
adenocarcinoma (clinically, ~77% of duodenal tumors) (3,4). In
terms of location, in the present study it was hypothesized that
the clinicopathological characteristics, diagnosis and methods of
treatment, comparing between ampulla and non-ampulla duodenal
tumors, were likely to exhibit certain differences. In the early
stages, the insidious clinical manifestation of duodenal tumors
easily leads to misdiagnosis and missed diagnoses, and therefore
this area warranted further investigation (5).
Materials and methods
Patients
The records of all patients with duodenal tumors
treated in our hospital, i.e. Guangzhou Hospital of Integrated
Traditional Chinese and Western Medicine between January 1, 1995
and December 31, 2012 were retrospectively reviewed. The diagnosis
of duodenal tumors was conducted by histological examination, and
confirmed by subsequent surgical pathology or endoscopic
biopsy.
Data on patients' age, gender, the tumor location,
symptoms, palliative check-up, cancer antigen 199 (CA199) and
carcinoembryonic antigen (CEA) biomarkers, treatment, tumor stage
and survival outcome were collected. Treatment data included the
type of resection and adjuvant treatment, and supportive therapy.
The patients' records/information was anonymized and de-identified
prior to the analysis.
The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki (6th revision,
2008), as reflected in a priori approval by the Medical
Ethics Committee of Guangzhou Hospital of Integrated Traditional
Chinese and Western Medicine.
Statistical analysis
Associations between tumor location and
clinicopathological variables were analyzed using the χ2
test. Survival curves were estimated using the Kaplan-Meier method,
and compared using the log-rank test. All statistical analyses were
performed with SPSS 13.0 software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). All tests were two-sided, and P<0.05 was
considered to indicate a statistically significant value.
Results
Patient characteristics
A total of 57 patients with small intestinal tumors
were admitted for treatment to Guangzhou Hospital of Integrated
Traditional Chinese and Western Medicine between January 1, 1995
and December 31, 2012, and of these, 44 patients (77.2%) were
diagnosed with duodenal tumors, among which 24 were located in the
ampulla and 20 were located in the non-ampulla (Table I). Gender distribution, age, smoking
and drinking habits, and any family history of cancer revealed no
significant differences (P>0.05) between ampulla and non-ampulla
duodenal tumors (Table I). The
majority of the patients were >60 years of age (Table II). A significant difference in the
symptoms of jaundice between patients with non-ampulla and ampulla
duodenal tumors was observed (P<0.05) (Table I), although the other clinical
manifestations of the two locations did not disclose any especial
differences compared with the other digestive tract tumors, which
provides one of the main reasons why duodenal tumors cannot be
diagnosed early.
 | Table I.General pathological features of
duodenal tumors. |
Table I.
General pathological features of
duodenal tumors.
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
| Characteristic | Non-ampulla | Ampulla | P-value |
|---|
| Gender |
|
| 1 |
| Male | 9 (45.0%) | 12 (50.0%) |
|
|
Female | 11 (55.0%) | 12 (50.0%) |
|
| Age, years |
|
| 0.813 |
|
<60 | 6 (30.0%) | 8 (33.3%) |
|
| ≥60 | 14 (70.0%) | 16 (66.7%) |
|
| Alcohol
consumption |
|
| 0.488 |
| No | 17 | 22 |
|
| Yes | 3 | 2 |
|
| Smoking |
|
| 0.284 |
| No | 15 | 21 |
|
| Yes | 5 | 3 |
|
| Family tumor
history |
|
| 0.268 |
| No | 19 | 24 |
|
| Yes | 1 | – |
|
| Clinical feature |
|
| 0.001 |
|
Bellyache | 9 | 8 |
|
|
Jaundice | 1 | 15 |
|
|
Bloating | 4 | 2 |
|
|
Gastrointestinal bleeding | 3 | 2 |
|
| Nausea,
vomiting | 6 | 2 |
|
| Abdominal
mass | – | – |
|
|
Anorexia | 1 | 2 |
|
| Acid
regurgitation, belching | 3 | 1 |
|
| Anal stop
exhaust defecation | 1 | 1 |
|
|
Diarrhea | 1 | – |
|
|
Other | 3 | 4 |
|
| No
obvious features | 4 | – |
|
 | Table II.Association between the patients' age
and tumor location. |
Table II.
