Introduction
Rectal cancer is the second and the third most
common type of cancer in developed countries and worldwide,
respectively, with a male: Female incidence ratio of (2–3):1
(1). Thus, a safe and feasible
treatment for rectal cancer is urgently required. In 1900,
laparoscopy was first used for right hemicolectomy, which was the
origin of laparoscopic surgery for colorectal cancer (2,3). It was
then used by Jacobs in 1991 (4),
which pioneered the era of laparoscopic rectal cancer surgery. At
present, laparoscopic surgery for rectal cancer is developing
rapidly in China, and is acknowledged by several doctors and
patients. However, it has not yet been widely approved or included
in international treatment guidelines. The aim of the present study
was to evaluate the curative effect of this type of surgery for
rectal cancer in order to promote its wider application
worldwide.
Patients and methods
Patient selection
A total of 64 cases of rectal cancer patients were
randomly divided into the laparoscopic surgery group (LS, n=31) and
the open surgery group (OS, n=33). A comparison was made between
the two groups according to the clinical data. The patient
selection criteria were as follows: All the patients consented to
participate in this study, they were all pathologically diagnosed
preoperatively via colonofiberscopic biopsy, without evidence of
distant metastases to the lung, abdominal cavity, liver or pelvic
cavity prior to and during the operation. In addition, the patients
were suitable candidates for radical cancer resection. Open or
laparoscopic surgery was selected according to the wishes of the
patients. The inclusion criteria for the study were as follows: All
the patients were diagnosed with rectal cancer, the standard
Karnofsky score was >70 prior to surgery, there was a definitive
pathological diagnosis and complete clinical data. The exclusion
criteria were patients with concomitant tumors that significantly
affected survival. Patients with pT4 and pN2 were not included in
the study, as such patients were only treated with open surgery in
our hospital at that time.
None of the two groups received neoadjuvant
chemotherapy. The surgeries were performed according to the total
mesorectal excision (TME) principle. The extent of tumor resection
of these two groups was determined with the aim to achieve negative
circumferential and distal margins. There was no statistically
significant difference between the two groups (P>0.05) regarding
age, gender, body mass index, localization and topography of the
tumor, pathological stage, type of surgery, status of the
circumferential and distal margins, quality of mesorectal excision,
pT and pN stage of the tumor. The comparison is summarized in
Table I. In both groups, a
proportion of the patients received anterior resection (Dixon),
whereas the remaining patients received abdominoperineal resection
(Miles). Thus, as shown in Tables
II and III, the comparisons
between these two operations were separately performed.
Furthermore, patients undergoing the open Dixon procedure received
more postoperative adjuvant chemotherapy compared with those
undergoing the laparoscopic Dixon procedure (P=0.019).
 | Table I.Comparison of general clinical data
between the LS and OS groups. |
Table I.
Comparison of general clinical data
between the LS and OS groups.
| Parameters | LS group (n=31) | OS group (n=33) | P-value |
|---|
| Age, years (mean ±
SD) | 65.6±10.9 | 63.0±1.8 | 0.448 |
| Gender
(male/female) | 18/13 | 19/14 | 0.968 |
| BMI, kg/m2
(mean ± SD) | 22.6±3.3 | 23.3±3.0 | 0.624 |
| Tumor location
(distance from anus, cm) | 7.8±4.8 | 8.6±3.4 | 0.669 |
| TNM stage |
|
| 0.999 |
| 0 | 1 | 1 |
|
| I | 14 | 15 |
|
| II | 10 | 11 |
|
| III | 6 | 6 |
|
| pT stage |
|
| 0.921 |
| Tis | 2 | 1 |
|
| T1 | 3 | 4 |
|
| T2 | 10 | 11 |
|
| T3 | 16 | 17 |
|
| pN stage |
|
| 0.911 |
| N0 | 22 | 23 |
|
| N1 | 9 | 10 |
|
| Type of surgery |
|
| 1.000 |
| Anterior
resection | 21 | 22 |
|
| AP
excision | 10 | 11 |
|
 | Table II.Comparison of general clinical data
between the LS and OS groups with the Dixon operation. |
Table II.
Comparison of general clinical data
between the LS and OS groups with the Dixon operation.
| Parameters | LS group (n=20) | OS group (n=21) | P-value |
|---|
| Age, years (mean ±
SD) | 67.8±10.4 | 64.4±10.4 | 0.376 |
| Gender
(male/female) | 15/5 | 15/6 | 0.796 |
| BMI, kg/m2
(mean ± SD) | 23.0±3.6 | 23.6±3.8 | 0.630 |
| Preoperative
anemia | 0 | 1 |
|
| Preoperative high
CEA | 10 | 8 | 0.443 |
| Tumor location
(distance from anus, cm, mean ± SD) | 12.3±2.5 | 8.9±3.3 | 0.116 |
| Tumor maximum
diameter (cm, mean ± SD) | 4.7±1.2 | 4.0±1.5 | 0.225 |
| TNM stage |
|
| 0.698 |
| 0 | 1 |
|
|
| I | 8 | 9 |
|
| II | 6 | 8 |
|
|
III | 5 | 4 |
|
| pT stage |
|
| 0.997 |
|
Tis | 1 | 1 |
|
| T1 | 2 | 2 |
|
| T2 | 6 | 7 |
|
| T3 | 11 | 11 |
|
| pN stage |
|
| 0.837 |
| N0 | 13 | 13 |
|
| N1 | 7 | 8 |
|
| Dukes' stage |
|
| 0.904 |
| A | 5 | 6 |
|
| B | 8 | 9 |
|
| C | 7 | 6 |
|
| Postoperative
adjuvant chemotherapy | 6 | 14 | 0.019 |
 | Table III.Comparison of general clinical data
between the LS and OS groups with the Miles operation. |
Table III.
