Introduction
The development of the magnifying scope is
associated with major benefits regarding diagnosis of early colon
cancer by enabling in vivo observation of the pit patterns
on the tumor surface (1,2). Recently, the invention of
ultramagnifying endocytoscopy (EC) has enabled observation at a
400-fold magnification. As the EC findings correspond well with the
pathological findings, it is possible to detect living tumor cells
and micro vessels in vivo (3–5) and
obtain a pathological image by simply applying the scope to the
target mucosa during an endoscopic examination, which serves as a
mode of on-site ‘optical biopsy’ (6,7).
However, a desmoplastic reaction (DR), characterized
by stromal myofibroblast infiltration of the outermost layers of
the tumor (8), is considered to be a
response to the invasion of carcinoma cells beyond the muscularis
mucosae into the submucosa (SM) or deeper layers (9). Detection of a DR may be useful for
predicting massive SM invasion by colorectal carcinoma (10). The fibrotic deposition of the cancer
cells is caused by stromal cells, principally myofibroblasts and
activated fibroblasts (11). These
fibroblasts synthesize collagen, namely types I, III and IV, and
proteoglycans that constitute the bulk of the DR. These collagens
accumulate around the tumor and directly affect the growth and
invasion of cancer cells. This process is considered to be a host
defense mechanism intended to confine the developing tumor.
However, DR has been associated with tumor progression and poor
prognosis in colorectal carcinoma (12).
The non-structural pit pattern V (type VN
pit pattern), which lacks a superficial microstructure, has been
associated with a superficial DR that reflects deeply invasive
submucosal components (an exposing DR) (13). However, to the best of our knowledge,
