Introduction
Intraductal papillary neoplasms of the bile duct
(IPNBs) are recognized as a distinct type of biliary tumor
(1,2). This entity includes previous
categories, such as biliary papillomatosis and mucin-producing bile
duct tumors (3). IPNBs share
clinicopathological characteristics with intraductal papillary
mucinous neoplasms (IPMNs) of the pancreas, as they both typically
present with dilatation of the affected ducts, predominantly
intraductal papillary masses and the overproduction of mucin
(3,4). The development of multiple separate
tumors is a characteristic feature potentially associated with
either condition. An index report of IPNBs included a case of
multiple IPNBs, in which three separate tumors developed in the
intrapancreatic bile duct 6 years after the initial tumor in the
cystic duct was resected (1).
However, multiple IPNBs have only been described in a small number
of reports, suggesting that a presentation with multiple biliary
tumors is less common compared with pancreatic IPMNs. The rarity of
multiple IPNBs has restricted the discussion on clinicopathological
characteristics and developmental mechanisms.
We herein describe another case of metachronous
multiple IPNBs and review the literature in order to establish
whether these cases represent true multicentric tumors or
intrabiliary tumor dissemination.
Case report
A 64-year-old woman with no particular previous
medical history presented with fever lasting for 1 week. No
remarkable findings were noted in a physical examination. The
serological tests revealed elevated levels of C-reactive protein
(13.4 mg/dl; normal range, <0.5 mg/dl), alanine aminotransferase
(62 IU/l; normal range, 5–36 IU/l), alkaline phosphatase (816 IU/l;
normal range, 110–370 IU/l) and γ-glutamyl transpeptidase (360
IU/l; normal range, 9–50 IU/l). The leukocyte count and serum
levels of aspartate aminotransferase, total bilirubin,
carcinoembryonic antigen and carbohydrate antigen 19–9 were within
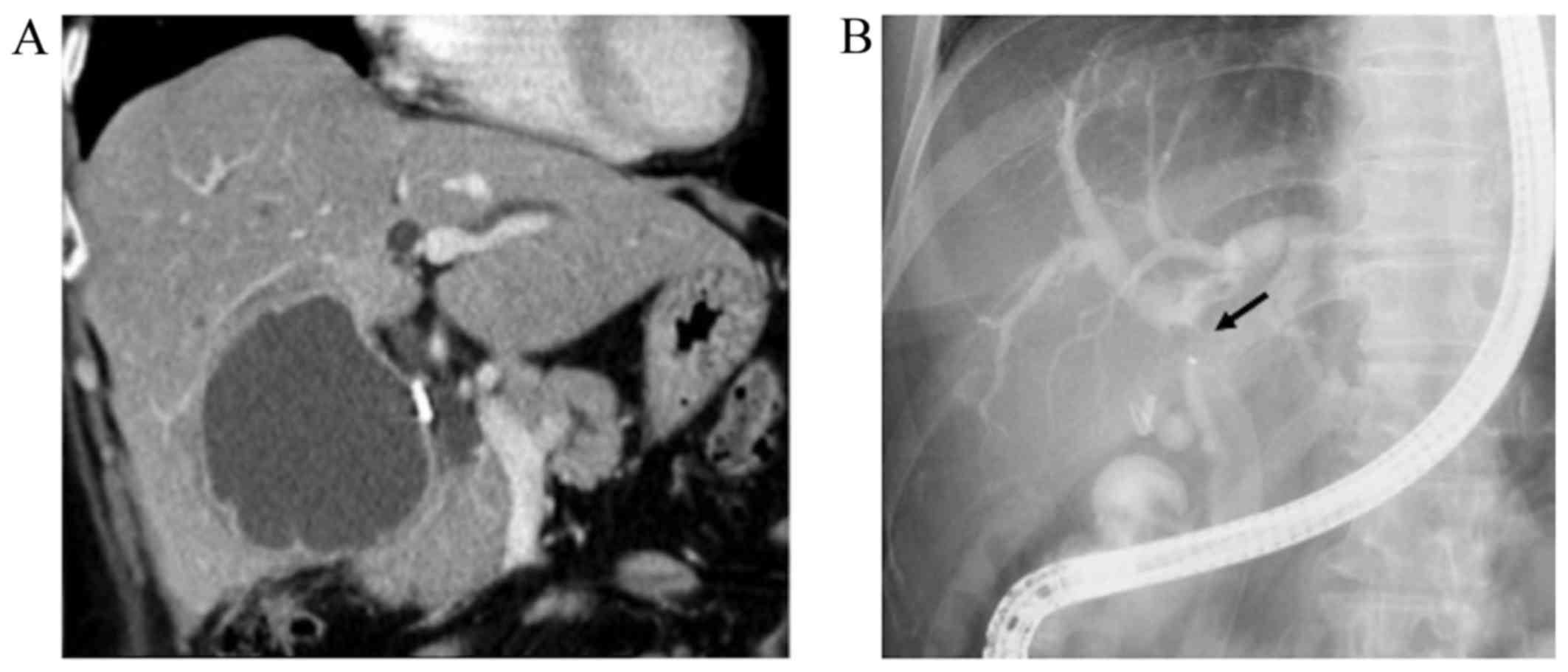
the normal range. On computed tomography (CT), a 75-mm cystic mass
in the right hepatic lobe and dilatation of the intrahepatic bile
duct were detected (Fig. 1A).
