Introduction
Spontaneous splenic rupture, also referred to as
atraumatic splenic rupture, is an uncommon serious complication of
acute leukemia, with very few reported cases since Rokitansky first
described spontaneous rupture of the spleen in a patient with
leukemia in 1861 (1–7). Although the precise occurrence of
spontaneous splenic rupture is not known, its actual morbidity may
be higher due to the difficulty of diagnosis in the past. In the
past, the mortality rate of splenic rupture was extremely high, due
to the patient's poor general condition, complications and
difficulty in diagnosis, with the major cause of death being
hypotensive shock from the extensive blood loss, whereas infection
and other complications also played a major role. However, with the
increased availability of imaging modalities, the mortality rate
has been reduced significantly over the past decades. In the
present study, we report a case of spontaneous splenic rupture
occurring in a patient with acute myeloid leukemia (AML).
Case report
A 58-year-old male patient was admitted to the Luhe
Hospital (Beijing, China) in July, 2015, with a 2-month history of
fever and cough, without symptoms of fatigue or night sweats. The
patient had no history of TB or human immunodeficiency virus
infection. The physical examination was unremarkable. The
peripheral blood smear revealed pancytopenia [hemoglobin (HGB)
level of 83 g/l, white blood cell (WBC) count of
2.92×109/l and platelet (PLT) count of
61×109/l], with 22% blasts. Certain blast cells included
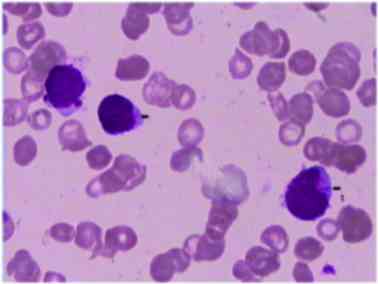
Auer rods. The bone marrow smear was characterized by
hypercellularity and patches of myeloblasts with Auer rods
(Fig. 1). These results were
consistent with AML of the M2 subtype. Following the diagnosis of
leukemia, leukemia genetic screening was performed. The gene
screening was negative. The c-KIT exon 8, c-KIT exon 17, FMS-like
tyrosine kinase 3 internal tandem duplication and nucleophosmin 1
were all wild-type, but Janus kinase (JAK) 2V617F or calreticulin
(CALR) gene detection was not performed. Bone marrow conventional
cytogenetic analysis on the G-banded metaphases revealed the
following: 46,XY, del (20)(q11)(20). The results of fluorescence in
situ hybridization were as follows: AML1/ETO:RUNX/RUNX1T1
0%.
The patient achieved complete remission after
induction chemotherapy with a regimen comprising daunorubicin 60
mg/m2 on days 1–3 and cytarabine 200 mg/m2 on
days 1–7; however, he developed severe myelosuppression,
agranulocytosis, and was febrile for 3 weeks. The galactomannan
assay was 56.10 pg/ml. Bronchoalveolar lavage revealed a few
bronchial epithelia and multifocal neutrophilic infiltration. An
abdominal and pelvic CT scan revealed no hepatosplenomegaly. The
patient was empirically treated with broad-spectrum antibiotics
(imipenem and cilastatin sodium for injection, vancomycin
hydrochloride for injection, cefoperazone sodium and sulbactam
sodium for injection) and antifungal agents [voriconazole for
injection (DSM Pharmaceuticals, Inc., Greenville, NC, USA) and
amphotericin B (North China Pharmaceutical Co., Ltd., Shijiazhuang,
China)]. A repeat chest CT showed improvement of the infiltrates
and his temperature normalized.
After the second course of chemotherapy, the
peripheral blood smear revealed a WBC count of
4.84×109/l with a small number of immature WBCs, an HGB
level of 106 g/l and a PLT count of 335×109/l. On repeat
bone marrow biopsy, dry tap aspiration, focal granulopoietic
progenitors and abnormal localization of immature precursors were
observed. The red cell population consisted mainly of intermediate-
and late-stage erythroblasts. The number of multinucleated
megakaryocytes was increased (8–12/high-power field). The reticulin
fibres were focally proliferated and were positive (++) on Gömöri
trichrome staining. On immunocytochemical staining, there was a
small number of CD34+ precursor cells, the leukemic
blasts were myeloperoxidase (MPO)+ and fractional
leukemic blasts were CD117+. The Ki-67 index was 30%
(Fig. 2). Interestingly, a
homozygous JAK2V617F mutation was detected by polymerase chain
reaction (PCR), as well as a missense mutation in exon 9 of the
CALR gene by bidirectional sequencing. The latter mutation has been
classified as c.1142A>C (p.E381A). The results were consistent
with AML with myelofibrosis (MF).