in vivo assessment of DR using EC has not been reported to
date.
EC enables the visualization of cellular membrane
structures and nuclear morphology. Therefore, the aim of the
present study was to identify specific EC findings that may be used
to detect DR histopathologically. Furthermore, we examined whether
EC enabled detection of DRs indicative of invasive tumors. To the
best of our knowledge, no previous study has described EC imaging
findings with regards to superficial DRs in colorectal cancer to
date.
Materials and methods
Patients and clinical data
A total of 49,990 patients underwent colonoscopy at
the Digestive Disease Center, Showa University Northern Yokohama
Hospital (Yokohama, Japan) between May, 2005 and August, 2013, and
a total of 17,331 lesions were detected. Patients with familial
adenomatous polyposis, tumor-related inflammatory bowel disease or
a history of chemotherapy were excluded. Of all included lesions,
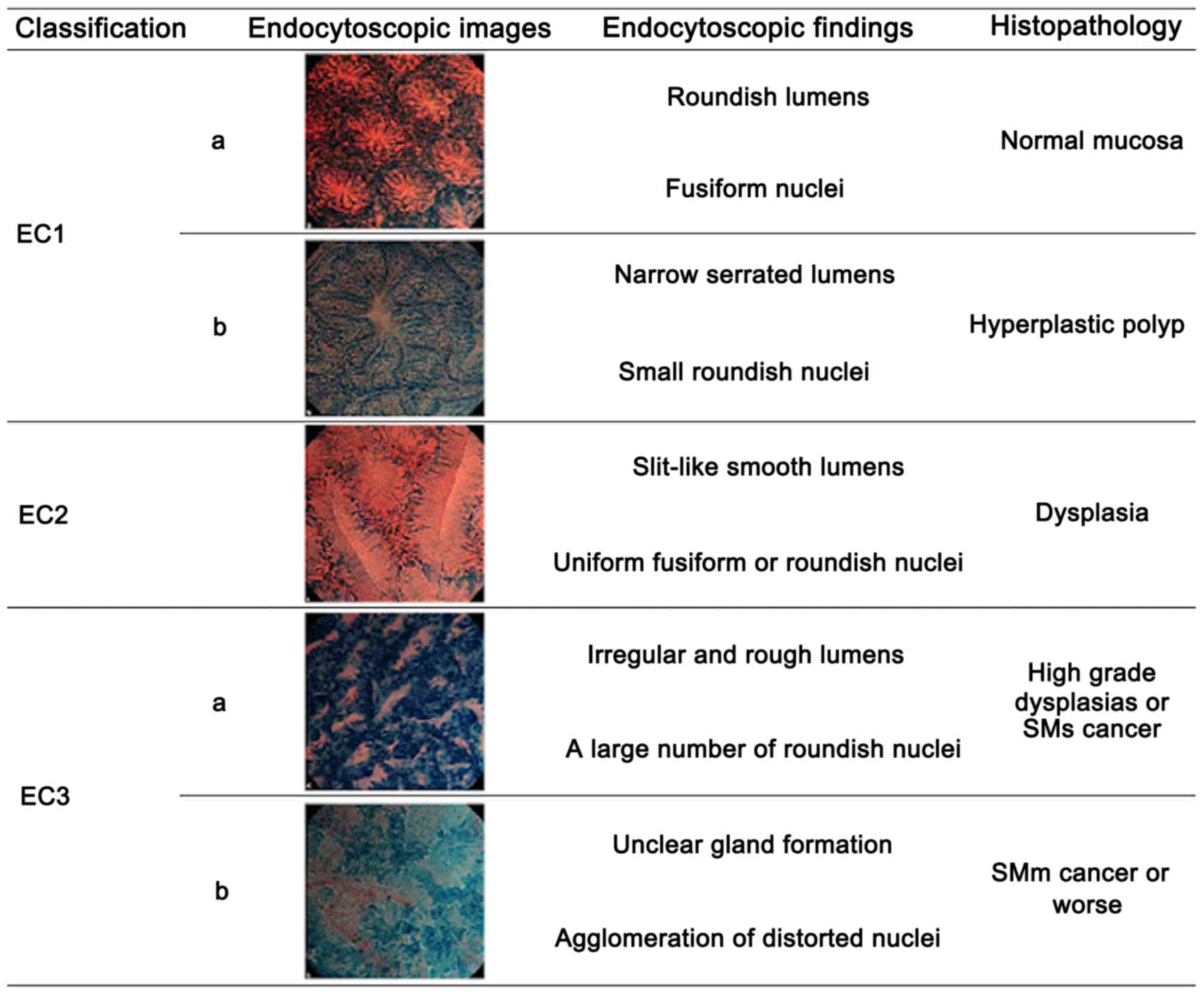
502 were identified using EC, with 72 diagnosed as EC3b according
to the EC classification (6),
suggesting invasion of the SM or deeper layers (Fig. 1). All the lesions were treated with
endoscopic mucosal resection/endoscopic submucosal dissection or
surgical resection. This study was granted ethical approval by the
local Ethics Review Committee (approval no. 1410-07) and informed
consent was obtained from all the participants prior to enrollment
in this clinical trial.
Evaluation of EC
All examinations were performed with an
integrated-type endocytoscope (XCF-260EC1; Olympus Co., Tokyo,
Japan). All endoscopic procedures were performed by any one of four
experienced endoscopists (Y.S., Y.M., M.M. and H.M.) who have
performed >100 EC procedures over a period of 2 years.