Comparison of general clinical data
between the LS and OS groups with the Miles operation.
| Parameters | LS group
(n=11) | OS group
(n=12) | P-value |
|---|
| Age, years (mean ±
SD) | 62.2±11.6 | 57.5±16.6 | 0.555 |
| Gender
(male/female) | 3/8 | 4/8 | 0.752 |
| BMI,
kg/m2 (mean ± SD) | 22.9±3.0 | 23.0±2.8 | 0.619 |
| Preoperative
anemia | 2 | 4 |
|
| Preoperative high
CEA | 8 | 6 | 0.265 |
| Tumor location
(distance from anus, cm; mean ± SD) | 4.4±2.4 | 4.0±1.0 | 0.814 |
| Tumor maximum
diameter, cm (mean ± SD) | 4.5±1.7 | 5.1±2.6 | 0.557 |
| TNM stage |
|
| 0.697 |
| 0 |
| 1 |
|
| I | 6 | 6 |
|
| II | 4 | 3 |
|
|
III | 1 | 2 |
|
| pT stage |
|
| 0.709 |
|
Tis | 1 | 0 |
|
| T1 | 1 | 2 |
|
| T2 | 4 | 4 |
|
| T3 | 5 | 6 |
|
| pN stage |
|
| 0.924 |
| N0 | 9 | 10 |
|
| N1 | 2 | 2 |
|
| Dukes' stage |
|
| 0.925 |
| A | 5 | 5 |
|
| B | 4 | 4 |
|
| C | 2 | 3 |
|
| Postoperative
adjuvant chemotherapy | 6 | 10 | 0.134 |
Patient records/information were anonymized and
de-identified prior to analysis. Our research was performed in
accordance with the principles outlined in the Declaration of
Helsinki and was approved by the Medical Ethics Committee of the
Guangzhou Hospital of Integrated Traditional Chinese and Western
Medicine.
Surgical procedures
The surgical operating principles of the LS and OS
groups were the same, following strict rules for achieving a
tumor-free status. In both groups, patients underwent the Miles or
Dixon operation. The Dixon operation was selected if the tumor was
located ≤5 cm from the anus, whereas the Miles operation was
selected if the tumor was located ≥5 cm from the anus.
Perioperative and postoperative
management protocols
On the day prior to the operation, the auxiliary
examinations and preparation were completed and there were no
obvious surgical contraindications. The blood pressure and blood
sugar levels were effectively controlled. The patients were
administered a full liquid diet, intravenous nutrition and oral
antibiotics and underwent an enema, skin preparation and
preoperative fasting for 12 h. On the day of the operation, a
nasogastric tube was inserted and intravenous prophylactic
antibiotics were administered 30 min prior to the operation. On the
day after the operation, the patients underwent gastrointestinal
decompression, indwelling catheterization and electrocardiographic
monitoring. The fluid draining from the peritoneal cavity via the
drainage tube was carefully observed. After the operation, the
patients received routine intravenous antibiotics and intravenous
nutrition. Early ambulation was encouraged. The patients were
fasted and received gastrointestinal decompression until they
passed gas, then were given a full liquid diet for 3 days, a
semi-liquid diet for another 3 days and a normal diet thereafter.
If the patients had been eating well without any feeling of
abdominal discomfort, the peritoneal drainage tube was removed and
the intravenous liquid was gradually reduced. The sutures were
removed ~1 week after the operation. Chemotherapy was considered 1
month after the operation on the basis of the pathological stage.
Chemotherapy in patients undergoing laparoscopic surgery was
initiated earlier compared with patients undergoing open surgery
due to the shorter recovery time following laparoscopy. The
chemotherapeutic regimen was generally FOLFOX or FOLFIRI. Two
patients with pathological stage I and 32 patients with
pathological stage ≥II disease received adjuvant chemotherapy
following surgery. In addition, patients with residual lesions were
treated with postoperative radiotherapy.
Observation index and follow-up
Indices such as operation time, intraoperative blood
loss, number of lymph nodes retrieved, time to oral intake of food,
duration of postoperative hyperthermia and hospitalization time
were recorded. The patients were observed for postoperative
complications, including pulmonary infection, formation of fistulas
and intestinal adhesions.
Clinical physical examination, measurement of
carcinoembryonic antigen levels, computed tomography and
colonoscopy were used for follow-up. The patients were followed up
yearly after the operation. Thorough follow-up protocols were
applied to assess local recurrence, pulmonary metastasis and
multiple metastases. Survival status was constantly assessed and
the 3-year tumor-free survival rate was calculated.
