Introduction
Bevacizumab (BEV), an inhibitor of vascular
endothelial growth factor A (VEGFA), has been used in Japan as an
insurance-covered drug for malignant gliomas since June, 2013. BEV
prolongs the overall survival (OS) of patients with various types
of cancer in other organs. However, phase III studies on BEV in
combination with chemoradiotherapy demonstrated significant
prolongation of the progression-free survival (PFS), but not of the
overall survival (OS), in newly diagnosed glioblastomas, apart from
a specific genomic subgroup (1–3). These
phase III studies included patients undergoing surgical removal of
varying extent. The extent of surgical removal of the tumor is
well-known to strongly affect OS in glioblastoma (4–6).
However, maximal surgical resection may be hindered by various
factors, including patient age, physical condition, tumor location
and extent, and multiplicity of the lesions. Biopsy via the
stereotactic approach or small craniotomy is often selected in
these settings. The number of patients with malignant gliomas who
undergo biopsy or limited resection may increase due to the aging
population. Limited resection was performed in 10–20% of the cases
in a large series of malignant gliomas. The prognosis in these
patients was reported to be rather poor (5,6).
However, patients who had undergone biopsy or limited resection,
referred to as a surgically nearly-naive population, may be an
adequate cohort for elucidating the independent effect of BEV on
OS, unaffected by the extent of surgical removal. The survival
curves of patients with unresectable malignant glioma were
retrospectively investigated and compared according to the use of
BEV.
Patients and methods
Patients
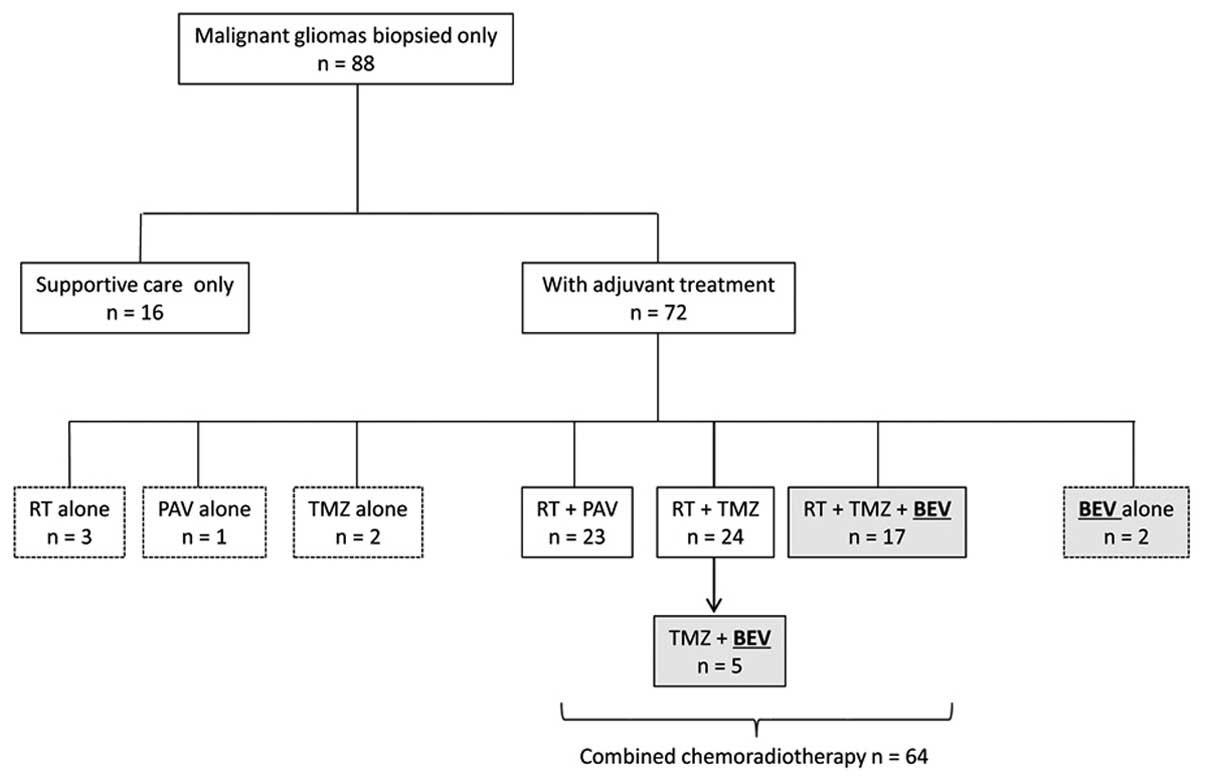
Of the 440 patients with malignant glioma who were
initially treated in our institute between December, 2000 and
January, 2016, 88 (44 men and 44 women) were targeted for biopsy
rather than radical surgical resection (Table I).
 | Table I.Baseline demographic characteristics
of the patients. |
Table I.