A third course of chemotherapy with mitoxantrone 4
mg/m2 on days 1–3 and cytarabine 150 mg/m2 on
days 1–7 was subsequently administered. On day 3, the patient
developed sudden-onset nausea, dizziness and severe abdominal pain,
he quickly became hypotensive and the HGB level decreased to 8.3
g/dl within 1 h. The coagulation tests (prothrombin time, activated
partial thromboplastin time, thrombin time and fibrinogen) were
within the normal range. The patient received aggressive fluid
resuscitation, vasopressors (dopamine; Shanghai Hefeng
Pharmaceutical Co., Ltd., Shanghai, China), and was intubated for
respiratory support. Chemotherapy was discontinued after a total
dose of 8 mg/m2 of mitoxantrone and 300 mg/m2
of cytarabine. An abdominal ultrasound revealed a considerable
amount of free fluid surrounding the liver and spleen, with a
density consistent with that of blood. An emergency laparotomy
confirmed splenic rupture and splenectomy was performed during the
procedure. Approximately 4,000 ml of fresh blood were evacuated
from the abdominal cavity. The patient was intubated and
transferred to the intensive care unit. After 1 week, the patient
was extubated and he eventually recovered fully.
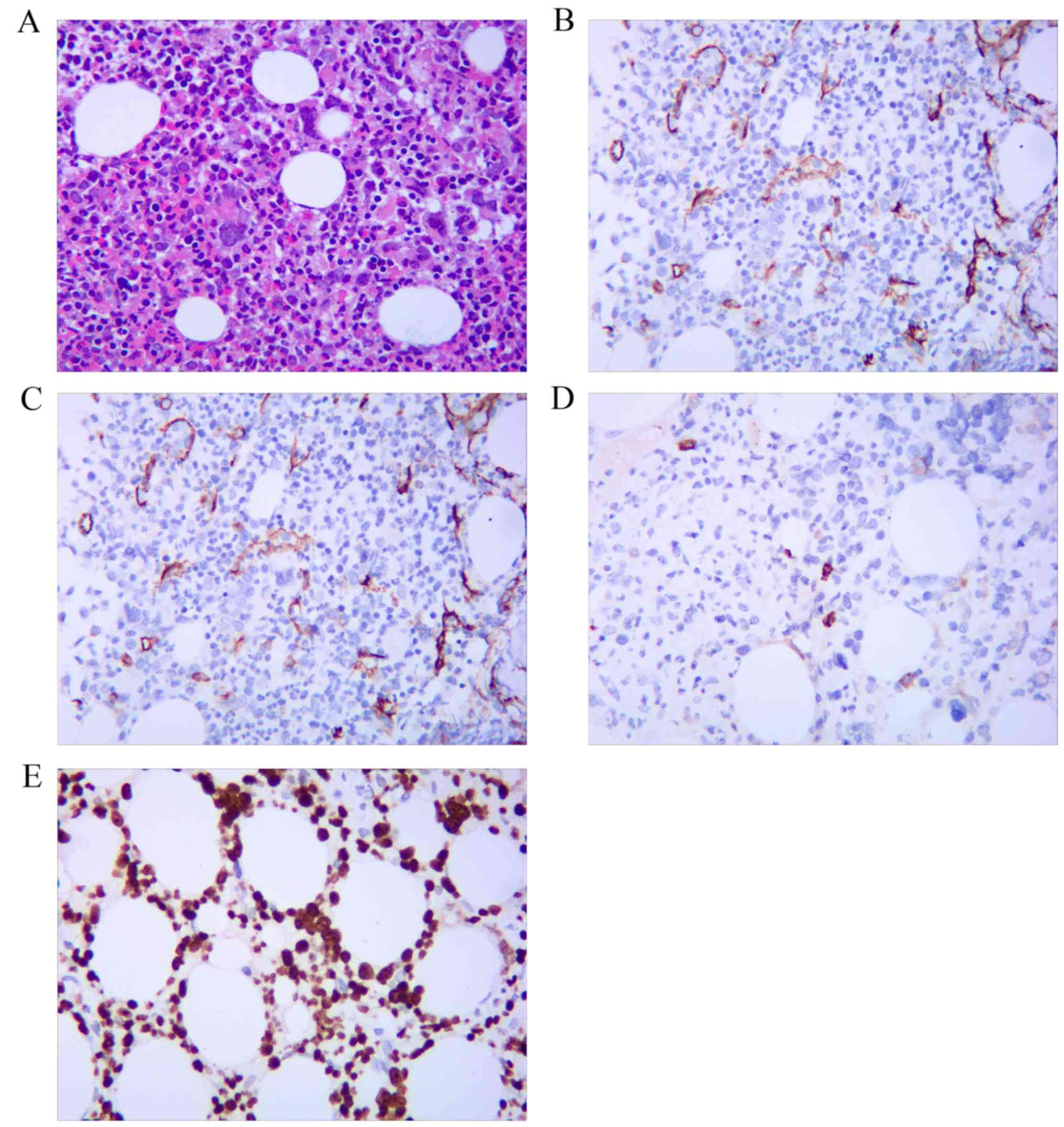
Pathological examination of the resected ruptured
spleen revealed increased red pulp, decreased white pulp and
multifocal granulomatosis with caseous necrosis, which raised the
suspicion of TB; however, the acid-fast staining was negative.