Statistical analysis
All obtained data were analyzed with the SPSS 19.0
statistical software (SPSS Inc., Chicago, IL, USA). Measurement
data were expressed as mean ± standard deviation. The Student's
t-test was used for continuous variables and the Chi-square test
for categorical variables. In addition, the Kaplan-Meir method was
used for survival comparison. P<0.05 indicated a statistically
significant difference.
Results
Comparison between the LS and OS
groups in patients undergoing the Dixon procedure
For patients undergoing the Dixon procedure, the LS
group had a longer operation time compared with the OS group
(271.2±56.2 vs. 216.0±62.7 min, respectively; P=0.036), and a
shorter time to oral intake (3.0±0.9 vs. 4.7±1.0 days,
respectively; P=0.000). There was no significant difference between
the LS and OS groups in terms of intraoperative blood loss
(107.1±69.4 vs. 166.4±81.6 ml, respectively; P=0.096), number of
lymph nodes retrieved (7±4 vs. 6±6, respectively; P=0.604),
duration of postoperative hyperthermia (2.6±0.5 vs. 2.6±1.6 days,
respectively; P=0.930) and hospitalization time (19.8±3.8 vs.
25.8±5.2 days, respectively; P=0.067). In addition, there was no
significant difference between the LS and OS groups with the Dixon
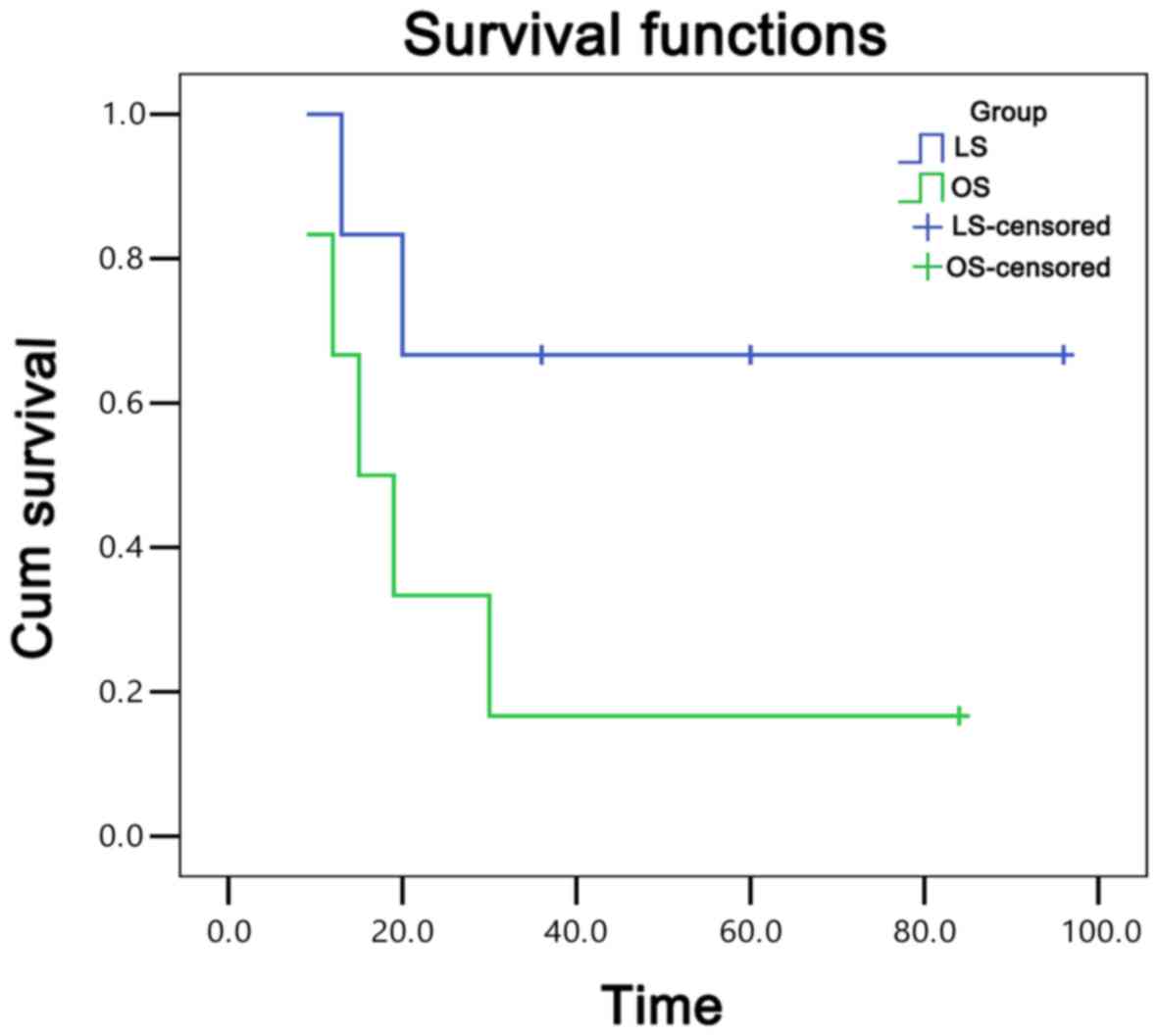
operation regarding 3-year tumor-free survival rate (35.0 vs.