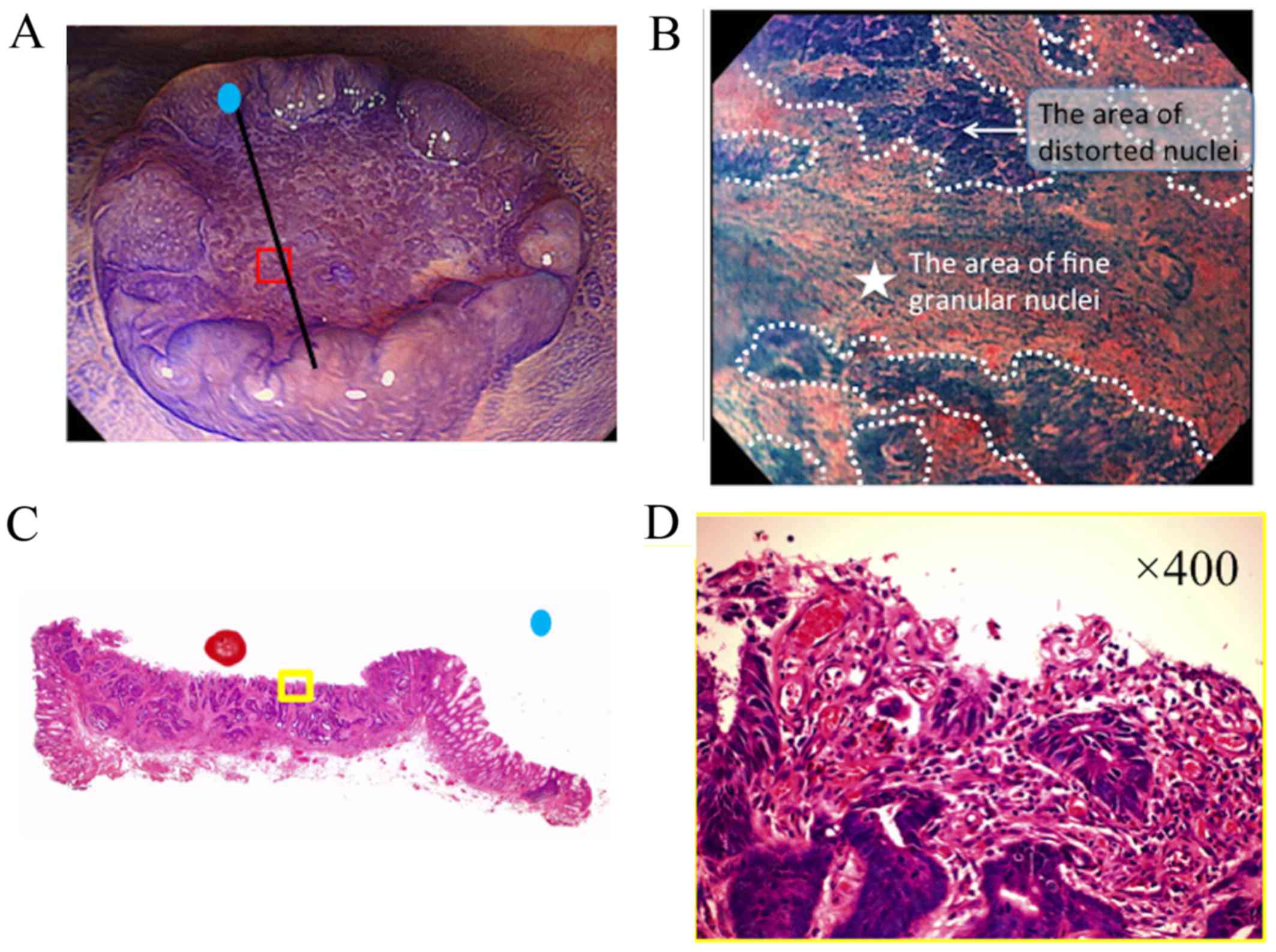
It was hypothesized that the presence of a fine
granular structure (FGS) may be a reliable indicator of DR
(Fig. 2). FGS identified via EC was
defined according to the following criteria: i) Presence of
distorted nuclei, indicative of cancer cells, around the FGS; and
ii) increased granularity of the FGS nuclei compared with the
surrounding cancer cells, indicative of myofibroblasts or
inflammatory cells. To ensure objectivity of the FGS findings,
independent external reviewers blinded to the patient information
calculated the interobserver agreement index regarding the
assessment of FGS on the basis of the EC histological
evaluations.
