Introduction
Trastuzumab (Herceptin®) is a recombinant
humanized immunoglobulin G1 monoclonal antibody against human
epidermal growth factor receptor 2 (HER2), which is amplified in
15–30% of primary breast cancer patients (1,2).
Incorporation of trastuzumab to adjuvant chemotherapy for
early-stage HER2-positive breast cancer has been shown to reduce
the risk of recurrence and to prolong survival (2–5). In
metastatic patients, the combination of an anti-HER2 antibody, such
as trastuzumab and/or pertuzumab, with a taxane has been
established as the standard primary chemotherapy (6). It is thus widely accepted that
trastuzumab is a backbone drug for the treatment of HER2-positive
breast cancer. Trastuzumab is generally well-tolerated, but a
proportion of patients develop serious complications, such as
cardiac dysfunction and anaphylactic reactions. Although the
association of interstitial lung disease (ILD) with trastuzumab has
only rarely been reported, clinicians should be aware of the
possibility of its serious clinical outcome (7). We herein report our recent experience
with 3 breast cancer patients in whom trastuzumab monotherapy was
complicated by ILD.
Case reports
Case 1
A 68-year-old female patient, with an Eastern
Cooperative Oncology Group performance status (ECOG PS) score of 0,
with no history of smoking or lung disease, underwent right breast
quadrant resection. Pathological examination revealed invasive
ductal carcinoma, scirrhous type, with negative sentinel node
biopsy (T1N0M0). The cancer cells were estrogen receptor
(ER)+, progesterone receptor (PR)+, and
HER2+, with a Ki-67 index of 30%. The patient received 4
courses of 21-day-cycle adjuvant chemotherapy with epirubicin and
cyclophosphamide, starting on the 46th postoperative day (POD),
followed by triweekly trastuzumab. Anastrozole and radiotherapy to
the right mammary area (total dose, 48 Gy) were started on the
185th and 192nd POD, respectively. The patient developed low-grade
fever and cough on the 4th day of the 5th course of trastuzumab,
corresponding to the day of 24/31 scheduled fraction of