Association between the patients' age
and tumor location.
|
| Mean age | 95% CI |
|---|
| Duodenum | 67.3±16.9 | (59.3, 75.2) |
| Ampulla | 66.5±12.3 | (61.3, 71.7) |
Auxiliary examination for
diagnosis
The most commonly used auxiliary examination
techniques for diagnosing all duodenal tumors are gastroscopy,
computed tomography (CT), ultrasound and digestive tract
radiography. Abdominal plain film is a routine examination,
although it is more suitable for intestinal obstructions,
particularly for non-ampullary duodenal tumors. For ampulla
duodenal neoplasms, magnetic resonance (MR)MR
cholangiopancreatography (MRCP) endoscopic retrograde CP
(ERCP)percutaneous transhepatic cholangial drainage (PTCD) may be
useful for revealing positive findings, which is also a common
examination for obstructive jaundice (Table III). Evidently, surgical pathology
is the technique best suited to offering the truest diagnosis.
 | Table III.Auxiliary examination for
diagnosis. |
Table III.
Auxiliary examination for
diagnosis.
|
| Number of
patients |
|---|
|
|
|
|---|
|
| Duodenum | Ampulla |
|---|
| Endoscopy | 12 (60.0%) | 14 (58.3%) |
| CT | 7 (35.0%) | 13 (54.2%) |
| Ultrasound | 2 | 8 |
| Digestive tract
radiography | 3 | 7 |
| Abdominal plain
film | 1 |
|
| MR |
| 7 |
| MRCP |
| 5 |
| ERCP |
| 6 |
| PTCD |
| 2 |
Role of tumor markers in
diagnosis
For ampulla and non-ampulla duodenal tumors, the
percentages of the high tumor markers, CA199 or CEA, reached