Histological evaluation
Each endoscopically or surgically resected specimen
was fixed in 10% formalin and embedded in paraffin wax. The tissue
specimens were then sliced into 2-mm sections and stained with
hematoxylin and eosin. All the specimens were routinely evaluated
to establish a pathological diagnosis. An experienced
gastrointestinal pathologist evaluated all the specimens and
assessed the presence of DR in the superficial layers of the tumor
according to the following criteria reported by Kimura et al
(14): i) Presence of carcinoma is
required for the detection of an exposing DR; and ii) a DR involves
an area of collagen fiber accumulation, myofibroblast proliferation
and inflammatory infiltration.
Statistical analysis
All analyses were performed using STATA software,
version 11.2 (StataCorp, College Station, TX, USA). The
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV) and overall accuracy of identifying DRs in
the superficial layer of the tumors were estimated. P-values
<0.05 were considered to indicate statistically significant
differences.
Validation study for FGS
Interobserver agreement was assessed using κ
statistics and interpreted as proposed by Landis and Koch (15). A κ value of 0 demonstrated the
absence of agreement; <0.20, slight agreement; 0.21–0.40, fair
agreement; 0.41–0.60; moderate agreement; 0.61–0.80, substantial
agreement; and >0.81, almost perfect agreement.
Results
Patient characteristics
A total of 72 consecutive patients diagnosed with
EC3b colorectal carcinoma by EC were enrolled in this study (median
age, 65 years; range, 40–85 years). Of the 72 patients, 46 were
men. The clinical and pathological characteristics are summarized
in Table I.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Characteristics | n (%) |
|---|
| Patients | 72 (100.0) |
| Median age, years
(range) | 65 (40–85) |
| Median tumor size, mm
(range) | 21 (4–94) |
| Gender |
|
|
Male/female | 46 (64.0)/26
(36.0) |
| Location |
|
|
Cecum | 2 (2.8) |
| Ascending
colon | 13 (18.1) |
|
Transverse colon | 5 (6.9) |
|
Descending colon | 3 (4.2) |
| Sigmoid
colon | 23 (31.9) |
|
Rectum | 27 (37.5) |
| Morphology |
|
|
Pedunculated | 5 (6.9) |
|
Nonpedunculated | 67 (93.1) |
| Histological
appearance |
|
|
Well-/moderately
differentiated | 52 (72.2) |
| Poorly
differentiated | 20 (27.8) |
Comparison of tumor invasion depth
between superficial exposing DRs and FGS
A comparison of the depth of tumor invasion among
lesions with a superficial exposing DR and FGS detected via EC is
shown in Table II. A close
association with tumor invasion depth was observed for DR-positive
and FGS-positive findings. Of the 72 lesions, 26 were FGS-positive.
As shown in Table III, the
majority of these lesions (23/26; 88.5%) exhibited an exposing DR
detected by the presence of FGS on EC, which indicates a
significant association. The overall accuracy, sensitivity,
specificity, PPV and NPV for the utility of FGS on EC imaging for
the detection of an exposing DR was 87.3, 91.9, 76.7, 90.1 and
80.2%, respectively.
 | Table II.Comparison of tumor invasion depth
between the superficial exposing DRs and FGS detected on
endocytoscopy. |
Table II.
Comparison of tumor invasion depth
between the superficial exposing DRs and FGS detected on
endocytoscopy.
|
| Superficial exposing
DR | Endocytoscopy |
|---|
|
|
|
|
|---|
| Depth of
invasion | Superficial exposure
(+), n (%) (n=30) | Superficial exposure
(−), n (%) (n=42) | FGS (+), n (%)
(n=26) | FGS (−), n (%)
(n=46) |
|---|
| M | 0 (0.0) | 3
(7.1) | 0 (0.0) | 3 (6.5) |
| SM | 23 (76.7) | 34 (80.1) | 19 (73.1) | 39 (84.8) |
| MP | 1 (3.3) | 2
(4.8) | 1 (3.8) | 2 (4.3) |
| SS | 5 (16.7) | 1
(2.4) | 5
(19.2) | 7 (15.2) |
| SE | 1 (3.3) | 0
(0.0) | 1 (3.8) | 2 (4.3) |
 | Table III.Correlation between the presence of
FGS and superficial exposing DR. |
Table III.