radiotherapy. Physical examination revealed coarse crackles on the
whole of the right chest and the left upper chest. The laboratory
tests revealed elevated levels of serum lactate dehydrogenase (LDH;
351 IU/l) and C-reactive protein (CRP; 15.3 mg/dl). The serum KL-6
level was within normal limits (247 IU/l). The PaO2 was
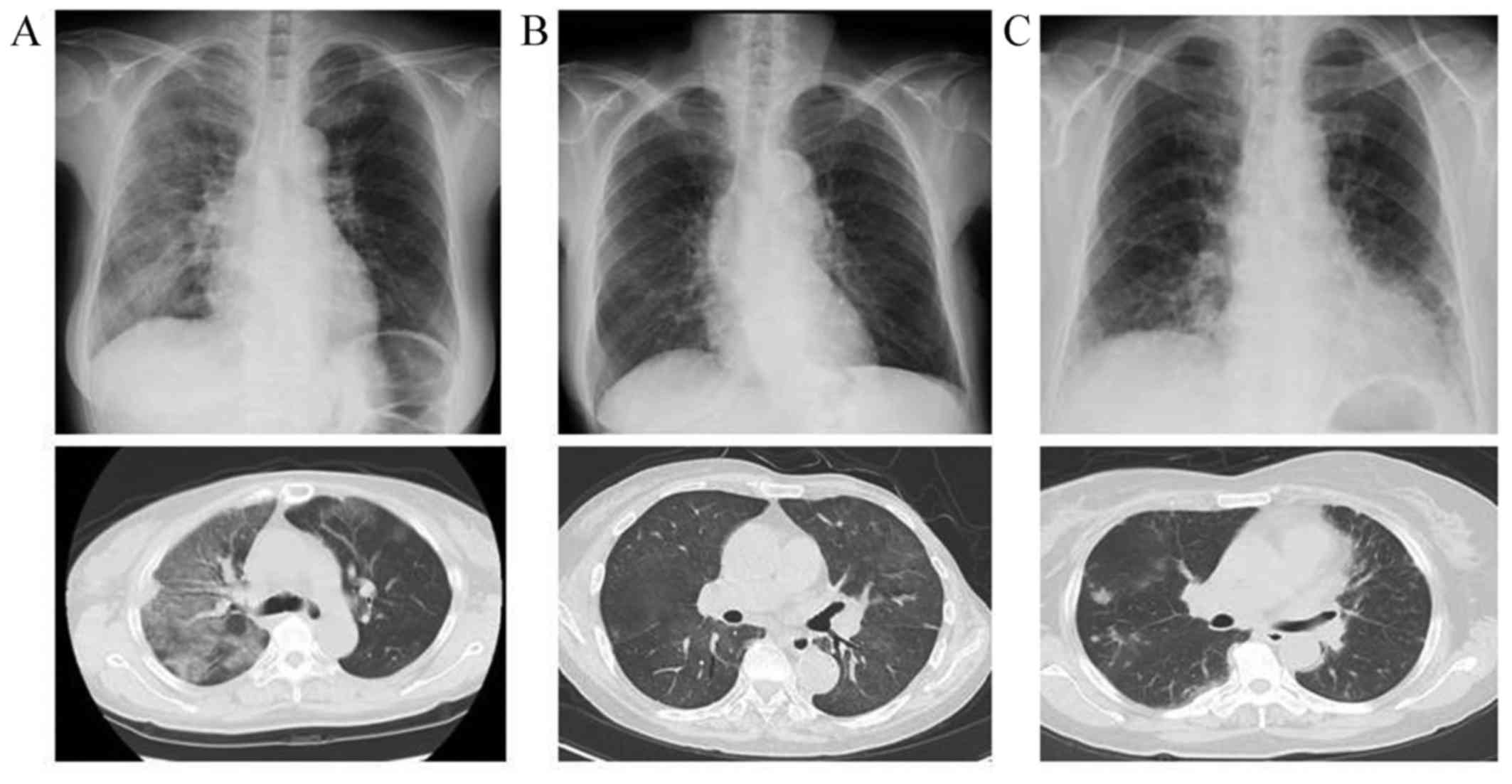
62 mmHg at room air (RA). The computed tomography (CT) scan
revealed right lung-dominant patchy infiltration with surrounding
ground-glass opacity (Fig. 1A).
Infectious lung diseases were excluded by sputum cultures,
Grocott's methenamine silver staining and polymerase chain reaction
analysis of tuberculosis. The blood cultures and circulating
galactomannan antigen tests were also negative. The patient was
diagnosed with interstitial pneumonitis and was treated with
steroid semi-pulse therapy with concurrent administration of
meropenem and sulfamethoxazole/trimethoprim. The symptoms subsided
within 7 days of the treatment.
Case 2
A 77-year-old female patient, with an ECOG PS score
of 1, with no history of smoking or lung disease underwent left
modified radical mastectomy. Pathological examination revealed
invasive ductal carcinoma, scirrhous type, with negative sentinel
node biopsy (T4bN0M0). The cancer cells were ER−,
PR− and HER2+, with a Ki-67 index of 60%. The
patient received 4 courses of 21-day-cycle adjuvant chemotherapy
with epirubicin and cyclophosphamide, starting on the 68th POD,
which was followed by trastuzumab monotherapy. After 3 h of the
first administration of trastuzumab, the SpO2 fell
rapidly to 81% at RA. Physical examination revealed a low-grade
fever and right lung-dominant coarse crackles. The laboratory tests
revealed elevated levels of serum LDH (552 IU/l), CRP (1.3 mg/dl)
and KL-6 (719 IU/l). The PaO2 was 48.8 mmHg at RA. A CT
scan revealed diffuse pale ground-glass opacities in the lungs
bilaterally (Fig. 1B). Based on the
radiological findings, the patient was diagnosed with interstitial
pneumonitis and was treated with 30 mg of prednisolone, resulting
in normalization of SpO2 within 7 days.