Baseline demographic characteristics
of the patients.
| Characteristics | No. |
|---|
| Total number | 88 |
| Gender
(male:female) | 44:44 |
| Age at biopsy
(years), mean ± SD (median) | 67.2±18.5 (74) |
| Route of biopsy |
|
|
Stereotactic biopsy | 75 |
| Small
craniotomy | 10 |
|
Endoscopic biopsy | 3 |
| PS (ECOG) |
|
| 0, 1,
2 | 43 |
| 3, 4 | 45 |
| Histopathological
diagnosis |
|
| WHO grade
III | 28 |
|
Anaplastic astrocytoma | 19 |
|
Anaplastic
oligodendroglioma | 9 |
| WHO grade
IV glioblastoma | 60 |
The patient age ranged from 5 to 88 years (median,
74 years). The reasons for cases deemed ‘unresectable’ were patient
age (n=36), physical and/or neurological condition (n=42),
unfavorable site and extent of the tumor for resective surgery
(n=45) and the patient's wishes (n=1), also including patients with
multiple reasons. Biopsy was performed via the stereotactic route
in 75, small craniotomy in 10 and endoscopic transventricular in 3
patients. The pathological diagnosis was glioblastoma in 60,
anaplastic astrocytoma in 19 and anaplastic oligodendroglioma in 9
patients.
Treatments
Radiotherapy (RT)
Extended local RT at 30–60 Gy with 15–30
fractionations was delivered 5 days/week. The clinical target
volume was the enhanced area on the T1-weighted image plus a 2-cm
margin. In patients with a small residual enhancement lesion
observed after 40 Gy of extended local RT, hypofractionated
stereotactic RT using a CyberKnife unit was delivered with 35
Gy/5-8 fractions (Fig. 1).
Primary adjuvant treatment
The time period during which the subjects underwent
initial treatment was divided into three periods according to the
chemotherapeutic agents used for primary adjuvant treatment as
follows: First period, December, 2000-June, 2006; second period,
July, 2006-July, 2013; and third period, July, 2013-present. During
the first period, procarbazine, nimustin and vincristine (PAV
regimen) were administered every 6 weeks as follows: Nimustin 80
mg/m2 intravenously on day 1; procarbazine 60
mg/m2 orally daily on days 8–21; and vincristine 1.4
mg/m2 up to 2 mg intravenously on days 8 and 29. During
the second period, oral temozolomide (TMZ) at 75
mg/m2/day was administered concomitantly with RT. During
the third period, oral TMZ at 75 mg/m2/day and BEV at 10
mg/kg intravenously every 2 weeks were administered concomitantly
with RT.
Maintenance treatment
As maintenance therapy, administration of the PAV
regimen was continued in the same manner as the primary PAV therapy
during the first period. TMZ was administered at 150–200
mg/m2 for 5 days every 4 weeks during the second and
third periods. In the third period, BEV was administered every 2
weeks concomitantly with maintenance TMZ therapy. These therapies
were continued until tumor progression leading to deterioration of
the patient's condition, rendering the patient unsuitable to
undergo maintenance therapy, or until the development of severe
adverse effects of grade 3 or 4 according to the Common Terminology
Criteria for Adverse Events (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40).
In total, combined chemoradiotherapy was
administered to 64 patients. Of these patients, the initial
cytotoxic chemotherapeutic agents used were PAV in 23 and TMZ in 41
patients. BEV was included in the initial therapy in 19 patients,
of whom 17 received BEV combined with TMZ and RT. BEV was
administered from the mid-course of the maintenance TMZ therapy in
5 patients. Thus, BEV was administered to 24 of the 88 patients
(shaded boxes in Fig. 1).
A total of 16 patients did not receive any adjuvant
treatment due to their age and/or general condition, and received
supportive care instead; 8 patients received either chemotherapy or
RT alone.