40–50%, which evidently has a certain significance in terms of the
diagnosis of duodenal tumor (Tables
IV and V).
 | Table IV.CA199 values of adenocarcinoma on
admission (or pre-operation). |
Table IV.
CA199 values of adenocarcinoma on
admission (or pre-operation).
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
| Location | High CA199 | Not high CA199 | Total | High CA199 ratio
(%) |
|---|
| Non-ampulla | 7 | 10 | 17 | 41.18 |
| Ampulla | 12 | 12 | 24 | 50.00 |
| Total | 19 | 22 | 41 | 46.34 |
 | Table V.CEA values of adenocarcinoma on
admission (or pre-operation). |
Table V.
CEA values of adenocarcinoma on
admission (or pre-operation).
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
| Location | High CEA | Not high CEA | Total | High CEA ratio
(%) |
|---|
| Non-ampulla | 8 | 9 | 17 | 47.06 |
| Ampulla | 10 | 14 | 24 | 41.67 |
| Total | 18 | 23 | 41 | 43.90 |
Pathological types of duodenal
tumors
Consistently with small intestine tumors, the
pathological types of the duodenal tumors were predominantly
adenocarcinoma. The pathological types of ampullary duodenal tumors
followed up were all adenocarcinoma; however, the non-ampullary
duodenal neoplasms were mainly adenocarcinoma, with rare
pathological types, including stromal tumor and large B-cell
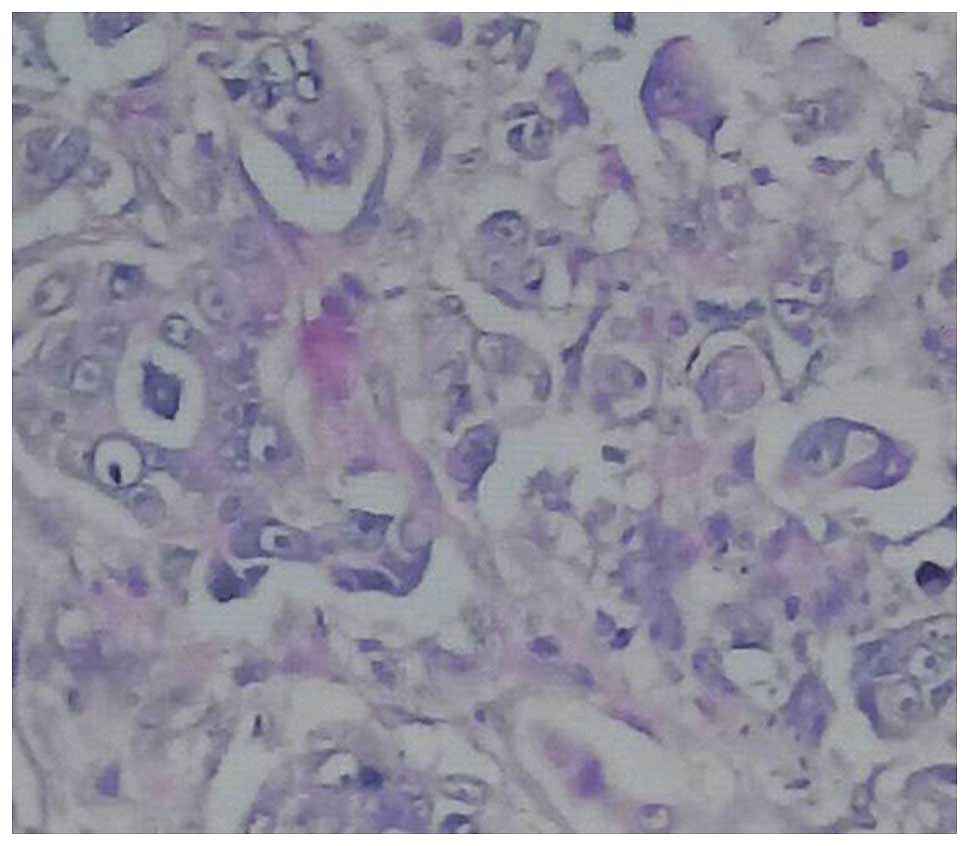
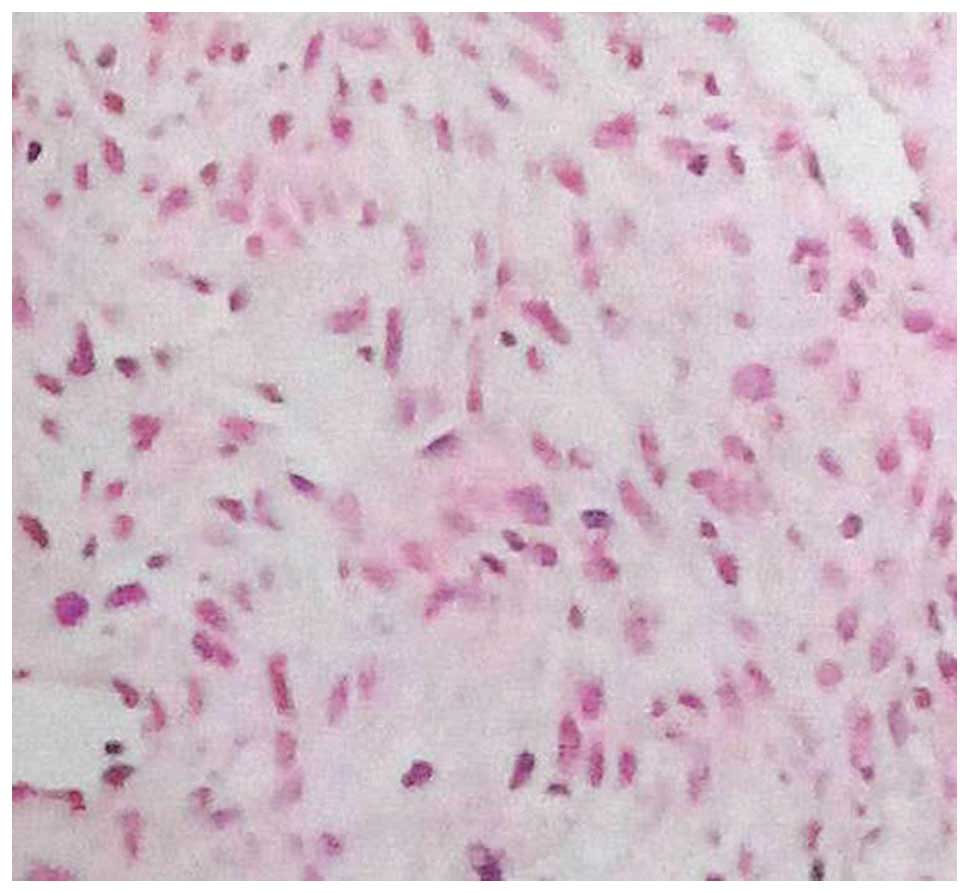
lymphoma (Table VI and Figs. 1–3).
 | Table VI.Pathological types. |
Table VI.