Furthermore, immunohistochemical staining of the splenic tissue
revealed the presence of some MPO-positive cells, but they were
negative for CD34 and CD117. The Ki-67 was sparsely positive in a
few splenic cells. The periodic acid-Schiff staining was negative
(Fig. 3). JAK2V617F mutation in the
splenic specimens was negative by the PCR method.
A purified protein derivative test was strongly
positive. The erythrocyte sedimentation rate was 128 mm/h. The
T-SPOT.TB test was negative. The patient was empirically treated
for TB with a combination of isoniazid, rifampicin, ethambutol and
pyrazinamide, despite the acid-fast staining being negative. On
bone marrow biopsy 1 month later, there was no dry tap aspiration
and complete remission was achieved. However, JAK2V617 and CALR
mutations were still detected. The patient then received
consolidation chemotherapy with standard-dose cytarabine (Pfizer
Italia Srl, Rome, Italy), daunorubicin (Haizheng Pharmaceutical
Co., Ltd., Taizhou, China) or mitoxantrone (Jiangsu Hengrui
Pharmaceutical Co., Lianyungang, China). The last follow-up was in
October, 2016, and the patient exhibited complete remission of the
bone marrow.
Discussion
Spontaneous splenic rupture refers to rupture of the
spleen without a history of blunt or penetrating trauma. Although
rare, spontaneous splenic rupture may be associated with neoplastic
(30.3%), infectious (27.3%), inflammatory, non-infectious (20.0%),
drug- and treatment-related (9.2%) and mechanical (6.8%) disorders;
it may also occur in the normal spleen (6.4%) (8). The major symptoms and signs of splenic
rupture include abdominal pain, tenderness and guarding,
hypotension, nausea, vomiting, dizziness and syncope (9). However, no characteristic clinical
manifestation may be used to definitively diagnose splenic rupture
without further investigation. The definitive treatment for
spontaneous splenic rupture is emergent splenectomy. Without
splenectomy, the mortality rate in such patients approaches 100%
(10).
While atraumatic splenic rupture most commonly
occurs in association with neoplastic diseases, its mechanism has
not been fully elucidated. In 1964, Hynes et al (11) proposed three possible mechanisms
underlying spontaneous splenic rupture, which remain widely
accepted to date: Mechanical effect of distention secondary to
leukemic infiltration of the spleen, particularly the capsule;
splenic infarct with capsular hemorrhage and subsequent rupture;
and blood coagulation defects. However, it appears that none of the
abovementioned mechanisms was responsible for the spontaneous
splenic rupture in our patient. First, his spleen was not
infiltrated by leukemic cells on pathological examination, which
was confirmed by negative immunohistochemical staining for CD117
and CD34, and the splenic tissue was negative for the JAK2V617F
mutation. Moreover, there was no evidence of blood coagulopathy or
splenic infarct. Therefore, the patient's splenic rupture cannot be
explained by leukemia or intracapsular hemorrhage.