Following aspiration due to a suspected infectious liver cyst, the
patient underwent endoscopic retrograde cholangiography (ERC),
which revealed filling defects within the dilated common hepatic
duct (Fig. 1B). On peroral
cholangioscopy (POCS), a papillary mucin-producing tumor was
identified around the orifice of the right hepatic duct, while no
obvious tumor was present in the left hepatic and common bile ducts
(Fig. 2). Although the small bile
duct biopsy from the papillary tumor was not conclusive, extended
right hepatectomy was performed for suspected IPNB. On
intraoperative frozen section examination, the common hepatic duct
margin was tumor-free, while the left hepatic duct appeared to be
positive for an intraepithelial tumor with high-grade dysplasia.
Despite additional resection of the left hepatic duct, the final
resection margin remained focally positive for an intraepithelial
neoplasm.
On macroscopic examination, the main tumor was
located in the right hepatic duct and exhibited mucin
overproduction. The tumor also extended into the dilated
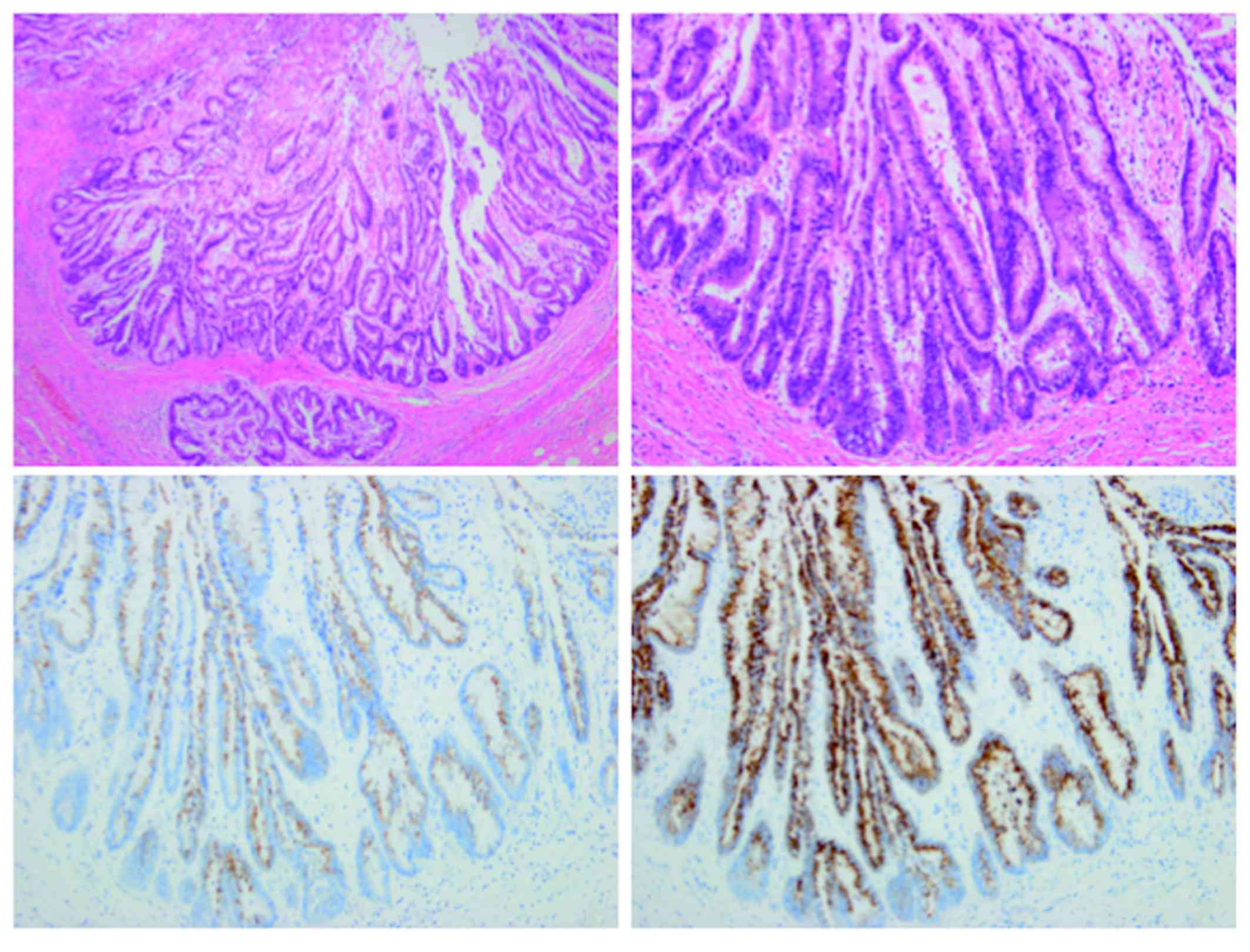
intrahepatic bile ducts. Microscopically, the biliary neoplasm
consisted of atypical epithelial cells arranged in a high papillary