Methods
Clinical data were retrieved from the medical
records, and information on survival was obtained from the medical
records or through telephone interviews with family members. The
effect of BEV on OS was investigated using the Kaplan-Meier method
and log-rank analysis in three patient groups as follows: In all 88
patients, in the 41 patients who underwent TMZ-based
chemoradiotherapy, and in the 31 patients with glioblastoma who
underwent TMZ-based chemoradiotherapy. The independent contributing
factors to survival were deduced using Cox proportional hazards
regression analysis.
Statistical analysis
The Statflex software program, version 6.0 (Artech
Co., Osaka, Japan) was used for the statistical analysis of the
results. The Kaplan-Meier method and Cox proportional hazard model
were used for survival analyses. The difference in survival was
assessed with the use of the log-rank test. The Chi-square test was
used to evaluate the distribution among the nominal variables. A
P-value of <0.05 was considered to indicate statistically
significant differences.
Ethical considerations
This retrospective study was approved by the Ethics
Committee of Kagoshima University Hospital (reference no. 27–160,
available at http://com4.kufm.kagoshima-u.ac.jp/information/department/015/015-02.html).
The study was conducted in accordance with the Declaration of
Helsinki of 1975 as revised in 2000 and the Ethical Guidelines for
Medical and Health Research Involving Human Subjects (effective
February 9, 2015) by the Ministry of Education, Culture, Sports,
Science and Technology, and the Ministry of Health, Labor and
Welfare of Japan. To protect the patients' privacy, all data were
collected and analyzed under anonymization in an unlinkable
manner.
Results
Survival analysis
The observation period was 10–2,417 days (median,
263 days). The median survival time (MST) of the 88 patients was
317 days. The Kaplan-Meier analysis and log-rank test results
revealed a significantly longer OS in patients with grade III
compared with grade IV gliomas (P=0.032; MST, 431 vs. 282 days,
respectively).
The effect of BEV was analyzed in the three patient