38.1%, respectively; P=0.837), as indicated in Table IV.
 | Table IV.Comparison of therapeutic effect
between the LS group and OS groups with the Dixon operation. |
Table IV.
Comparison of therapeutic effect
between the LS group and OS groups with the Dixon operation.
| Parameters | LS group
(n=20) | OS group
(n=21) | P-value |
|---|
| Operation time, min
(mean ± SD) | 271.2±56.2 | 216.0±62.7 | 0.036 |
| Intraoperative
blood loss, ml (mean ± SD) | 107.1±69.4 | 166.4±81.6 | 0.096 |
| Number of lymph
nodes retrieved (mean ± SD) | 7±4 | 6±6 | 0.604 |
| Time to oral
intake, days (mean ± SD) | 3.0±0.9 | 4.7±1.0 | 0.000 |
| Duration of
postoperative hyperthermia, days (mean ± SD) | 2.6±0.5 | 2.6±1.6 | 0.930 |
| Postoperative
complications |
|
|
|
|
Pulmonary infection | 0 | 1 |
|
|
Intestinal adhesion | 0 | 1 |
|
|
Fistula | 1 | 0 |
|
| Hospitalization,
days (mean ± SD) | 19.8±3.8 | 25.8±5.2 | 0.067 |
| 3-year tumor-free
survival rate (%) | 35.0 | 38.1 | 0.837 |
| Postoperative
recurrence/metastasis |
|
|
|
| Local
recurrence | 1 | 1 |
|
|
Pulmonary metastasis | 0 | 1 |
|
|
Multiple metastasis | 0 | 1 |
|
Comparison between the LS and OS
groups in patients undergoing the Miles procedure
For patients undergoing the Miles procedure, there
was no significant difference between the LS and OS groups in terms
of operation time (310.5±93.2 vs. 284.2±77.2 min, respectively;
P=0.551), intraoperative blood loss (211.1±74.0 vs. 280.0±164.3 ml,
respectively; P=0.416), number of lymph nodes retrieved (5±7 vs.
8±4, respectively; P=0.429), time to oral intake (3.1±0.4 vs.
3.7±1.0, respectively; P=0.203), duration of postoperative
hyperthermia (2.4±1.2 vs. 3.2±1.0 days, respectively; P=0.210) and
hospitalization time (26.6±7.8 vs. 31.5±10.0 days, respectively;
P=0.314). Furthermore, there was no significant difference between
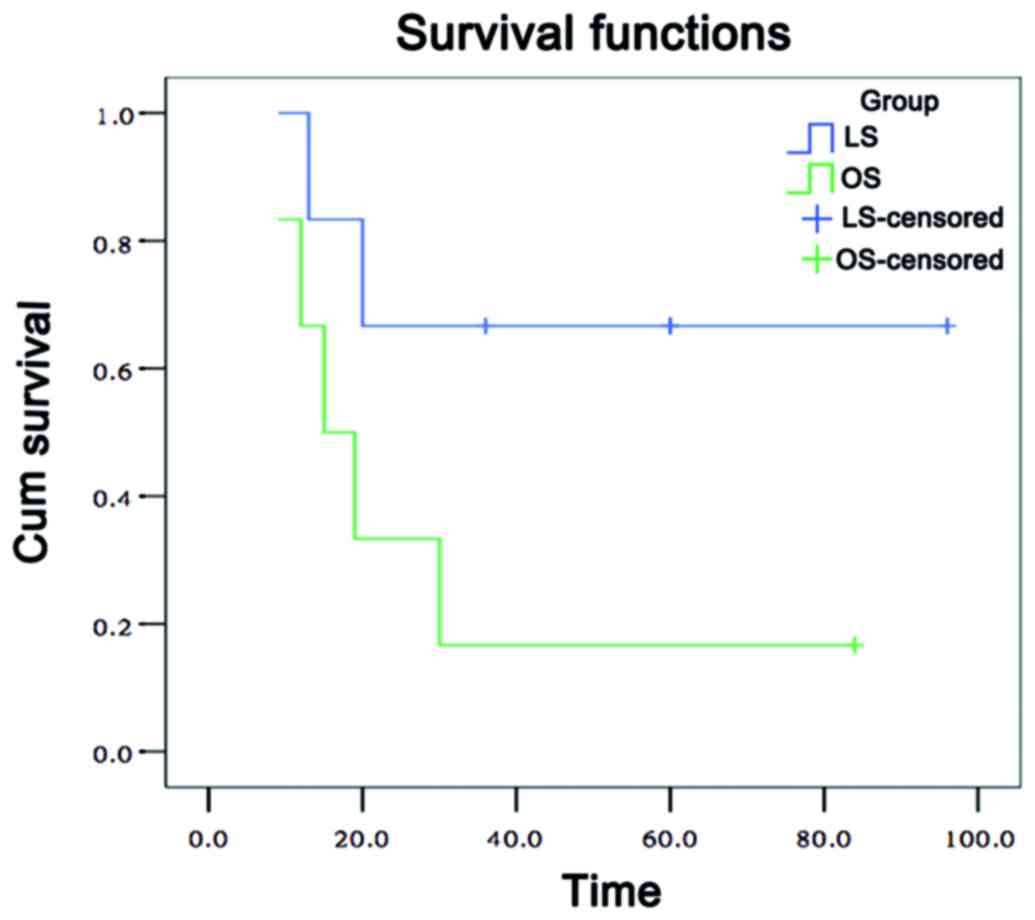
the LS and OS groups regarding 3-year tumor-free survival rate
(36.4 vs. 33.3%, respectively; P=0.879), as indicated in Table V.
 | Table V.Comparison of therapeutic effect
between the LS and OS groups with the Miles operation. |
Table V.