architecture along thin fibrovascular stalks (Fig. 3). The tumor cells exhibited
intestinal-type morphology (e.g., nuclear stratification and goblet
cells), with enlarged nuclei and loss of polarity, characteristics
consistent with high-grade dysplasia. No invasive growth was
observed. On immunostaining, the cells were diffusely positive for
mucin core protein (MUC)2 and MUC5AC (Fig. 3), focally positive for MUC1, MUC6 and
cytokeratin (CK)20, and negative for CK7. Based on the histological
findings, the tumor was diagnosed as intestinal-type IPNB with
high-grade dysplasia. Since the left hepatic duct margin was
positive for tumor cells, gemcitabine 1,000 mg/m2 was
administered every 2 weeks for a total of 15 courses.
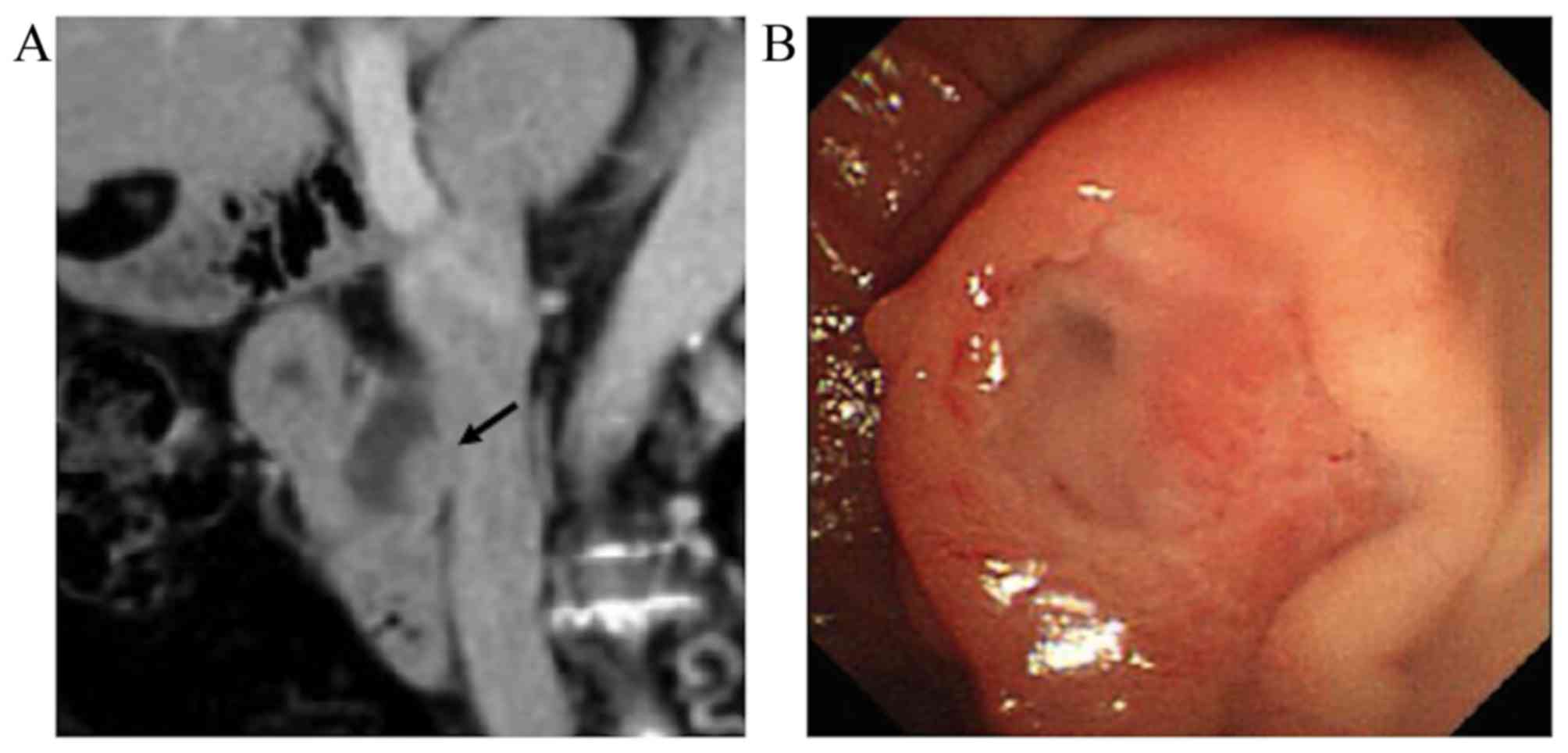
Twenty-six months later, the patient underwent a
follow-up CT, which revealed no recurrence in the left hepatic
duct, but detected a papillary tumor in the common bile duct
(Fig. 4A). On ERC, the tumor
appeared to be a mucin-producing neoplasm with abundant mucus
secreted from the dilated ampulla of Vater (Fig. 4B). Pancreatoduodenectomy was thus
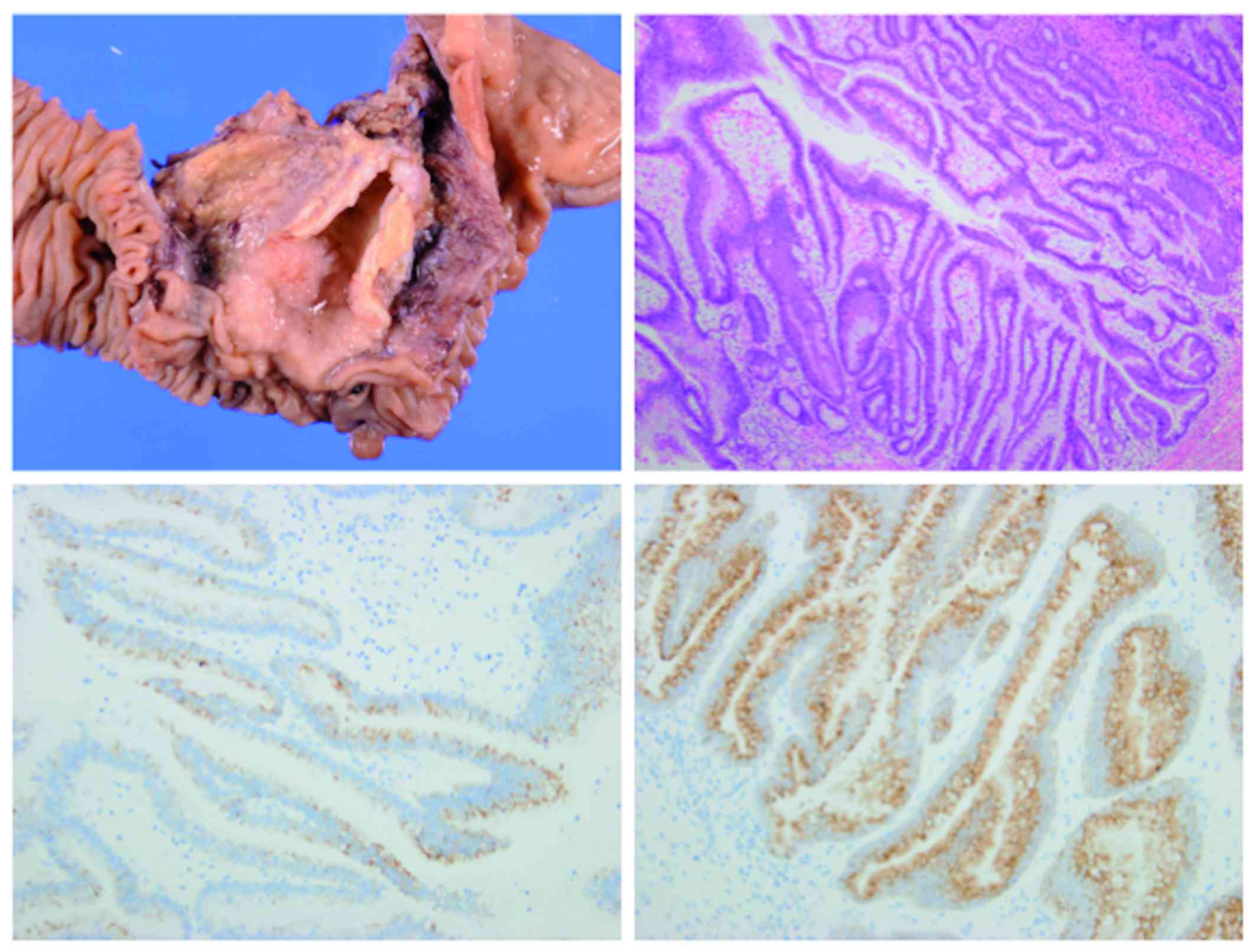
performed for suspected recurrent IPNB. In the resected specimen,