groups. The clinical characteristics of the patients who received
BEV and those who did not in the three patient groups are
summarized in Table II. In all 88
patients and in the 41 patients with malignant glioma who received
TMZ-based chemoradiotherapy, the non-BEV group included more
patients with grade III tumors compared with the BEV group. The
differences were statistically significant (P=0.004 and 0.014,
respectively, Chi-square test; Table
IIA and B). Among the 88 patients, those who received BEV more
frequently underwent cytotoxic chemotherapy and RT, including the
use of the CyberKnife (Table
IIA).
 | Table II.Clinical factors of the three patient
groups. |
Table II.
Clinical factors of the three patient
groups.
| A, A total of 88
patients with unresectable malignant gliomas |
|---|
|
|---|
| Characteristics | BEV | non-BEV | P-value |
|---|
| Gender
(male:female) | 10:14 | 34:30 | 0.338 |
| Age at biopsy
(years), mean ± SD | 65.3±21.2 | 67.9±17.5 | 0.282 |
| PS (0–2:3,4) | 13:11 | 30:34 | 0.542 |
| WHO grade
(III:IV) | 2:22 | 26:38 | 0.004 |
| Location |
|
| 0.427 |
| Dominant
side | 14 | 27 |
|
|
Non-dominant side | 5 | 14 |
|
|
Bilateral | 3 | 18 |
|
| Posterior
fossa | 2 | 5 |
|
| Cytotoxic
chemotherapy (yes:no) | 22:2 | 46:18 | 0.048 |
| Radiotherapy
(yes:no) | 22:2 | 45:19 | 0.036 |
| CyberKnife
(yes:no) | 10:14 | 10:54 | 0.009 |
|
|---|
| B, A total of 41
patients with unresectable malignant gliomas who received TMZ-based
chemoradiotherapy |
|
|---|
| Characteristics | BEV | non-BEV | P-value |
| Gender
(male:female) | 9:13 | 10:9 | 0.453 |
| Age at biopsy
(years), mean ± SD | 64.9±21.8 | 69.5±18.3 | 0.236 |
| PS (02:3,4) | 13:9 | 10:9 | 0.678 |
| WHO grade
(III:IV) | 2:20 | 8:11 | 0.014 |
| Location |
|
| 0.787 |
| Dominant
side | 12 | 9 |
|
|
Non-dominant side | 5 | 3 |
|
|
Bilateral | 3 | 4 |
|
| Posterior
fossa | 2 | 3 |
|
| CyberKnife
(yes:no) | 10:12 | 4:15 | 0.189 |
|
|---|
| C, A total of 31
patients with unresectable glioblastomas who received TMZ-based
chemoradiotherapy |
|
|---|
|
Characteristics | BEV | non-BEV | P-value |
| Gender
(male:female) | 8:12 | 4:7 | 0.842 |
| Age at biopsy
(year), mean ± SD | 67.9±20.4 | 67.2±21.9 | 0.463 |