The splenic rupture in our patient likely involved
other underlying mechanisms. First, the pathological examination of
the ruptured spleen revealed findings considered to be typical for
TB. Splenic TB is an extremely rare cause of splenic rupture, with
only 3 cases of splenic abscess and rupture as a consequence of TB
infection described in the literature to date (12,13). TB
mostly locates primarily in the lungs, and splenic TB is a rare
form of extrapulmonary localization. Immunocompromised patients,
such as those with acquired immunodeficiency syndrome, have been
reported to be at high risk for splenic TB (14), but the diagnosis may be challenging
due to the non-specific clinical manifestations. The patient may
suffer from fever, left upper quadrant abdominal pain, weight loss,
diarrhea and, occasionally, ascites (15). However, in one-third of the cases,
there were no abdominal symptoms, despite sonographic confirmation
of a splenic lesion (16).
Therefore, the diagnosis of splenic TB is difficult and is often
missed or delayed. In patients with TB, splenic TB may present as
multiple small hypoechoic lesions on abdominal sonography, or
hypodense lesions on abdominal CT (17). Diagnosis is established by
pathological examination of the fine-needle aspirate, splenic
biopsy, or examination of the surgical specimen following
splenectomy (18). However, invasive
diagnostic procedures are difficult to perform in hematological
patients due to the increased risk of bleeding and marked
neutropenia following intensive chemotherapy. Therefore, management
is largely dependent on clinical diagnosis when limited
microbiological data are available (19).
On the other hand, the patient may be diagnosed with
AML with primary MF (PMF). The bone marrow biopsy revealed dry tap
aspiration, increased megakaryocyte number and focal hyperplasia of
the fibrous tissue, and was positive for Gömöri trichrome staining,
JAK2V617 and CALR mutations. The activating mutation JAK2V617F is
frequently found in myeloproliferative neoplasms (MPNs), including
polycythemia vera, essential thrombocytosis and PMF (20). MPNs share an increased risk of
thrombotic and hemorrhagic complications. Thrombosis occurs in less
typical sites, including cerebral sinus vessels and splanchnic
vessels (hepatic, portal, mesenteric and splenic veins). Emerging
risk factors may include leukocytosis and presence of the JAK2V617F
mutation, or an increase in its allelic burden. Hemorrhagic
complications are likely associated with acquired platelet defects,
such as acquired von Willebrand syndrome (21). Whether thrombotic and hemorrhagic
complications caused by MPNs are associated with splenic rupture
remains to be further elucidated. Although splenectomy is an
effective treatment for MPN-related symptoms, it is also associated
with significant operative morbidity, increased mortality, and even
a potential increased risk of blast phase transformation in MF
(22,23). Most clinicians manage to avoid
splenectomy in the majority of the patients.
In conclusion, it is hypothesized that the splenic
rupture in our patient was caused by the subcapsular localization
of tuberculous granulomatous tissue infiltrating the red pulp of
the spleen, predisposing to tearing of the splenic capsule. The
role of MPN in the splenic rupture may be another, although
unlikely, possibility, since there was no evidence of coagulopathy.
Regardless of the underlying mechanism, sudden onset of abdominal
pain associated with symptomatic anemia or hypotension in a patient
with leukemia warrants further investigation to exclude fatal
splenic rupture.
References
|
1
|
Han JS, Oh SY, Kim SH, Kwon HC, Hong SH,
Han JY, Park KJ and Kim HJ: A case of pathologic splenic rupture as
the initial manifestation of acute myeloid leukemia M2. Yonsei Med
J. 51:138–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimmer BM, Berdel WE, Ludwig WD, Notter M,
Reufi B and Thiel E: Fatal spleen rupture during induction
chemotherapy with rh GM-CSF priming for acute monocytic leukemia.