the tumor was located in the lower common bile duct (Fig. 5). The histological appearance of the
tumor was nearly identical to that of the original neoplasm, with a
similar immunohistochemical phenotype (Fig. 5). No invasive cancer was found. The
tumor was surrounded by non-neoplastic epithelium. The
postoperative course was uneventful, with no signs of recurrence at
the time of writing of this manuscript (follow-up of 12
months).
Verbal consent for publication of the case details
was obtained from the patient.
Discussion
In the present case, two separate IPNBs developed
over a ~2-year period. Although the left hepatic duct margin in the
initial surgery was focally positive for intraepithelial neoplasia,
the second tumor developed in the lower common bile duct. The
recurrent tumor was surrounded by non-neoplastic epithelium,
suggesting that the two IPNBs were separate, with no tumor cells
between them. This case was originally considered to be
multicentric IPNBs. However, we also hypothesized that the second
tumor represented intrabiliary tumor dissemination, as both tumors
had a nearly identical histological appearance, and the second
tumor developed in the lower part of the bile duct compared with
the primary tumor. Since intrabiliary dissemination is not
considered as progression in IPNBs, a literature review was
performed to investigate this possibility.
Large studies were first reviewed, in which >20
cases of IPNBs were investigated, in terms of how often multiple
tumors develop in patients with IPNBs. Among ~10 large studies
reported to date from Asia, Europe and the USA, two provided
detailed descriptions of patients with multiple biliary tumors
(5,6). In a study by Paik et al
(5), 4 of the 25 investigated cases
with IPNBs were found to have tumor recurrence after complete
resection with negative surgical margins. Two patients had
recurrence in the liver parenchyma and lymph nodes, representing
remote metastasis of the invasive components. In the remaining 2
patients, the primary IPNBs were located in the intrahepatic bile
ducts, whereas the recurrent tumors developed in the distal bile
duct, similar to our patient. Kang et al (6) also reported multifocal tumors in 43 of
84 (51%) patients with IPNBs; however, this number appears to be
markedly higher compared with that in other studies, which leads us
to question the definition of multiple IPNBs used in that study. No
or limited information was available in terms of multiple tumors in
the remaining studies (7–10).