| PS (02:3,4) | 12:8 | 3:8 | 0.081 |
| Location |
|
| 0.554 |
|
Dominant side | 11 | 5 |
|
|
Non-dominant side | 4 | 1 |
|
|
Bilateral | 3 | 4 |
|
|
Posterior fossa | 2 | 1 |
|
| CyberKnife
(yes:no) | 8:12 | 1:10 | 0.106 |
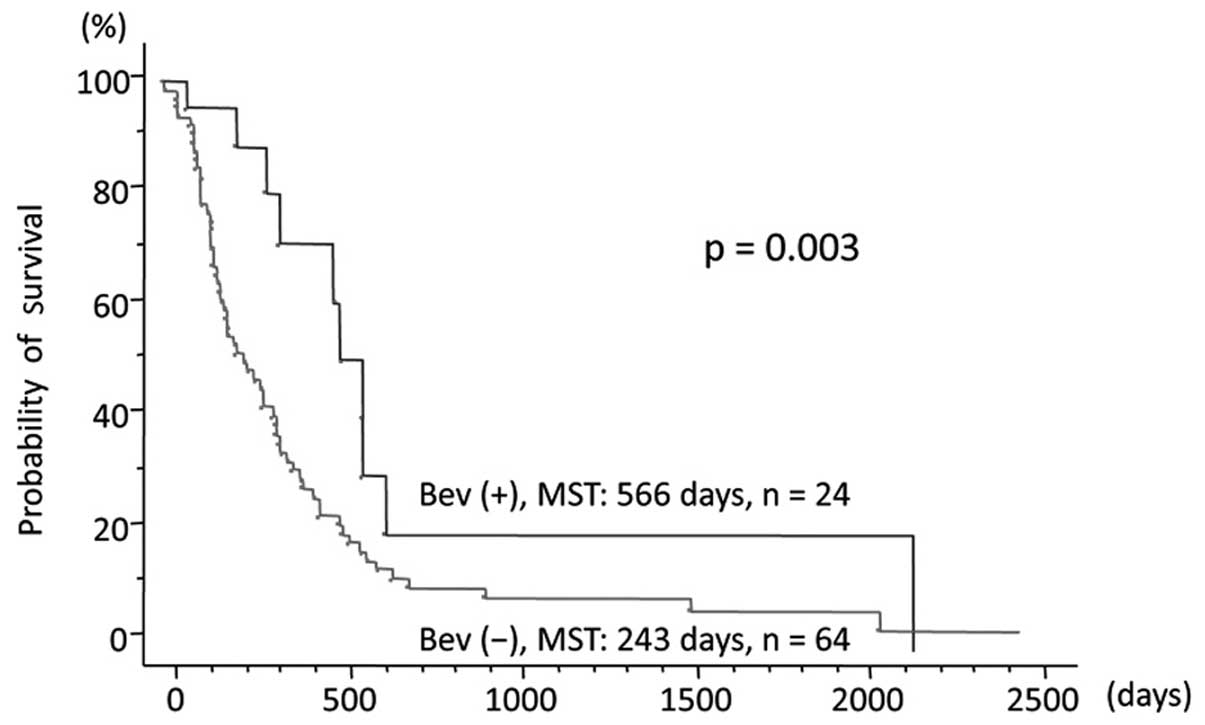
Of the 88 patients, OS was longer in the 24 patients
treated with BEV compared with that in the 64 patients who did not
receive BEV [MST, 566 vs. 243 days; hazard ratio (HR)=0.413; 95%
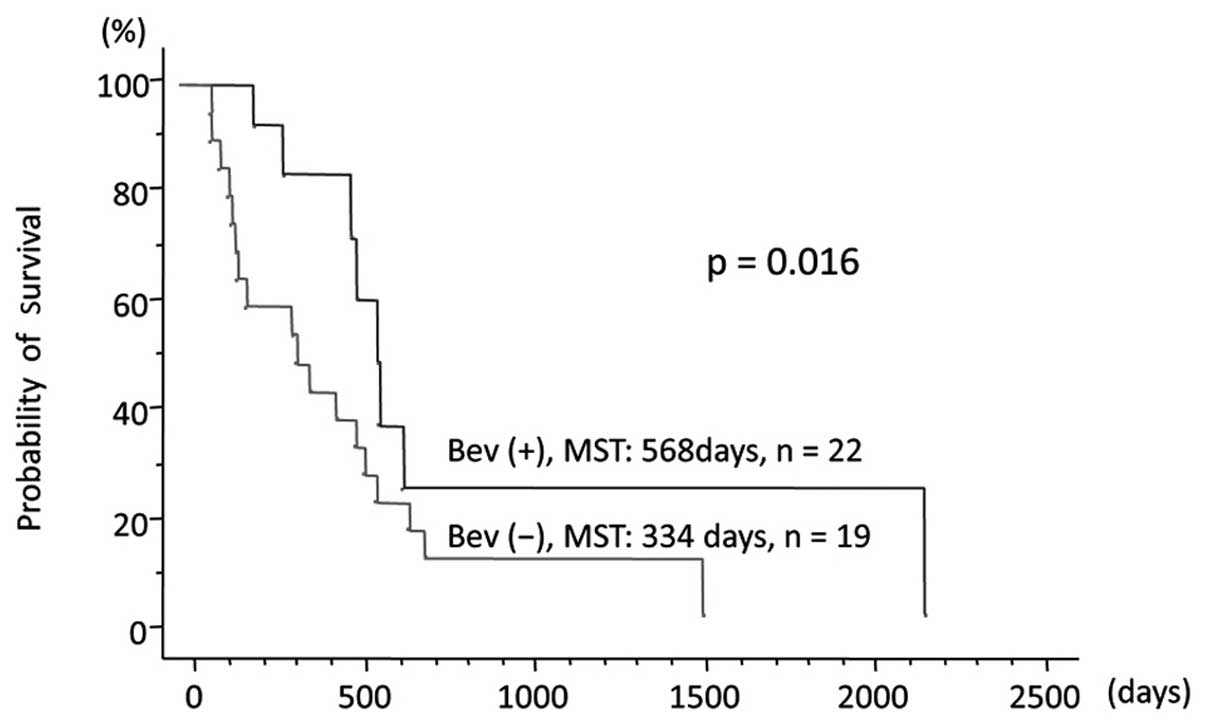
confidence interval (CI): 0.216–0.787; P=0.003; Fig. 2]. In the 41 patients who received
TMZ-based chemoradiotherapy, OS was longer in the 22 patients
treated with BEV compared with that in the 19 patients not treated
with BEV (MST, 568 vs. 334 days, respectively; HR=0.404; 95% CI:
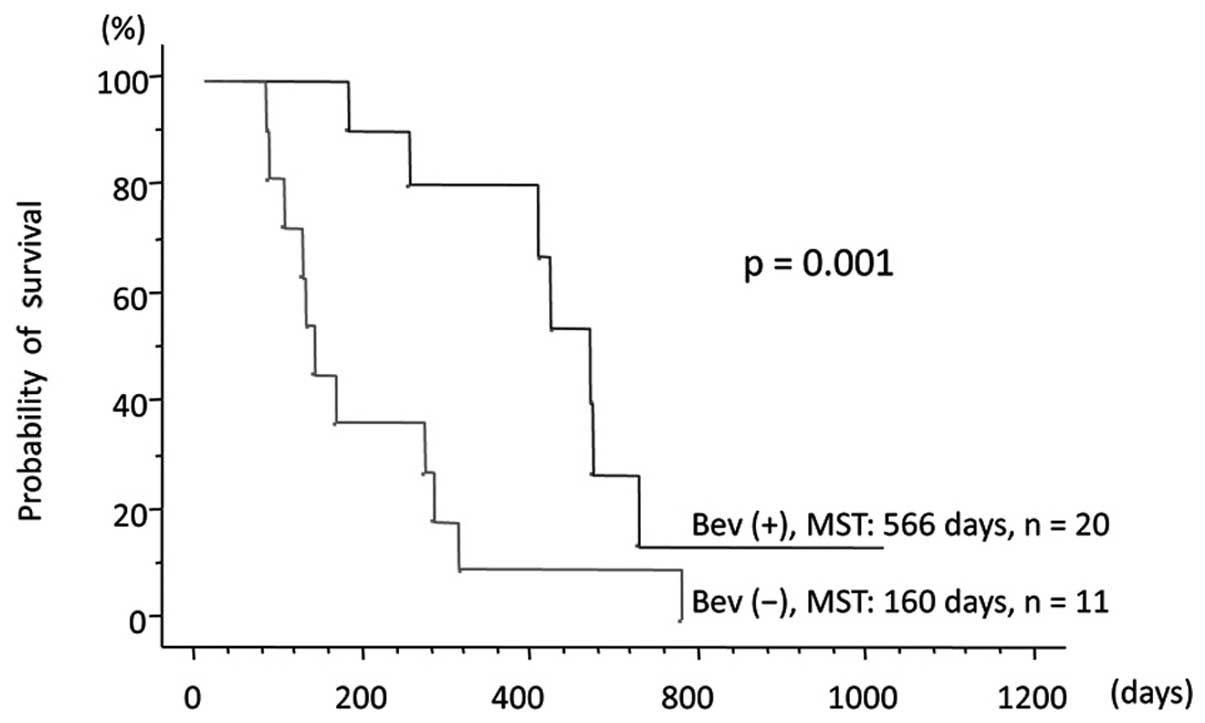
0.175–0.933; P=0.016; Fig. 3). In
the 31 patients with glioblastomas who received TMZ-based
chemoradiotherapy, OS was longer in the 20 patients treated with
BEV compared with that in the 11 patients not treated with BEV
(MST, 566 vs. 160 days, respectively; HR=0.253; 95% CI:
0.099–0.646; P=0.001; Fig. 4). The
independent contributing factors for OS were deduced using Cox
proportional hazards regression models. In 72 patients who
underwent any type of adjuvant therapy, Cox proportional hazard
analysis using the World Health Organization (WHO) grade, age,
Eastern Cooperative Oncology Group (ECOG) performance scale score
after biopsy, radiation, CyberKnife, nimustin, TMZ and BEV as
covariates, revealed that WHO grade III, good PS (0–2) and use of
BEV were independent prognostic factors of survival (HR<1;
P<0.05; Table III). In the 41
patients who underwent TMZ-based chemoradiotherapy, Cox
proportional hazard analysis using WHO grade, age, ECOG performance
scale score after biopsy, CyberKnife and use of BEV as covariates,
revealed that WHO grade III, good PS (0–2) and use of BEV were also
independent prognostic factors of survival (HR<1; P<0.05;
Table IV). The use of BEV was the
strongest favourable factor (HR=0.101; P=0.0002) among the three
factors. No BEV-specific adverse events, such as wound dehiscence,