Pathological types.
|
| Number of
patients |
|---|
|
|
|
|---|
| Location | Adenocarcinoma | Stromal tumor | Large B-cell
lymphoma |
|---|
| Non-ampulla | 17 (85%) | 2 (10%) | 1 (5%) |
| Ampulla | 24 | – | – |
Tumor location and pathological
stage
Ampulla and non-ampulla tumors of the duodenum may
be clearly assigned to pathological staging. In the analysis,
neither the TNM stage nor T- staging demonstrated any obvious
differences, comparing between the ampulla and non-ampulla tumors.
However, pathological N staging and the M stage were late for
non-ampulla tumors compared with ampulla duodenal tumors in the
diagnosis, which might be associated with more atypical symptoms of
non-ampulla duodenal tumors, which render an early diagnosis
difficult (Table VII).
 | Table VII.Evaluation of the pathological stage
of adenocarcinoma. |
Table VII.
Evaluation of the pathological stage
of adenocarcinoma.
|
| Number of
patients |
|
|---|
|
|
|
|
|---|
|
| Duodenum | Ampulla | P-value |
|---|
| TNM stage |
|
| 0.078 |
| I |
| 3 |
|
| II | 2 | 6 |
|
|
III | 2 | 2 |
|
| IV | 9 | 4 |
|
| T-stage |
|
| 0.365 |
|
Tis |
|
|
|
| T1 |
|
|
|
| T2 |
| 3 |
|
| T3 | 2 | 3 |
|
| T4 | 4 | 5 |
|
| N-stage |
|
| 0.007 |
| N0 |
| 9 |
|
| N1 | 1 | 2 |
|
| N2 | 2 |
|
|
| M-stage |
|
| 0.031 |
| M0 | 3 | 10 |
|
| M1 | 9 | 5 |
|
Treatment of duodenal tumors
As for other cancer types, the treatment included
surgery, surgery plus chemotherapy and supportive therapy (see
Table VIII). The reasons for the
choice of supportive therapy were as follows: Advanced metastases
unable to be operated on (7 cases, including 2 cases of ampulla and
5 cases of non-ampulla); patients or their families refused surgery
(7 cases, including 3 cases of ampulla and 4 cases of non ampulla);
or reason unknown (3 cases, including 1 case of ampulla and 2 cases
of non ampulla).
 | Table VIII.Treatment options. |
Table VIII.
Treatment options.
|
| Number of
patients |
|---|
|
|
|
|---|
|
| Surgery | Surgery plus
chemotherapy | Supportive
therapy |
|---|
| Non-ampulla | 8 | 2 | 11 |
| Ampulla | 12 | 5 | 6 |
Surgical method of duodenal tumor
Although the predominant surgical approach was
pancreatoduodenectomy, a difference remained in terms of the
surgical procedure for ampulla and non-ampulla duodenum tumors
(Table IX). Duodenal ampulla tumor
radical resections were all performed as pancreatoduodenectomy.
However, for non-ampulla duodenal tumors, partial resection of the
duodenum and Billroth II subtotal gastrectomy also were able to
attain the purpose of radical cure resection according to the tumor
location, although pancreatoduodenectomy was assigned priority. The
probability of a palliative operation for duodenal ampulla cancer
was also higher. As for the tumor margins, the authors consider
that, as long as the tumor is removed cleanly, the spacing is not
constant, although a larger sample would be required to analyze
this further.
 | Table IX.Operation mode. |
Table IX.
Operation mode.
|
| Number of
patients |
|---|
|
|
|
|---|
|
|
Pancreatoduodenectomy | Partial duodenum
resection | Billroth II
subtotal gastrectomy | Palliative
operation |
|---|
| Non-ampulla | 3 | 3 | 2 | 2 |
| Ampulla | 12 | 0 | 0 | 5 |
Survival of duodenal
adenocarcinoma
To facilitate comparison, only the pathological
types of duodenal adenocarcinoma were included for a comparison of
the various treatment methods and to draw the survival curve. As
shown in Table X and Figs. 4–7,
the survival rate of patients receiving radical surgery was the
best, and chemotherapy had little effect on the survival of the
patients. It was unexpected that no difference would be observed in
terms of the survival rate of patients receiving palliative surgery
and conservative treatment, although a larger sample size would be
required to substantiate these findings.
 | Table X.The survival time of different
treatment for patients with adenocarcinoma (month). |
Table X.