An additional literature review was conducted with a
focus on case reports. The PubMed database was searched using the
terms ‘IPNB’, ‘mucin-producing bile duct tumor’, ‘biliary
papillomatosis’, ‘multiple’, ‘multiplicity’ and ‘recurrence’, and
the selected manuscripts were reviewed. A similar search was also
conducted through ICHUSHI (http://login.jamas.or.jp/), which enables searching
for medical literature and abstracts written in Japanese. Cases of
recurrent tumors at the anastomotic site were excluded, as they
were most likely to be local recurrence rather than multiple
tumors. A total of 9 additional case reports describing multiple
separate IPNBs were eventually retrieved (11–19). The
clinicopathological characteristics of these cases and our patient
are summarized in Table I. Of the 10
patients, 8 initially underwent liver resection for IPNBs that
developed in the hilar or intrahepatic bile duct, while the
remaining 2 received bile duct resection for a cystic duct neoplasm
or cholecystectomy for a gallbladder IPNB. Multiple tumors were
identified in the original resected specimens in 2 patients (cases
4 and 9, Table I). Recurrence
occurred over a median period of 25 months. Recurrent tumors
developed in more distal parts of the bile duct compared with the
primary tumors in 8 of the 10 patients.
 | Table I.Reported cases of multiple recurrent
intraductal papillary neoplasms of the bile duct. |
Table I.
Reported cases of multiple recurrent
intraductal papillary neoplasms of the bile duct.
|
| Primary tumor | Recurrent tumor |
|
|---|
|
|
|
|
|
|---|
| Case | Site | No. of tumors | Surgical
procedure | Resection margin | No. of episodes | Site | No. of tumors | Treatment | Interval
(months) | Refs. |
|---|
| 1 | IHBD (B2/3) | 1 | Left hepatectomy | Negative | 1 | Lower CBD | 1 | Whipple | 36 | (11) |
| 2 | Cystic duct to
CBD | 1 | Choledochectomy and
cholecystectomy | Negative | 1 | Lower CBD | 3 | Whipple | 57 | (12) |
| 3 | IHBD (B4) | 1 | Left hepatectomy | Negative | 4 | IHBD (B1) | 1 | Laser ablation | 6 | (13) |
|
|
|
|
|
|
| Upper CBD | 1 | Enucleation | 7 |
|
|
|
|
|
|
|
| RHD | 1 | Choledochectomy | 28 |
|
|
|
|
|
|
|
| IHBD (right posterior
segmental duct) | 1 | Laser ablation | 2 |
| 4 | IHBD (B2/3) | 2 | Left hepatectomy | Not described | 1 | Lower CBD | 1 | Whipple | 24 | (14) |
| 5 | RHD | 1 | Left hepatectomy and
choledochectomy | Negative | 1 | Lower CBD | 1 | Whipple | 48 | (15) |
| 6 | Gallbladder | 1 | Cholecystectomy | Negative | 1 | Lower CBD | 3 | Whipple | 10 | (16) |
| 7 | IHBD (B2) | 1 | Left hepatectomy | Negative | 2 | Upper CBD | 1 | Choledochectomy | 15 | (17) |
|
|
|
|
|
|
| Lower CBD | 1 | Whipple | 35 |
| 8 | IHBD (B8) | 1 | Right hepatectomy and
choledochectomy | Negative | 1 | Lower CBD | 1 | Whipple | 16 | (18) |
| 9 | LHD | 2 | Left hepatectomy | Negative | 1 | IHBD (right
lobe) | 1 | Drainage stent
only | 29 | (19) |
| 10 | RHD | 1 | Right
hepatectomy | Positive | 1 | Lower CBD | 1 | Whipple | 26 | Present |
Although the majority of the patients had a single
episode of tumor recurrence, 2 patients had multiple episodes
(cases 3 and 7 in Table I) (13,17).
Miyata et al (17) described
a case that developed recurrent IPNB twice. A 66-year-old woman
originally had IPNB in the left hepatic lobe and underwent left
hepatectomy with a negative surgical margin. Fifteen months later,
a recurrent tumor was detected in the upper common bile duct, which
was surgically resected. Although the bile duct margin in the
second surgery was also tumor-free, another papillary tumor was
detected in the intrapancreatic bile duct 35 months later,
requiring the Whipple procedure.