Case 3
A 62-year-old female patient, with an ECOG PS score
of 1, with no history of smoking or lung disease, underwent right
modified radical mastectomy. The patient had a history of
gallbladder cancer, for which she underwent curative surgery 2
years prior to the onset of breast cancer. Pathological examination
revealed invasive ductal carcinoma, solid-tubular type, with
negative sentinel node biopsy (T2N0M0). The cancer cells were
ER−, PR− and HER2+, with a Ki-67
index of 30%. The patient received 4 courses of 21-day-cycle
adjuvant chemotherapy with docetaxel, cyclophosphamide and
trastuzumab, starting on the 43rd POD. Although she experienced
transient flu-like symptoms after the third course of the
chemotherapy, the 4 courses of chemotherapy were completed,
followed by trastuzumab monotherapy. On the 6th day of the first
course of trastuzumab, a CT scan scheduled for a follow-up of the
gallbladder cancer coincidentally revealed multiple patchy
consolidations and ill-defined nodules in the lungs bilaterally
(Fig. 1C). Physical examination
revealed fine crackles bilaterally in the precordial chest, with
SpO2 96% at RA. The laboratory tests revealed elevated
levels of serum LDH (293 IU/l) and KL-6 (911 IU/l). Bronchoscopic
lung biopsy revealed inflamed bronchial mucosa infiltrated by
lymphocytes and histiocytes. Infection was excluded by various
microbial tests of sputum and bronchoalveolar lavage specimens. The
patient was diagnosed with drug-induced organizing pneumonia and
was treated with 30 mg of prednisolone. The serum levels of KL-6
decreased gradually over 8 months.
Discussion
Although trastuzumab is indispensable to the
treatment of HER2-positive breast cancer, physicians scarcely
encounter trastuzumab-associated ILD. Previous large-scale trials,
namely the B-31 and N9831 trials, which confirmed the efficacy of
trastuzumab combined with paclitaxel after completing 4 courses of
chemotherapy with doxorubicin and cyclophosphamide, reported that
interstitial pneumonitis or pulmonary infiltrate was observed in
0.46 and 0.61% of patients, respectively (3). Although those studies suggested that
trastuzumab possibly induces ILD when combined with cytotoxic
anticancer drugs, the incidence of trastuzumab
monotherapy-associated ILD has not been elucidated. In the
large-scale phase 3 HERA trial, which verified the efficacy of
trastuzumab monotherapy in an adjuvant setting, trastuzumab was
administered to 3,105 patients without reports of ILD (2,4). Thus,
although trastuzumab monotherapy-associated ILD is a recognized
entity, it is a rare complication.
We herein reported the cases of 3 patients with
trastuzumab monotherapy-associated ILD. Patient 1 developed ILD
following administration of 5 courses of trastuzumab. Since breast
radiotherapy was concurrently administered at the onset of ILD, the
possibility of radiation pneumonitis cannot be excluded. However, a
CT scan revealed a diffuse alveolar damage (DAD) pattern, spreading
beyond the irradiated area. Furthermore, the onset of ILD was
within 1 month of radiotherapy initiation, which is different from
the typical clinical course of radiation pneumonitis. We thus
considered trastuzumab to be the most likely cause of ILD in this
patient. Patient 2 developed ILD 3 h after the first administration
of trastuzumab, which was scheduled 3 weeks after the last
cytotoxic chemotherapy with epirubicin and cyclophosphamide.
Although the clinical symptoms were suggestive of an infusion
reaction, the patient was diagnosed with trastuzumab-associated
pneumonitis based on the findings of a CT scan, which corresponded
well with the hypersensitivity pneumonitis (HP) pattern. Patient 3
developed ILD after administration of the first course of
trastuzumab monotherapy following 4 cycles of combination
chemotherapy with docetaxel, cyclophosphamide and trastuzumab.
Although a scheduled CT scan as follow-up for gallbladder cancer
coincidentally revealed ILD, it should be noted that this patient
had experienced flu-like symptoms of unknown origin after the third
course of the combination chemotherapy with docetaxel,
cyclophosphamide and trastuzumab, and the LDH level was elevated 27
days prior to the emergence of ILD. Considering this clinical
course, we hypothesized that the pre-existing drug-associated
pulmonary inflammation became obvious and manifested as ILD after
the initiation of trastuzumab monotherapy.
Thus far, several cases of ILD have been reported in
association with combination chemotherapy with trastuzumab and
cytotoxic drugs, such as paclitaxel and docetaxel (8,9). Those
case reports mostly concluded that paclitaxel or docetaxel, rather
than trastuzumab, was responsible for the development of ILD, as
ILD is a well-known complication of taxanes. However, according to
our literature survey, there have been 4 English-language case
reports of trastuzumab monotherapy-associated ILD (10–13).