Comparison of therapeutic effect
between the LS and OS groups with the Miles operation.
| Parameters | LS group
(n=11) | OS group
(n=12) | P-value |
|---|
| Operative time, min
(mean ± SD) | 310.5±93.2 | 284.2±77.2 | 0.551 |
| Intraoperative
blood loss, ml (mean ± SD) | 211.1±74.0 | 280.0±164.3 | 0.416 |
| Number of lymph
nodes retrieved (mean ± SD) | 5±7 | 8±4 | 0.429 |
| Time to oral
intake, days (mean ± SD) | 3.1±0.4 | 3.7±1.0 | 0.203 |
| Duration of
postoperative hyperthermia, days (mean ± SD) | 2.4±1.2 | 3.2±1.0 | 0.210 |
| Postoperative
complications |
|
|
|
|
Pulmonary infection | 1 | 1 |
|
|
Intestinal adhesion | 0 | 1 |
|
|
Fistula | 0 | 0 |
|
| Hospitalization
time, days (mean ± SD) | 26.6±7.8 | 31.5±10.0 | 0.314 |
| 3-year tumor-free
survival rate, % | 36.4 | 33.3 | 0.879 |
| Postoperative
recurrence/metastasis |
|
|
|
| Local
recurrence | 0 | 0 |
|
|
Pulmonary metastasis | 2 | 1 |
|
|
Multiple metastasis | 1 | 0 |
|
The operation was successfully performed in all the
patients, without damage to the adjacent organs and supplying blood
vessels. Only 1 case of laparoscopic Dixon operation was converted
to the open Dixon procedure due to bleeding. There was no
circumferential margin positivity in any of the patients. All cases
of preoperative anemia were corrected prior to surgery. There was 1
patient with a distal surgical margin at a distance of only 1 cm
from the lesion (pathological TNM stage III, pathological Dukes'
stage C), who was treated postoperatively with the FOLFOX
chemotherapeutic regimen; the patient survived for 4 years. Apart
from patients undergoing the Miles operation, no patient received a
diverting loop ostomy. Intestinal decompression measures were
commonly applied; a plastic thread tube with negative pressure was
used intraoperatively to suction the intestinal contents when
necessary. Every effort was made to secure the bowel anastomosis
during the first phase of the operation. Therefore, unless there
was severe inflammation or edema in the bowel wall caused by
obstruction, a diverting loop stoma was seldom adopted. In
addition, there was 1 case of postoperative pulmonary infection
among LS group patients undergoing the Miles operation, 2 cases of
postoperative pulmonary infection in the OS group (1 Dixon and 1
Miles operation) and 2 cases of postoperative intestinal adhesion
in the OS group (1 Dixon and 1 Miles operation). Only 1 patient
undergoing the laparoscopic Miles operation developed the
postoperative complication of chronic perineal fistula; the patient
recovered following fistulectomy. No other patients developed
fistula. There was 1 case of local recurrence (descending colon
cancer) 1.5 years later in the LS group with the Dixon operation,
and 1 case of local recurrence (cancer of the hepatic flexure) 1
year later in the OS group with the Dixon operation. There were 2
cases of lung metastasis in the LS group with the Miles operation
(1 at 6 years and 1 at 5 years after the operation), and 2 cases of
lung metastasis in the OS group (1 within the first year after the
Dixon operation, and 1 in the second year after the Miles
operation). Furthermore, there was 1 case of multiple metastases
(lung, liver and urethra) in the LS group >3 years after the
Miles operation, and 1 case of multiple metastases (bladder and
lung) in the OS group >1 year after the Dixon operation.
In the 3 years after the operation, 1 case was lost
to follow-up from the LS group and 2 cases from the OS group. The
95% confidence interval of the LS and OS groups with the Dixon
operation were 78–116 and 109–184 months, respectively. There was
no significant difference between the two groups regarding
postoperative chemotherapy (P>0.05). The survival curves of the
OS and LS groups with the Dixon operation are shown in Fig. 1. Similarly, the 95% confidence
interval of the LS and OS group with Miles operation were 39–100
and 7.4–48 months, respectively. There was no significant
difference in postoperative chemotherapy between these two groups
(P>0.05). The survival curves of the OS and LS groups with the
Miles operation are shown in Fig.
2.
Discussion
Rectal cancer is the third most common type of
cancer worldwide, with a yearly increasing incidence. Surgical
resection is the preferred curative approach to cancer treatment.