The literature review clearly demonstrated that
recurrent tumors typically develop in the lower bile duct compared
with the primary IPNBs. Of note, 84% of primary IPNBs develop in
the intrahepatic or hilar bile duct (20), in contrast to recurrent IPNBs, 80% of
which developed in the common bile duct (Table I). These findings suggest that
intrabiliary dissemination is a more likely mechanism for multiple
IPNBs, rather than true multicentricity in the majority of the
patients. However, multicentric IPNBs remain a possibility in a
specific proportion of patients, particularly those with risk
factors for this biliary neoplasm, such as hepatolithiasis. Future
molecular studies comparing gene abnormalities in multiple IPNBs
are required to elucidate the frequency of true multicentric IPNBs
developing in the biliary tree.
Matsubara et al (21) recently reported a case of papillary
adenocarcinoma of the ampulla of Vater, which was subsequently
complicated by retrograde intraductal dissemination in the
pancreas. Although this is the only report raising the possibility
of cancer cells possibly disseminating along the pancreatobiliary
duct system, we have observed similar cases, in which ampullary
cancers or intrapancreatic cholangiocarcinomas were intraductally
disseminated to the pancreas. To the best of our knowledge, the
present study is the first report suggesting that a similar pattern
of tumor extension may also occur in the bile duct. Multiple IPMNs,
particularly of the branch-duct type, are common; however, the
majority are suspected to represent true multicentric tumors. One
reason is that the majority of branch-duct type IPMNs are
low-grade, with a low propensity for dissemination. Another
potential factor is that the pancreatic duct is located
horizontally, in contrast to the bile duct, which is positioned
vertically.
In conclusion, the results of our case indicate that
multiple IPNBs may be attributed to the biliary dissemination of
tumor cells. This hypothesis is supported by the literature review.
True multicentric IPNBs appear to be less common than initially
considered, given that the extrahepatic lower bile duct is
typically affected by recurrent IPNBs.
Glossary
Abbreviations
Abbreviations:
|
IPNBs
|
intraductal papillary neoplasms of the
bile duct
|
|
IPMNs
|
intraductal papillary mucinous
neoplasms
|
|
CT
|
computed tomography
|
|
ERC
|
endoscopic retrograde
cholangiography
|
|
POCS
|
peroral cholangioscopy
|
References
|
1
|
Zen Y, Fujii T, Itatsu K, Nakamura K,
Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A,
Masuda S, et al: Biliary papillary tumors share pathological
features with intraductal papillary mucinous neoplasm of the
pancreas. Hepatology. 44:1333–1343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zen Y, Sasaki M, Fujii T, Chen TC, Chen
MF, Yeh TS, Jan YY, Huang SF, Nimura Y and Nakanuma Y: Different
expression patterns of mucin core proteins and cytokeratins during
intrahepatic cholangiocarcinogenesis from biliary intraepithelial
neoplasia and intraductal papillary neoplasm of the bile duct-an
immunohistochemical study of 110 cases of hepatolithiasis. J
Hepatol. 44:350–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohtsuka M, Shimizu H, Kato A, Yoshitomi H,
Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O and Miyazaki M:
Intraductal papillary neoplasms of the bile duct. Int J Hepatol.
2014:4590912014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zen Y, Fujii T, Itatsu K, Nakamura K,
Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S, et al:
Biliary cystic tumors with bile duct communication: A cystic
variant of intraductal papillary neoplasm of the bile duct. Mod
Pathol. 19:1243–1254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paik KY, Heo JS, Choi SH and Choi DW:
Intraductal papillary neoplasm of the bile ducts: The clinical
features and surgical outcome of 25 cases. J Surg Oncol.
97:508–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang MJ, Jang JY, Lee KB, Han IW and Kim
SW: Impact of macroscopic morphology, multifocality, and mucin
secretion on survival outcome of intraductal papillary neoplasm of
the bile duct. J Gastrointest Surg. 17:931–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung G, Park KM, Lee SS, Yu E, Hong SM and
Kim J: Long-term clinical outcome of the surgically resected
intraductal papillary neoplasm of the bile duct. J Hepatol.
57:787–793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SS, Kim MH, Lee SK, Jang SJ, Song MH,
Kim KP, Kim HJ, Seo DW, Song DE, Yu E, et al: Clinicopathologic
review of 58 patients with biliary papillomatosis. Cancer.