intracranial hemorrhage, or extracranial hemorrhage, were
reported.
 | Table III.Cox proportional hazard model
analysis of the 72 patients who underwent adjuvant therapy after
biopsy. |
Table III.
Cox proportional hazard model
analysis of the 72 patients who underwent adjuvant therapy after
biopsy.
|
Characteristics | Degree of
freedom | P-value | Hazard ratio |
|---|
| WHO grade III | 1 | 0.0110 | 0.453 |
| Age | 1 | 0.9662 | 1.000 |
| PS (0,1,2) | 1 |
<0.0001 | 0.201 |
| Radiotherapy | 1 | 0.2538 | 0.537 |
| CyberKnife | 1 | 0.2596 | 1.510 |
| Nimustin | 1 | 0.1092 | 0.449 |
| TMZ | 1 | 0.2021 | 0.545 |
| Bevacizumab | 1 | 0.0002 | 0.199 |
 | Table IV.Cox proportional hazard model
analysis of the 41 patients who underwent TMZ-based
chemoradiotherapy after biopsy. |
Table IV.
Cox proportional hazard model
analysis of the 41 patients who underwent TMZ-based
chemoradiotherapy after biopsy.
|
Characteristics | Degree of
freedom | P-value | Hazard ratio |
|---|
| WHO grade III | 1 | 0.0038 | 0.207 |
| Age | 1 | 0.2811 | 1.013 |
| PS (0,1,2) | 1 | 0.0017 | 0.135 |
| CyberKnife | 1 | 0.1518 | 2.242 |
| Bevacizumab | 1 | 0.0002 | 0.101 |
Discussion
The results of this retrospective analysis using
Kaplan-Meier and Cox hazard model analyses suggest a strong
beneficial effect of BEV on the OS of patients with malignant
glioma. Among all 88 patients and the 41 patients who underwent
TMZ-based chemoradiotherapy, the proportion of patients with grade
III tumors was higher among the non-BEV patients; however, this
does not invalidate the results showing an effect of BEV on OS, as
WHO grade III was an independent prognostic factor for OS according
to the results of the Cox proportional hazards model analysis. The
16 patients who did not receive any adjuvant therapy were included
in the total 88 patients; thus, the frequencies of cytotoxic
chemotherapy and RT were not balanced between the BEV and non-BEV
patients. If the 16 patients were omitted, no significant
difference in treatment frequency would have been found between the
BEV and non-BEV groups. In addition, these treatments were not
independent prognostic factors for OS (Tables III and IV).