Correlation between the presence of
FGS and superficial exposing DR.
|
| FGS |
|
|---|
|
|
|
|
|---|
| DR exposure | Positive | Negative | Total |
|---|
| Positive | 23 (31.9) | 7 (9.7) | 30 (41.7) |
| Negative | 3 (4.17) | 39 (54.1) | 42 (58.3) |
| Total | 26 (36.1) | 46 (63.9) | 72 (100.0) |
The mean κ score for interobserver agreement between
the two independent reviewers was 0.720, indicating that the
observers' evaluation of the FGS had substantial accuracy. The mean
sensitivity, specificity, PPV and NPV for the detection of an FGS
predictive of DR histopathology was 85.5, 73.6, 93.1 and 75.4%,
respectively.
Discussion
To the best of our knowledge, no previous study has
reported EC imaging findings that may be used to detect a
superficial exposing DR in colorectal cancer. In the present study,
we observed that the presence of FGS on the EC image was a reliable
marker of a DR, without the need for pathological examination.
Thus, EC may have the potential to be a ‘one-step’ diagnostic tool
for detecting superficial exposing DRs in colorectal cancer based
on FGS findings.
The detection of DRs in pretreatment biopsy
specimens has been reported to be useful for the prediction of
early colorectal cancer invading the SM; therefore, DR status in a
pathology report allows patients to be directed towards appropriate
therapy, such as endoscopic mucosal resection/endoscopic submucosal
dissection treatment or surgical resection (10).
Invasive carcinoma is characterized by the
interruption of normal cellular compartments. The invasion field
between pre-existing epithelial and stromal compartments is a
critical interface in carcinogenesis. Stromal fibroblasts in a DR
are critical in the development of digestive tract cancers
(16). Accordingly, we hypothesized
that there are several promising clinical implications of stromal
fibroblast research for the prevention, diagnosis and management of
digestive tract cancers. The presence of FGS detected via EC may be
easily confirmed in one step, and this may significantly contribute
to the future of this line of research.
A limitation of the present study was that the
evaluation of FGS was subjective; however, an interobserver
agreement index was obtained from multiple independent specialists.
Although the obtained index value was significant (0.720), it is
not adequate to provide sufficient objectiveness for the evaluation
of the presence of FGS. Further studies are required to validate
these findings and minimize interobserver variations. Prospective
randomized studies, comparing the efficacy of identifying FGS by
using EC with the histopathological assessment of DR as a
diagnostic tool used for predicting treatment and patient outcomes
after endoscopic or surgical resection may confirm the usefulness
of EC. These future studies may improve the diagnosis of colorectal
cancer and support the utility of ‘one-step’ diagnosis without the
need for biopsy.
In conclusion, histological evaluation of biopsy
specimens remains the standard approach for identifying DR.
However, the presence of FGS identified on EC is likely to indicate
an exposing DR. A DR is of vital importance to cancer cell
migration and invasiveness. We consider that EC may improve the
diagnosis of colorectal cancer and become the gold standard
approach in the future, replacing the combination of conventional
endoscopy and biopsy.
Acknowledgements
The authors express their gratitude to all the staff
members at the Digestive Disease Center and the Department of
Pathology, Showa University Northern Yokohama Hospital, for their
excellent assistance.