Laparoscopic surgery is a rapidly advancing, minimally invasive
operative procedure. Laparoscopic colon surgery was first reported
in 1991 (5), after which time it has
been increasingly performed worldwide for the treatment of
colorectal cancer. In 1993, laparoscopic colectomy was introduced
in China, and it has become a proficient and widely used method
over the past 10 years. Laparoscopic colectomy has been generally
acknowledged as an effective therapy (6). However, laparoscopic surgery for rectal
cancer had not been recommended outside a clinical trial (7), and remains controversial due to the
limited availability of long-term data on survival and recurrence
(8). As a therapy for malignant
tumors, the operative safety and long-term effects of laparoscopic
surgery are under constant scrutiny.
In this study, the operation time with the
laparoscopic Dixon operation was longer compared with that with
open surgery. The difference was statistically significant, as
laparoscopic surgery was more complicated and the operators did not
have adequate technical proficiency. Laparoscopic radical resection
of rectal carcinoma is an operation with distinct anatomical
characteristics and it is crucial to determine the accurate
anatomical plane and distinguish anatomic landmarks during
laparoscopy. Operative laparoscopy mainly consists of three steps,
namely vessel separation, lymph node dissection and ensuring a
tumor-free resection margin. In this study, there was no
significant difference between laparoscopic and open surgery with
the Miles operation for rectal cancer treatment. Although
technically challenging, laparoscopic rectal cancer surgery may
provide a more magnified view of the pelvic cavity compared with
open surgery, which may facilitate the resection of the mesorectum
with a higher accuracy and greater ease (9). The duration of postoperative
hyperthermia reflects the degree of trauma. In this study, there
was no significant difference in the duration of postoperative
hyperthermia between laparoscopic and open surgery, although the
operation time of laparoscopic surgery was longer compared with
that of open surgery, possibly due to the minimal invasiveness of
the laparoscopic procedure. Furthermore, compared with open
surgery, the laparoscopic method is associated with smaller
incisions, less tissue damage, less postoperative discomfort,
shorter time to ambulation and a lower incidence of postoperative
complications. The visual field under a laparoscope is broader,
which is helpful for intraoperative exploration, particularly in
patients with tumors of the lower rectum. In addition, the
laparoscope may be inserted into the pelvis minor and provide a
better view of the surgical field, which may be helpful in
distinguishing and preserving key structures, such as the pelvic
nerve plexus, so as to avoid postoperative urinary retention and
sexual dysfunction. Moreover, the cavitation effect produced by the
ultrasonic knife on the tissue plane may enable faster and more
accurate dissection of the rectum, so that the pelvic nerve plexus
is protected and the risk of damage to the sacral venous plexus is
reduced. With accumulation of technical experience and an
increasing number of skilled operators, the operation time for
laparoscopic rectal cancer resection may be further shortened.
Dural et al (8) reported that patients undergoing
laparoscopic surgery for rectal cancer had similar incidence rates
of complications, circumferential resection margin involvement, and
long-term outcomes compared with those undergoing open surgery.
However, laparoscopic surgery for rectal cancer had the advantage
of minimal invasiveness. These conclusions were in agreement with
ours. Although laparoscopic surgery may require more advanced
technical skills, the same long-term oncological results were
obtained with this technique compared with open surgery (8). In this study, the postoperative
complications with laparoscopic surgery, such as pulmonary
infection and intestinal adhesions, were similar to those of open
surgery. The time to oral intake of the LS group was shorter
compared with that of the OS group, which meant faster
gastrointestinal recovery following laparoscopic surgery. Patients
in the LS group had a shorter hospital stay, which reduced the cost
of medical treatment and the burden of the patients. In the
literature, laparoscopy was found to be cost-effective for rectal
cancer surgery, improving health care expenditure as well as
patients' outcomes. For selected patients, laparoscopic rectal
cancer resection may shorten the hospitalization time, operating
time and resource utilization (10).
In addition, oncologic clearance of the resection margins and the
number of lymph nodes removed were similar between the laparoscopic
and open surgery groups (9,11–14).
This was consistent with our results. Distal margins remain the
most widely debated pathological parameter for total mesorectal
excision surgery. Initially, a 5-cm distal margin was considered to
be necessary. However, this criterion was refuted in the 1980s when
the distal tumor spread was found to be <5 cm (15). Recently, several studies suggested
that <1 cm or even a merely negative margin may be acceptable
(16,17). The extent of tumor removal and
metastatic lymph node dissection are key to rectal cancer surgery
and are associated with the quality of life, recurrence rate and
survival rate postoperatively.
Two recent meta-analyses demonstrated that a clear
benefit was conferred by the construction of a stoma, with
significantly low leakage and reoperation rates (18,19).
Although a stoma was not constructed in our patients, there was
only 1 case of chronic perineal fistula in a male patient with a
stage III tumor undergoing the laparoscopic Miles operation. The
patient recovered following fistulectomy. The inherent drawbacks of
laparoscopic rectal resection, such as the narrow space in which to
insert the stapling device, inadequate traction and the oblique
cutting angle, may require multiple applications of the linear
stapler. A recent study reported that an unduly long transection
line with multiple staples may result in anastomotic leakage
(20,21). In this study, one-stage anastomosis
of the colon and rectum was feasible in the majority of the
patients, which requires full preoperative upper intestinal
preparation (emptying), sufficient broad anastomosis with good
blood supply and without tension, and distal intestinal patency.