The survival time of different
treatment for patients with adenocarcinoma (month).
| Treatment | Survival time
(months) |
|---|
| Radical surgery (no
chemotherapy) | 9, 40.6, 18+, 33+,
4.7, 12.5, 12-, 60, 50, 35+, 7, 25 |
| Radical surgery +
chemotherapy | 71, 26.8, 15, 19.7,
181, 30+ |
| Supportive therapy
(no chemotherapy) | 3, 3, 0.7, 6, 4.8,
2.5, 6, 1, 12-, 4, 6, 0.4, 2, 8, 10, 12- |
| Palliative therapy
(no chemotherapy) | 5, 13, 11.4, 1.3,
1, 1 |
| Palliative therapy
+ chemotherapy | 25.4 |
Case analysis of patients with
duodenal tumors with long-term survival
A total of 20 patients underwent radical operation.
A total of seven patients experienced long-term survival (over 3
years), and all of these underwent radical operation.
Fifteen cases received radical
pancreatoduodenectomy, of which four cases experienced long-term
survival. There were five cases of other radical operations,
including three cases that experienced long-term survival.
A total of seven patients with long-term survival
included three cases of duodenal ampulla adenocarcinoma (17 cases
of duodenal ampulla cancer surgery, including 12 cases of radical
surgery, all underwent pancreatoduodenectomy). Four cases were
long-term survival were associated with non-ampulla duodenal
neoplasms (10 cases of duodenal non-ampulla cancer surgery,
including eight cases of radical surgery), including one case of
stromal tumor, one case of large B-cell lymphoma and two cases of
adenocarcinoma. Only one case was operated with the operation of
pancreatoduodenectomy.
If it was operable, the prognosis of the
non-papillary tumor was improved, which may be associated with the
long-term survival of the pathological types of non-gland cancer,
which accounted for 50%. The operation effect of stromal tumors was
also better.
Regarding the long-term survival of seven patients,
three had no option of a pancreatoduodenectomy. In fact, performing
a pancreatoduodenectomy has itself also been questioned due to the
difficulty of the operation, the procedure is not fully
standardized because of the complexity of the operation, or the
scope of the surgery or cleaning requires further improvement.
Discussion
The majority of the malignant tumors of the small
intestine are located in the duodenum, which are predominantly
adenocarcinoma (6). Primary duodenal
adenocarcinoma is observed only rarely in the clinic, and this
accounts for ~43% of all small bowel adenocarcinoma (7). Differences are clearly evident in
different sections of the duodenum with regard to the incidence of
primary duodenal adenocarcinoma, with the majority located in the
area surrounding the nipple (8). The
present case study suggests that duodenal adenocarcinoma
predominantly accounts for the vast majority of malignant tumors of
the small intestine, slightly more so for the ampulla than the
non-ampulla.
Notably, the clinical manifestations of primary
duodenal adenocarcinoma were lacking in specificity, and the
incidence rate was low, therefore this may easily escape the
attention of clinicians, making early diagnosis difficult. The
first diagnosis easily becomes a misdiagnosis of the common
digestive tract diseases, including gastritis, gallstones, and so
forth. Improvements in the ability of doctors and patients to
understand the disease would also help to improve vigilance, in
order to assist in forming a correct, early diagnosis. The present
study has also determined that, with the exception of the symptoms
of jaundice in patients with tumors of the duodenal ampulla, which
offer certain insights into possible diagnostic ranges, the
clinical manifestations of duodenal tumors were not specific to
other digestive tract diseases.
For adenocarcinomas surrounding the area of nipple,
ERCP currently offers the best means of examination (9). Upper digestive tract radiography has an
important use for the diagnosis of tumors of the duodenal 3 and 4
segments, which may indicate that tumors of the distal duodenum are
difficult to reach using an endoscope. B-ultrasound, CT and MR
imaging (MRI) are not very specific for the diagnosis of this
disease, and it is not easy to differentiate bile duct stones from
cancer around the ampulla. However, they are useful as early
screening measures. At the same time, they are very useful as
techniques in terms of tumor staging and operation mode selection.