Those cases and the 3 cases presented herein are summarized in
Table I. The review of these cases
suggests that ILD commonly manifests 3–4 months after trastuzumab
treatment initiation. Furthermore, in all but 1 patient,
trastuzumab monotherapy was initiated at an adjuvant setting after
completing cytotoxic chemotherapy. It may be thus reasonable to
hypothesize that the preceding cytotoxic chemotherapy in
association with trastuzumab monotherapy may have affected the
development of ILD. However, ILD appears to be a true complication
of trastuzumab treatment, as at least some of the reported cases
clearly suggest that trastuzumab was responsible for the
development of ILD. Another important point is that radiological
findings and clinical symptoms of trastuzumab-associated ILD appear
to be variable. Indeed, our case series exhibited DAD, HP and OP
pattern on CT. A previous review of antineoplastic drug-induced ILD
estimated the incidence of trastuzumab-induced ILD to be as low as
0.4–0.6% (7). Despite its low
incidence, clinicians should bear in mind that trastuzumab may
induce ILD, which may manifest with variable non-specific
patterns.
 | Table I.Cases of trastuzumab
monotherapy-induced ILD. |
Table I.
Cases of trastuzumab
monotherapy-induced ILD.
| Age/gender | Setting | Previous CRT | Duration after Tmab
initiation | Pattern on CT | Treatment | Recovery | Refs. |
|---|
| 49/F | Adjuvant | AC, RT, D | 3 months | Focal (OP) | Steroid,
antibiotics | Yes | (10) |
| 72/F | Palliative | None | 3 months | Diffuse (DAD) | Steroid | No | (11) |
| 65/F | Adjuvant | EC, PTX | 5 weeks | Diffuse (DAD) | Steroid,
antibiotics | Yes (slow
recovery) | (12) |
| 56/F | Palliative | DT | 19 weeks | Diffuse (CT: np) | Steroid | Yes | (13) |
| 68/F | Adjuvant | EC, RT | 3 months | Diffuse (DAD) | Steroid,
antibiotics | Yes | Case 1 |
| 77/F | Adjuvant | EC | On first a
administration | Diffuse (HP) | Steroid | Yes | Case 2 |
| 62/F | Adjuvant | DCT | 3 months | Focal (OP) | Steroid | Yes (slow
recovery) | Case 3 |
Following diagnosis of drug-associated ILD,
trastuzumab was discontinued in all 3 patients in the present study
and steroid therapy was immediately initiated, resulting in
recovery from drug-induced ILD. As shown in Table I, our literature survey suggests that
trastuzumab-associated ILD is mostly reversible, resulting in a
relatively favorable prognosis. Thus, early withdrawal of
trastuzumab and immediate commencement of corticosteroid therapy
are considered mandatory for a favorable outcome.
References
|
1
|
Slamon DJ, LeylandJones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piccart-Gebhart MJ, Procter M,
LeylandJones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldhirsch A, Gelber RD, PiccartGebhart
MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D,
Weber HA, Heinzmann D, et al: 2 years versus 1 year of adjuvant
trastuzumab for HER2-positive breast cancer (HERA): An open-label,
randomised controlled trial. Lancet. 382:1021–1028. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J
Med. 365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vahid B and Marik PE: Pulmonary
complications of novel antineoplastic agents for solid tumors.
Chest. 133:528–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abulkhair O and El Melouk W: Delayed
paclitaxel- trastuzumab-induced interstitial pneumonitis in breast
cancer. Case Rep Oncol. 4:186–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuip E and Muller E: Fatal pneumonitis
after treatment with docetaxel and trastuzumab. Neth J Med.
67:237–239. 2009.PubMed/NCBI
|
|
10
|
Radzikowska E, Szczepulska E, Chabowski M
and Bestry I: Organising pneumonia caused by transtuzumab
(Herceptin) therapy for breast cancer. Eur Respir J. 21:552–555.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vahid B and Mehrotra A: Trastuzumab
(Herceptin)-associated lung injury. Respirology. 11:655–658. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bettini AC, Tondini C, Poletti P, Caremoli
ER, Guerra U and Labianca R: A case of interstitial pneumonitis
associated with Guillain-Barré syndrome during administration of
adjuvant trastuzumab. Tumori. 94:737–741. 2008.PubMed/NCBI
|
|
13
|
Pepels MJ, Boomars KA, van Kimmenade R and
Hupperets PS: Life-threatening interstitial lung disease associated
with trastuzumab: Case report. Breast Cancer Res Treat.
113:609–612. 2009. View Article : Google Scholar : PubMed/NCBI
|