During the Dixon operation, prior to inserting the stapling
apparatus, the anus was stretched to 4–6 fingers. A drainage tube
was inserted into the pelvic cavity. All those measures were
effective in preventing anastomotic fistula formation. Furthermore,
Park et al reported that male gender, low anastomosis,
preoperative chemoradiation, advanced tumor stage, perioperative
bleeding and multiple firings of the linear stapler increased the
risk of anastomotic leakage following laparoscopic surgery for
rectal cancer. A diverting stoma may be mandatory in patients with
≥2 of the abovementioned risk factors (22).
In recent years, accumulating evidence from single-
and multicenter randomized trials indicated that laparoscopic
surgery for rectal cancer was associated with earlier postoperative
recovery, lower morbidity and better short-term quality of life
compared with open surgery (23).
Thus, it was concluded that laparoscopic surgery was associated
with a greater short-term benefit compared with open surgery.
However, this approach was not widely accepted, partly due to its
technical difficulties and certain doubts regarding its long-term
oncological outcomes. Recent randomized controlled trials have
demonstrated that laparoscopic surgery for colon cancer is similar
to open surgery in terms of oncological outcome, and several
advantages of laparoscopic surgery have been reported regarding
short-term outcomes. However, controversy persists regarding the
appropriateness of laparoscopic surgery for patients with rectal
carcinoma due to concerns regarding the safety of the procedure and
the uncertainty of the long-term outcome (24). The first randomized study describing
long-term oncological outcomes was reported by Braga et al
(14), who observed that there was
no difference between the two approaches in terms of 5-year overall
and disease-free survivals. Laurent et al (25) reported that laparoscopic surgery was
an independent predictor of better overall survival, but not
cancer-specific survival, for rectal cancer. The authors concluded
that the type of surgery did not affect cancer outcome. Yamamoto
et al reported that laparoscopic surgery may be used for
safe and radical resection of clinical stage 0/I rectal cancer,
without increasing the short-term surgical and oncological risks
(26). The study of Lacy et
al (27) demonstrated that,
compared with open surgery, laparoscopic surgery may confer a
survival benefit in patients with rectal cancer of Duke's stage C.
These may be attributed to the reduced contact of the tumor with
the abdominal organs during extraction, minimizing the risk of
spread, vision amplification with the laparoscopic camera, vascular
ligation of higher precision, and more thorough regional lymph node
dissection. In our study, the postoperative survival rate of
laparoscopic surgery was similar with that of open surgery,
indicating that laparoscopic surgery for rectal cancer is safe and
feasible.
In this study, although there was a difference in
postoperative chemotherapy between patients in the LS and the OS
groups undergoing the Dixon operation, there was no significant
difference in long-term outcome between the two surgeries. The
effect of chemotherapy on long-term survival will be addressed in
another study. In this study, there were significant differences
between laparoscopic and open surgery for rectal cancer in terms of
short-term results, such as operation time and hospitalization.
However, there was no significant difference regarding long-time
survival rate. In addition, the minimal invasiveness and safety of
laparoscopic surgery are acknowledged by an increasing number of
doctors and patients. It is considered that, with the continuous
advances in technology, accumulation of clinical experience and
improving team collaboration, laparoscopic surgery will be more
widely applied by more medical centers specializing on rectal
cancer treatment. We consider laparoscopic surgery to be a safe and
feasible option for rectal cancer treatment.
Acknowledgements
We would like to thank our colleagues H.J.S., L.S.J.
and Z.J. for their assistance and collaboration.
References
|
1
|
Lai SQ, Ju FH and Wang GQ: The clinical
epidemiological characteristics of 704 cases of colorectal cancer
from 2004–2008. China Cancer. 19:111–113. 2010.
|
|
2
|
Zhou XY, Zhang JL and Wang ZK: Comparative
study of laparoscopic and open surgery for colorectal cancer:
Short-term results. J Laparosc Surg. 17:199–203
|
|
3
|
Wang SY and Chen ZY: Short-term outcomes
of laparoscopy surgery for colorectal cancer: A comparative study.
Fudan Univ J Med Sci. 37:92–99. 2010.
|
|
4
|
Pugliese R, DiLernia S, Sansonna F,
Ferrari GC, Maggioni D, Scandroglio I, Costanzi A, Magistro C and
De Carli S: Outcomes of laparoscopic Miles' operation in very-low
rectal adenocarcinoma. Analysis of 32 cases. Eur J Surg Oncol.
33:49–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacobs M, Verdeja JC and Goldstein HS:
Minimally invasive colon resection (laparoscopic colectomy). Surg
Laparosc Endosc. 1:144–150. 1991.PubMed/NCBI
|
|
6
|
Lezoche E, Feliciotti F, Paganini AM,
Guerrieri M, De Sanctis A and Campagnacci R: Laparoscopic colonic
resection. J Laparoendose Adv Surg Tech A. 11:401–408. 2004.