CT is recommended as the first choice for the diagnosis of primary
duodenal adenocarcinoma (10). The
present study has revealed that the diagnosis of duodenal tumors by
endoscopy and CT was valuable, and MRMRCPERCPPTCD may have an
applicability for tumors of the duodenal ampulla. In addition, CEA
and CA199, tumor markers in the gastrointestinal tract, provide a
reference for the diagnosis of duodenal tumor.
Duodenal papilla carcinoma is a rare tumor. Duodenal
papilla carcinoma accounts for <1% of all digestive system
malignant tumors. Duodenal papilla cancer, due to the special
location of the lesion, usually causes an obstruction to an early
diagnosis with respect to biliary tract symptoms, and the clinical
manifestations of progressive painless jaundice. In the present
study, it has been demonstrated that the N and M stages of
adenocarcinoma of the duodenal papilla occurred earlier compared
with those of the non-duodenal papilla, and this could be
associated with the influence of the jaundice particularly
associated with adenocarcinoma of the duodenal papilla.
The treatment of duodenal adenocarcinoma largely
depends on surgical resection. The present study has also revealed
that radical surgery was more effective compared with other
treatments. Surgical procedures include pancreatoduodenectomy,
duodenal segment resection, palliative bypass surgery, and so
forth. The choice of surgical approach depends predominantly on the
location of the tumor, tumor staging, the patient's general
situation and the level of experience. If the patient's health
status is good, radical resection of the duodenum is the first
choice for the treatment of duodenal papilla carcinoma (11). A number of surgeons also consider
that duodenal papillary tumor local excision embodies the
principles of modern, minimally invasive surgery after the
resection of tumors, and to the greatest extent this: i) leads to a
retention of normal levels of human bile and pancreatic juice; and
ii) is mainly applicable to duodenal papilla in patients with
benign tumors and for patients with malignant tumors where a
pancreatoduodenectomy could not be tolerated; therefore, this
method is gaining in popularity in the clinic (12). In the present study, the use of
pancreatoduodenectomy for duodenal papilla carcinoma was more
reliable. In our group of 12 patients with duodenal papilla
carcinoma who underwent radical operation, three patients
experienced long-term survival. Radical resection of duodenal
cancer is the most effective method of treatment;
pancreatoduodenectomy is preferred as the method of choice for the
tumor area around the nipple, and it may also be used in duodenum
primary adenocarcinoma. For adenocarcinoma located in segments 3 or
4 of the duodenum, with no local lymph node metastasis, and where
the patients are in poor general condition and are unable to
tolerate large-scale surgery, duodenal segment resection may be an
option. There are reports that the two types of operation are not
significantly different, suggesting that duodenal segment resection
should be the first choice for adenocarcinoma located in segments 3
or 4 of the duodenum (13). For
patients with advanced cancer tumors that are not able to be
resected, feasibly, palliative bypass surgery can be implemented,
for example, a stomach jejunum anastomosis, or internal and
external drainage of the bile duct. Based on the present study, our
analysis indicates that pancreatoduodenectomy would be a radical
operation for duodenal papilla carcinoma located in the ampulla.
However, for non-ampulla duodenal gland cancer, duodenum partial
resection or subtotal gastrectomy may also be able to achieve the
radical goal.
The majority of commentators suggest that adjuvant
radiotherapy and chemotherapy are able to improve the prognosis of
duodenal adenocarcinoma, although it has been reported that
adjuvant chemotherapy cannot improve the prognosis of patients
(14). In our study, there was no
survival benefit for adjuvant chemotherapy in duodenal
adenocarcinoma. In conclusion, the incidence of duodenal
adenocarcinoma is low, the diagnosis is difficult and the prognosis
is poor. Early diagnosis and radical resection are the key to an
improved prognosis.
Gastrointestinal stromal tumor (GIST) is a type of
non-directional differentiation tumor that independently originates
from the primitive mesenchymal tissues of the gastrointestinal
tract. Duodenal stromal tumors are derived in Cajal interstitial
cells or more primitive mesoderm mesenchymal stem cells with
multipotential differentiation, and these are clinically rare. The
incidence of GIST is ~1–2 per 200,000, and it may occur across the
length of the entire digestive tract, from the esophagus to the
anus, also including the retina, the mesentery, the peritoneum and
the retroperitoneal area. Most commonly however, it occurs in the
stomach and the small intestine, while duodenal stromal tumors are
less frequent, accounting for only 10% of the small intestine
(15). Duodenal stromal tumors are
predominantly concentrated in the descending and horizontal portion
of the duodenum, and less so in the bulb and the ascending portion
(16). Certain researchers have
reported that recurrent black stool is the most common clinical
symptom of duodenal stromal tumor (17). The invasion of the stroma is not
strong, rarely leading to obstruction of the bile duct or the
pancreatic duct. In our study, of 44 cases of duodenal tumor, only
two cases were stromal tumors, both of which were located in the
non-ampulla duodenum.