BEV is an anti-VEGFA molecule that strongly inhibits
the action of the angiogenic factors spontaneously produced by
tumor cells and/or tumor-associated stroma in malignant tumors
(7). Through its inhibitory effect,
BEV reduced the peritumoral edema in human malignant brain tumors,
including glioblastomas and metastatic lesions. BEV also suppressed
glioblastoma growth in an in vivo xenograft model (8,9).
BEV is well-known to significantly prolong PFS in a
number of cancer types. However, in terms of OS, it showed some
benefit in certain types of primary and metastatic cancers, but not
in breast, pancreatic, gastric, or ovarian cancer (10–13). The
difference in the benefit of BEV regarding OS among various types
of malignant tumors has been attributed to the variation and
effects of cytotoxic chemotherapeutic agents concomitantly used
with BEV on neoplasms (12,13). Rapid changes in the biological nature
of cancers induced by anti-VEGF therapy and/or the cessation of BEV
treatments defined by treatment protocols may induce rebound
effects on angiogenesis and tumor growth. These effects may
eventually curtail the BEV benefit for OS (10,12).
Differences in disease-specific OS and length of survival after
progression between malignancies may play a role in this difference
(11,12,14,15).
Various additional lines of treatment after progression, including
crossover-allowed design, may also obscure the survival-improving
benefit of anti-VEGF therapy (10,13).
Two precedent randomized controlled trials (AVAglio
and RTOG-0825) reported longer median PFS with BEV compared with
that with placebo, but did not observe a prolongation of OS with
the addition of BEV to RT plus TMZ (1,2). One of
the suggested reasons for failure to exert an effect on OS is the
crossover design of the studies on glioblastoma (1,2,16). Another suspected reason for the
absence of a prolonging effect on OS was omission of patients with
a Karnofsky performance status of <70 in the study cohort
(1,2). Omission of such patients may cancel the
potential OS-prolonging effect that may be gained through
improvement of performance by BEV administration (16).
The advantage of our study is that the subjects were
limited to those who underwent biopsy alone. Between 2000 and 2015,
which is the period during which our patients underwent surgery,
the 5-aminolevulinic acid-induced fluorescence technique and
intraoperative magnetic resonance imaging to identify residual
tumor were introduced in our hospital. Thus, the resection rate
improved over time (5). This made it
difficult to compare the OS of all the patients with malignant
glioma in the BEV era (June, 2013-present) with that of those in
the pre-BEV era, as the resection rates differed significantly.
In our study design, the resection rate was
universally <10% and would not have affected the therapeutic
results. Therefore, we hypothesized that the independent effect of
BEV on malignant gliomas could be extracted through OS comparison
between patients who received BEV and those who did not, both
groups being almost ‘surgically naïve’. Biopsy-only cases were
omitted from the RTOG-0825 study, which comprised only 11.3% of the
AVAglio study cohort (1,2).
Although our study suggested a prolonging effect on
OS in unresectable malignant glioma cases, a recent report of a
phase II trial on unresectable glioblastomas (TEMAVIR study) did
not demonstrate prolongation of OS with BEV (17). It should be noted that the starting
ratio of radiation was 66.7% in the experimental arms and 97.6% in
the control arms in the latter studies. As regards adjuvant
therapy, BEV/irinotecan without TMZ was allocated to the
experimental arm; not only TMZ, but also salvage BEV was permitted
in the control arm. The inequality in the use of concomitant
chemotherapeutic agents, the infeasibility of treatment protocols
for the experimental arm, and the use of salvage BEV may have made
the benefit of BEV on OS difficult to identify. In addition, these
adjuvant treatments were continued for only 6 months or
discontinued upon disease progression. Our policy to continue
adjuvant treatment, including BEV, after progression was identified
may have contributed to the prolongation of OS in the BEV treatment
groups. In fact, BEV was continued after progression was diagnosed
in 8 patients of our series.