View Article : Google Scholar
|
|
7
|
Bipat S, Glas AS, Slors FJ, Zwinderman AH,
Bossuyt PM and Stoker J: Rectal cancer: Local staging and
assessment of lymph node involvement with endoluminal US, CT, and
MR imaging-a meta-analysis. Radiology. 232:773–783. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dural AC, Keskin M, Balik E, Akici M,
Kunduz E, Yamaner S, Asoglu Q, Gulluoglu M and Bugra D: The role of
the laparoscopy on circumferential resection margin positivity in
patients with rectal cancer: Long-term outcomes at a single
high-volume institution. Surg Laparosc Endosc Percutan Tech.
25:129–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lujan J, Valero G, Hernandez Q, Sanchez A,
Frutos MD and Parrilla P: Randomized clinical trial comparing
laparoscopic and open surgery in patients with rectal cancer. Br J
Surg. 96:982–989. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keller DS, Champagne BJ, Reynolds HL Jr,
Stein SL and Delaney CP: Cost-effectiveness of laparoscopy in
rectal cancer. Dis Colon Rectum. 57:564–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guillou PJ, Quirke P, Thorpe H, Walker J,
Jayne DG, Smith AM, Heath RM and Brown JM: MRC CLASICC trial group:
Short-term endpoints of conventional versus laparoscopic-assisted
surgery in patients with colorectal cancer (MRC CLASICC trial):
Multicentre, randomised controlled trial. Lancet. 365:1718–1726.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang SB, Park JW, Jeong SY, Nam BH, Choi
HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, et al: Open versus
laparoscopic surgery for mid or low rectal cancer after neoadjuvant
chemoradiotherapy (COREAN trial): Short-term outcomes of an
open-label randomised controlled trial. Lancet Oncol. 11:637–645.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng
Z, Li L, Shu Y and Wang TC: Laparoscopic versus open total
mesorectal excision with anal sphincter preservation for low rectal
cancer. Surg Endosc. 18:1211–1215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braga M, Frasson M, Vignali A, Zuliani W,
Capretti G and Di Carlo V: Laparoscopic resection in rectal cancer
patients: Outcome and cost-benefit analysis. Dis Colon Rectum.
50:464–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollett WG and Nicholls RJ: The
relationship between the extent of distal clearance and survival
and local recurrence rates after curative anterior resection for
carcinoma of the rectum. Ann Surg. 198:159–163. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernstein TE, Endreseth BH, Romundstad P
and Wibe A: Norwegian Colorectal Cancer Registry: What is a safe
distal resection margin in rectal cancer patients treated by low
anterior resection without preoperative radiotherapy? Colorectal
Dis. 14:e48–e55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fitzgerald TL, Brinkley J and Zervos EE:
Pushing the envelope beyond a centimeter in rectal cancer:
Oncologic implications of close, but negative margins. J Am Coll
Surg. 213:589–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan WS, Tang CL, Shi L and Eu KW:
Meta-analysis of defunctioning stomas in low anterior resection for
rectal cancer. Br J Surg. 96:462–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huser N, Michalski CW, Erkan M, Schuster
T, Rosenberg R, Kleeff J and Friess H: Systematic review and
meta-analysis of the role of defunctioning stoma in low rectal
cancer surgery. Ann Surg. 248:52–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JS, Cho SY, Min BS and Kim NK: Risk
factors for anastomotic leakage after laparoscopic intracorporeal
colorectal anastomosis with a double stapling technique. J Am Coll
Surg. 209:694–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito M, Sugito M, Kobayashi A, Nishizawa Y,
Tsunoda Y and Saito N: Relationship between multiple numbers of
stapler firings during rectal division and anastomotic leakage
after laparoscopic rectal resection. Int J Colorectal Dis.
23:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JS, Choi GS, Kim SH, Kim HR, Kim NK,
Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, et al: Multicenter
analysis of risk factors for anastomotic leakage after laparoscopic
rectal cancer excision: The Korean laparoscopic colorectal surgery
study group. Ann Surg. 257:665–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak
TW, Leung WW and Leung KL: Long-term oncologic outcomes of
laparoscopic versus open surgery for rectal cancer: A pooled
analysis of 3 randomized controlled trials. Ann Surg. 259:139–147.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jayne DG, Guillou PJ, Thorpe H, Quirke P,
Copeland J, Smith AM, Heath RM and Brown JM: UK MRC CLASICC Trial
Group: Randomized trial of laparoscopic-assisted resection of
colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial
Group. J Clin Oncol. 25:3061–3068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laurent C, Leblanc F, Wütrich P, Scheffler
M and Rullier E: Laparoscopic versus open surgery for rectal
cancer: Long-term oncologic results. Ann Surg. 250:54–61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto S, Ito M, Okuda J, Fujii S,
Yamaguchi S, Yoshimura K, Sugihara K and Watanabe M: Japan Society
of Laparoscopic Colorectal Surgery: Laparoscopic surgery for stage
0/I rectal carcinoma: Short-term outcomes of a single-arm phase II
trial. Ann Surg. 258:283–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lacy AM, García-Valdecasas JC, Delgado S,
Castells A, Taurá P, Piqué JM and Visa J: Laparoscopy-assisted
colectomy versus open colectomy for treatment of non-metastatic
colon cancer: A randomised trial. Lancet. 359:2224–2229. 2002.
View Article : Google Scholar : PubMed/NCBI
|