Surgical resection is the first choice for treatment
of duodenal stromal tumors, and the goal is to achieve the R0
removal of negative tissue margins. Common operational techniques
include partial duodenum resection, pancreatoduodenectomy, distal
subtotal gastrectomy and duodenal segment resection. A study has
shown that the incidence of postoperative complications associated
with pancreatoduodenectomy was greater compared with the other
surgical procedures, and an expanded resection range could not
improve a survival rate of 5 years following the surgery (18). Therefore, considered from two
different aspects, namely, to ensure the safety of the surgery and
to improve the survival time, blind implementation of
pancreatoduodenectomy should be avoided. Pancreaticoduodenectomy is
suitable for tumors of diameter ≥5 cm located in the bulb or the
descending portion of the duodenum, where there is duodenal nipple
involvement, or in cases of recurrence after surgery. Usually,
GISTs rarely occur in lymph node metastasis, and it is thought
that, in duodenal stromal tumor surgery, unless there is evidence
to support such a practice, cleaning of the lymph nodes is not
required (19). For stromal tumors
where there is more expansive growth or rare lymph node metastasis,
excessive expansion excision would not prolong the survival time of
the patients. In a study that advocated ensuring a negative margin
necessary to perform the required surgical resection, a wide range
of lymph node dissection was not advocated (20). In our study, there were two cases of
non-ampullary duodenal tumors. One case was located in the
descending part, and the patient refused an operation. One case was
located in the horizontal section, and partial resection of the
duodenum was performed; the pathological stage was III,
PT3G1M0. This patient has survived
for more than 10 years, and the operation therefore effected a
radical cure.
Our study also identified one case of duodenal large
B-cell lymphoma, which is clinically rare and has not previously
reported by others in the literature, which was categorized as
gastrointestinal lymphoma, clinical stage II E period. The patient
underwent a Billroth II subtotal gastrectomy, and the patient has
survived for 3 years following the operation.
Primary melanoma of the duodenum has also been
reported in the literature (21),
although it was not identified in the present study.
In conclusion, the present study has shown that
duodenal tumors are a rare disease, although they accounted for the
vast majority of small intestinal tumors. The pathological type was
mainly adenocarcinoma; periampullary duodenal tumors were nearly
all adenocarcinoma. Non-ampulla duodenal tumors also included rare
pathological types, such as stromal tumors and large B-cell
lymphoma, in addition to predominantly adenocarcinoma. The clinical
manifestations of duodenal tumor were not specific compared with
other diseases of the digestive tract, leading to difficult early
diagnosis and treatment. Due to the clinical manifestation of
jaundice, periampullary duodenal tumors were diagnosed at an
earlier stage compared with non-ampulla duodenal tumors. Endoscopy
and CT examination are valuable in diagnosis, and may be used as a
means of screening. CEA and CA199 also are very important in terms
of diagnosis. Radical surgery is the most effective treatment, and
pancreaticoduodenectomy applicable for all duodenal tumors. For
non-ampulla duodenal tumors, partial duodenum resection and
subtotal gastrectomy may be selected. No survival benefit was
identified for adjuvant chemotherapy. It is expected that there
will be further, larger scale retrospective studies to further
explore the relevant issues.
Acknowledgements
We would like to thank, with their permission, our
colleagues, W.J.R., L.S.J. and H.J.S., who assisted in or
collaborated with us in this study, and who did not meet with the
criteria for full authorship. No sources of funding were available
for any of authors.
References
|
1
|
Chen J-R, Zhang Y-Q and Chen K-N: Imaging
diagnosis of intestinal tumors. Chinese Medical Computer Imaging
Journal. 7:93–102. 2001.
|
|
2
|
Cortese AF and Cornell GN: Carcinoma of
the duodenum. Cancer. 29:1010–1015. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia R-M, Zhang S-Z and Zhang H-L: CT
diagnosis and differential diagnosis of duodenal malignant tumors.