Clinical case report and in vitro studies. Leuk Res. 17:277–283.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kasper C, Jones L, Fujita Y, Morgenstern
GR, Scarffe JH and Chang J: Splenic rupture in a patient with acute
myeloid leukemia undergoing peripheral blood stem cell
transplantation. Ann Hematol. 78:91–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernández R, del Cañizo MC, López C,
González MI, Vázquez ML, Caballero MD and San Miguel JF: Pathologic
rupture of the spleen during induction with ATRA in a patient with
acute promyelocytic leukemia. Med Oncol. 17:337–339. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan A, Ziari M, Salman H, Ortega W and
Cortese C: Spontaneous rupture of the spleen in the presentation of
acute myeloid leukemia. J Clin Oncol. 25:5519–5520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singhal V, Kuiper J, Chavda K and Kashmer
D: Spontaneous splenic rupture as first manifestation of acute
myeloid leukemia: Case report and review of literature. J Clin
Oncol. 29:e576–e578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeidan AM, Mitchell M, Khatri R, Itani D,
Boikos S, Bose S, Lipsett P, Efron D, King KE, Gerber J and DeZern
A: Spontaneous splenic rupture during induction chemotherapy for
acute myeloid leukemia. Leuk Lymphoma. 55:209–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renzulli P, Hostettler A, Schoepfer AM,
Gloor B and Candinas D: Systematic review of atraumatic splenic
rupture. Br J Surg. 96:1114–1121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giagounidis AA, Burk M, Meckenstock G,
Koch AJ and Schneider W: Pathologic rupture of the spleen in
hematologic malignancies: Two additional cases. Ann Hematol.
73:297–302. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Canady MR, Welling RE and Strobel SL:
Splenic rupture in leukemia. J Surg Oncol. 41:194–197. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hynes HE, Silverstein MN and Fawcett KJ:
Spontaneous rupture of the spleen in acute leukemia. Report of 2
cases. Cancer. 17:1356–1360. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeo HJ, Lee SY, Ahn E, Kim EJ, Rhu DG,
Choi KU, Lee SE, Cho WH, Jeon D and Kim YS: Spontaneous splenic
rupture as a paradoxical reaction during treatment for splenic
tuberculosis. Tuberc Respir Dis (Seoul). 75:218–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Safioleas MC, Stamatakos MC, Safioleas CM,
Diab AI and Agapitos EB: Co-existence of spontaneous splenic
rupture and tuberculosis of the spleen. Saudi Med J. 27:1588–1590.
2006.PubMed/NCBI
|
|
14
|
Gotor MA, Mur M, Guerrero L, Aspiroz C,
Romero D and Gimeno E: Tuberculous splenic abscess in an
immunocompetent patient. Gastroenterol Hepatol. 18:15–17. 1995.(In
Spanish). PubMed/NCBI
|
|
15
|
Pramesh CS, Tamhankar AP, Rege SA and Shah
SR: Splenic tuberculosis and HIV-1 infection. Lancet. 359:3532002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dixit R, Arya MK, Panjabi M, Gupta A and
Paramez AR: Clinical profile of patients having splenic involvement
in tuberculosis. Indian J Tuberc. 57:25–30. 2010.PubMed/NCBI
|
|
17
|
Zhan F, Wang CJ, Lin JZ, Zhong PJ, Qiu WZ,
Lin HH, Liu YH and Zhao ZJ: Isolated splenic tuberculosis: A case
report. World J Gastrointest Pathophysiol. 1:109–111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nayyar V, Ramakrishna B, Mathew G,
Williams RR and Khanduri P: Response to antituberculous
chemotherapy after splenectomy. J Intern Med. 233:81–83. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon JC, Kim SH, Park SH, Choi SM, Lee DG,
Choi JH, Yoo JH, Kim YJ, Lee S, Kim HJ, et al: Clinical
characteristics and the usefulness of the QuantiFERON-TB Gold
In-Tube test in hematologic patients with hepatic or splenic
lesions. Korean J Intern Med. 28:187–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshiki Y, Asai T, Ichikawa M, Hangaishi
A, Ota S, Imai Y, Takahashi T and Kurokawa M: A case of myeloid
sarcoma with correlation to JAK2V617F mutation, complicated by
myelofibrosis and secondary acute myeloid leukemia. Intern Med.
50:2649–2652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McMahon B and Stein BL: Thrombotic and
bleeding complications in classical myeloproliferative neoplasms.
Semin Thromb Hemost. 39:101–111. 2013.PubMed/NCBI
|
|
22
|
Santos FP, Tam CS, Kantarjian H, Cortes J,
Thomas D, Pollock R and Verstovsek S: Splenectomy in patients with
myeloproliferative neoplasms: Efficacy, complications and impact on
survival and transformation. Leuk Lymphoma. 55:121–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melikian AL, Kolosova L, Sokolova MA,
Kovrigina AM, Silaev MA, Giliazitdinova EA, Gemdzhian ÉG and
Karagiulian SR: Role of splenectomy in the treatment of
myelofibrosis. Ter Arkh. 85:69–76. 2013.(In Russian). PubMed/NCBI
|