Glossary
Abbreviations
Abbreviations:
|
DR
|
desmoplastic reaction
|
|
EC
|
endocytoscopy
|
|
FGS
|
fine granular structure
|
|
NPV
|
negative predictive value
|
|
PPV
|
positive predictive value
|
|
SM
|
submucosa
|
References
|
1
|
Kudo S, Hirota S, Nakajima T, Hosobe S,
Kusaka H, Kobayashi T, Himori M and Yagyuu A: Colorectal tumors and
pit pattern. J Clin Pathol. 47:880–885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kudo S, Rubio CA, Teixeira CR, Kashida H
and Kogure E: Pit pattern in colorectal neoplasia: Endoscopic
magnifying view. Endoscopy. 33:367–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inoue H, Kazawa T, Sato Y, Satodate H,
Sasajima K, Kudo SE and Shiokawa A: In vivo observation of living
cancer cells in the esophagus, stomach, and colon using
catheter-type contact endoscope, ‘Endo-Cytoscopy system’.
Gastrointest Endosc Clin N Am. 14:589–594, x-xi. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasajima K, Kudo SE, Inoue H, Takeuchi T,
Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J and Shiokawa
A: Real-time in vivo virtual histology of colorectal lesions when
using the endocytoscopy system. Gastrointest Endosc. 63:1010–1017.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo SE, Misawa M, Wada Y, Nakamura H,
Kataoka S, Maeda Y, Toyoshima N, Hayashi S, Kutsukawa M, Oikawa H,
et al: Endocytoscopic microvasculature evaluation is a reliable new
diagnostic method for colorectal lesions (with video). Gastrointest
Endosc. 82:912–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mori Y, Kudo S, Ikehara N, Wakamura K,
Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, et
al: Comprehensive diagnostic ability of endocytoscopy compared with
biopsy for colorectal neoplasms: A prospective randomized
noninferiority trial. Endoscopy. 45:98–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudo SE, Wakamura K, Ikehara N, Mori Y,
Inoue H and Hamatani S: Diagnosis of colorectal lesions with a
novel endocytoscopic classification-a pilot study. Endoscopy.
43:869–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin M, Pujuguet P and Martin F: Role of
stromal myofibroblasts infiltrating colon cancer in tumor invasion.
Pathol Res Pract. 192:712–717. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirose M, Fukui H, Igarashi Y, Fujimori Y,
Katake Y, Sekikawa A, Ichikawa K, Tomita S, Imura J, Ajioka Y, et
al: Detection of desmoplastic reaction in biopsy specimens is
useful for predicting the depth of invasion of early colorectal
cancer: A Japanese collaborative study. J Gastroenterol.
45:1212–1218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto Y, Fujimori T, Ohkura Y, Sugai T,
Arai T, Watanabe G, Wada R, Ueno H, Togashi K, Yao T, et al:
Histological assessment of intra- and inter-institutional
reliabilities in detection of desmoplastic reaction in biopsy
specimens of early colorectal carcinomas. Pathol Int. 63:539–545.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunz LA and Knuechel R: Tumor-associated
fibroblasts (part I): Active stromal participants in tumor
development and progression? Histol Histopathol. 17:599–621.
2002.PubMed/NCBI
|
|
12
|
Angeli F, Koumakis G, Chen MC, Kumar S and
Delinassios JG: Role of stromal fibroblasts in cancer: Promoting or
impeding? Tumour Biol. 30:109–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudo SE, Sugihara Y, Kida H, Ishida F,
Miyachi H, Mori Y, Misawa M, Hisayuki T, Kodama K, Wakamura K, et
al: Depressed-type colonic lesions and ‘de novo’ cancer in familial
adenomatous polyposis: A colonoscopist's viewpoint. ISRN
Gastroenterol. 2013:8381342013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimura R, Fujimori T, Ichikawa K, Ajioka
Y, Ueno H, Ohkura Y, Kashida H, Togashi K, Yao T, Wada R, et al:
Desmoplastic reaction in biopsy specimens of early colorectal
cancer: A Japanese prospective multicenter study. Pathol Int.
62:525–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Worthley DL, Giraud AS and Wang TC:
Stromal fibroblasts in digestive cancer. Cancer Microenviron.
3:117–125. 2010. View Article : Google Scholar : PubMed/NCBI
|