Pract Radiol J. 21:48–50. 2005.
|
|
4
|
Hu F and Ma Y-J: Diagnosis and treatment
experience of 30 cases primary malignant duodenal tumors. Chin Med
J. 22:41–42. 2009.
|
|
5
|
Ge X-B: Comparative study of X-ray and CT
diagnosis of duodenal adenocarcinoma. Chin CT and MRI J. 12:84–86.
2014.
|
|
6
|
Liang L, Ma L, Xiao Y, Zhang Y and Li H-Y:
Clinical analysis of 55 cases of primary small bowel malignant
tumor. Tumor. 29:589–591. 2009.
|
|
7
|
Zhang S-S, Sun D-L, Liu D, Li J-M and Chen
L: Clinical analysis of 56 cases of primary duodenal
adenocarcinoma. Chin J Gen Surg. 20:534–535. 2005.
|
|
8
|
He Y-N, Ye M-X and Lei Z-M: Analysis of 77
cases of primary duodenal tumors. Diagnosis & Treatment of
Cancer Prevention & Treatment. 24:154–156. 2011.
|
|
9
|
He Z-H: Comparison of detection rate of
four methods of duodenal papilla carcinoma (24 cases analysis). J
Pract Oncol. 19:347–348. 2004.
|
|
10
|
Tang Z-Y, Wang Y-F, Chen Y-R, Zhou E-H and
Shan X-H: Value of CT in the diagnosis of primary duodenal
malignant tumors. Radiol Pract. 27:880–884. 2012.
|
|
11
|
Zhou F, Huang M-W, Xu Z, Luo Z-Q, Zhou
S-B, Shao J-H and Wang K: Application and evaluation of
Laparoscopic assisted pancreaticoduodenectomy in the treatment of
duodenal papilla carcinoma. Chin J Clin Oncol. 38:166–169.
2011.
|
|
12
|
Wang J and Yang L-H: Local excision of
duodenal papilla tumor. Chin J Digest Surg. 12:520–523. 2013.
|
|
13
|
Zhang S-S, Chen L, Leng X-S, Zhang X-F and
Shao Y-F: Clinical analysis of 89 cases of duodenal adenocarcinoma.
Chin J Gen Surg. 26:543–545. 2011.
|
|
14
|
Struck A, Howard T, Chiorean EG, Clarke
JM, Riffenburgh R and Cardenes HR: Non-ampullary duodenal
adenocarcinoma: Factors important for relapse and survival. J Surg
Oncol. 100:144–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yildirgan MI, Basoghu M, Atamanalp SS,
Albayrak Y, Gürsan N and Onbaş O: Duodenal stromal tumor: Report of
a case. Surg Today. 37:426–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q-N, Peng C-H, Wang J-C, Han B-S,
Cheng D-F, Lu Z and Wang X-M: Clinical analysis of 16 cases of
duodenal stromal tumors. J Hepatobiliary Surg. 15:108–110.
2007.
|
|
17
|
Jin X-B and Wang D-Q: Experience of
diagnosis and treatment of 12 cases of duodenal stromal tumors.
Pract Med. 24:1009–1010. 2008.
|
|
18
|
Lu Z-H, Wu X-J, Fang Y-J, Pan Z-Z and Wan
D-S: Surgical treatment and prognosis of gastrointestinal stromal
tumor. Chin J Gastrointestinal Surg. 14:778–780. 2011.
|
|
19
|
Li Y-J, Zhou J-P, Kong F-M, Tian Y-L and
Dong M: Operation mode study of duodenal stromal tumors and
literature review of 219 cases. Shandong Medicine. 54:85–86.
2014.
|
|
20
|
Lanuke K, Bathe OF and Mack LA: Local
excision of duodenal gastrointestinal stromal tumor. J Surg Oncol.
95:267–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korkolis DP, Apostolaki K, Gontikakis E,
Plataniotis GD, Siskos D, Xinopoulos D, Dimitroulopoulos D,
Papantoniou N, Biteli M and Vassilopoulos PP: Primary malignant
melanoma of the duodenum: Aggressive management and long-term
survival of an unusual oncologic entity. South Med J. 101:836–839.
2008.http://doodle.com/poll/9533dgd7in3ebd5e#
View Article : Google Scholar : PubMed/NCBI
|