Among the 27 patients who received initial radiation
+ TMZ treatment in our study, 90.9% of patients in the group that
received additional BEV also received TMZ maintenance therapy; this
percentage was only 43.8% in the group without additional BEV.
Adding BEV to the initial chemoradiotherapy may protect the general
and neurological status of our senior population, which would
eventually facilitate maintenance chemotherapy and contribute to
the OS benefit.
This study has several disadvantages inherent to the
nature of small retrospective cohorts as follows: Lack of i)
randomization, ii) consistency of concomitant chemotherapeutic
agents, iii) dose and timing of BEV administration, and iv)
stratification according to isocitrate dehydrogenase-1 gene status
and gene profiling (3). A future
study protocol is currently being prepared with consideration of
the aforementioned factors.
This retrospective survey of 88 unresectable
malignant gliomas demonstrated the potential of BEV to prolong the
OS of patients with disadvantageous conditions. Considering the
rapidly aging population, the beneficial effect of BEV on OS should
be verified by prospective studies, particularly in subsets of
patients who are elderly and/or in poor general condition.
Acknowledgements
We would like to thank Editage (www.editage.jp) for the English language editing. This
study was partially aided by research grants, Kiban Research Grant
C (15K10338 to H.H.) from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandmann T, Bourgon R, Garcia J, Li C,
Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R,
et al: Patients with proneural glioblastoma may derive overall
survival benefit from the addition of bevacizumab to first-line
radiotherapy and temozolomide: Retrospective analysis of the
AVAglio trial. J Clin Oncol. 33:2735–2744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawano H, Hirano H, Yonezawa H, Yunoue S,
Yatsushiro K, Ogita M, Hiraki Y, Uchida H, Habu M, Fujio S, et al:
Improvement in treatment results of glioblastoma over the last
three decades and beneficial factors. Br J Neurosurg. 29:206–212.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Committee of Brain Tumor Registry of
Japan, . Report of Brain Tumor Registry of Japan (2001–2004). 13th
ed. Neurol Med Chir (Tokyo). 54:(Suppl). 1–102. 2014.
|
|
7
|
Ferrara N, Hillan KJ and Novotny W:
Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody
for cancer therapy. Biochem Biophys Res Commun. 333:328–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathieu V, De Nève N, Le Mercier M,
Dewelle J, Gaussin JF, Dehoux M, Kiss R and Lefranc F: Combining
bevacizumab with temozolomide increases the antitumor efficacy of
temozolomide in a human glioblastoma orthotopic xenograft model.
Neoplasia. 10:1383–1392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: Impact on invasion, disease progression and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ocaña A, Amir E, Vera F, Eisenhauer EA and
Tannock IF: Addition of bevacizumab to chemotherapy for treatment
of solid tumors: Similar results but different conclusions. J Clin
Oncol. 29:254–256. 2011. View Article : Google Scholar
|
|
12
|
Amit L, Ben-Aharon I, Vidal L, Leibovici L
and Stemmer S: The impact of bevacizumab (avastin) on survival in
metastatic solid tumors-a meta-analysis and systematic review. PLoS
One. 8:e517802013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Yan H, Zhao P, Yang Y and Cao B:
Efficacy and safety of bevacizumab combined with chemotherapy for
managing metastatic breast cancer: A meta-analysis of randomized
controlled trials. Sci Rep. 5:157462015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broglio KR and Berry DA: Detecting an
overall survival benefit that is derived from progression-free
survival. J Natl Cancer Inst. 101:1642–1649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morita S, Sakamaki K and Yin G: Detecting
overall survival benefit derived from survival postprogression
rather than progression-free survival. J Natl Cancer Inst.
107:djv1332015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Narita Y: Bevacizumab for glioblastoma.
Ther Clin Risk Manag. 11:1759–1765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chauffer B, Feuvret L, Bonnetain F,
Taillandier L, Frappaz D, Taillia H, Schott R, Honnorat J, Fabbro
M, Tennevet I, et al: Randomized phase II trial of irinotecan and
bevacizumab as neo-adjuvant and adjuvant to temozolomide-based
chemoradiation compared with temozolomide-chemoradiation for
unresectable glioblastoma: Final results of the TEMAVIR study from
ANOCEF. Ann Oncol. 25:1442–1447. 2014. View Article : Google Scholar : PubMed/NCBI
|