100:783–793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rocha FG, Lee H, Katabi N, DeMatteo RP,
Fong Y, D'Angelica MI, Allen PJ, Klimstra DS and Jarnagin WR:
Intraductal papillary neoplasm of the bile duct: A biliary
equivalent to intraductal papillary mucinous neoplasm of the
pancreas? Hepatology. 56:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen
TC, Jan YY and Chen MF: Cholangiographic spectrum of intraductal
papillary mucinous neoplasm of the bile ducts. Ann Surg.
244:248–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano H, Nakamura M, Yoshikawa T, Araida
T, Azuma T, Ohta T, Takasaki K and Hanyu F: A Recent Case of
Mucin-producing Distal Bile Duct Carcinoma Recurred 3 Years after
Resection of Mucin-producing Intrahepatic Bile Duct Carcinoma. Tan
to Sui. 17:497–502. 1996.(In Japanese).
|
|
12
|
Fujioka M, Mitsui T, Terada T, Takehara A,
Uno A, Kawaguchi M, Munemoto Y, Asada Y, Miura S and Zen Y: A Case
of Biliary Papillomatosis with Asynchronous Recurrence. Tan to Sui.
28:231–236. 2007.(In Japanese).
|
|
13
|
Kurahara H, Shinchi H, Mataki Y, Maeda S,
Natsugoe S and Takao S: A long-term survival case of
mucin-producing bile duct carcinoma treated with repetitive
surgical procedure. Jpn J Gastroenterol Surg. 42:510–515. 2009.(In
Japanese). View Article : Google Scholar
|
|
14
|
Fukuda S, Koide K, Mukai S, Oishi K,
Fujisaki S, Arita M, Sakimoto H, Eto T and Takahashi M: A case of
biliary papillomatosis with asynchronous recurrence after curative
operation. Jpn J Gastroenterol Surg. 43:815–821. 2010.(In
Japanese). View Article : Google Scholar
|
|
15
|
Ito E, Watanabe J, Hatano M, Kushihata F
and Takada Y: A case of intraductal papillary neoplasm of bile
duct, which recurred 4 years after primary curative resection. J
Jpn Surg Assoc. 74:791–796. 2013.(In Japanese). View Article : Google Scholar
|
|
16
|
Sato H, Sato Y, Harada K, Sasaki M, Hirano
K and Nakanuma Y: Metachronous intracystic and intraductal
papillary neoplasms of the biliary tree. World J Gastroenterol.
19:6125–6126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyata T, Kawamukai J, Sakurai K, Terakawa
H, Matsui D, Watanabe T, Kawahara Y, Kinoshita J, Amaya S, Terada
I, et al: A Case of Intraductal Papillary Neoplasm of Bile Duct,
which Recurred 2 Times after Primary Curative Resection. Tan to
Sui. 35:1319–1326. 2014.(In Japanese).
|
|
18
|
Ohgi K, Sugiura T, Kanemoto H, Okamura Y,
Ito T, Kuribara T, Asida R, Sasaki K, Nakamura Y and Uesaka K: A
case of intraductal papillary neoplasm of the bile duct recurred at
the remnant lower bile duct after curative liver resection. JJBA.
29:271–278. 2015.(In Japanese).
|
|
19
|
Narita M, Endo B, Mizumoto Y, Matsusue R,
Hata H, Yamaguchi T, Otani T and Ikai I: Multicentric recurrence of
intraductal papillary neoplasms of bile duct in the remnant
intrahepatic bile duct after curative resection. Int J Surg Case
Rep. 12:123–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujikura K, Fukumoto T, Ajiki T, Otani K,
Kanzawa M, Akita M, Kido M, Ku Y, Itoh T and Zen Y: Comparative
clinicopathological study of biliary intraductal papillary
neoplasms and papillary cholangiocarcinomas. Histopathology. (In
press). PubMed/NCBI
|
|
21
|
Matsubara A, Nara S, Sekine S, Ojima H,
Kosuge T, Shimada K, Kushima R, Kanai Y and Hiraoka N: Intraductal
dissemination of papillary adenocarcinoma of the ampulla of Vater
in the pancreatic duct. Pathol Int. 64:39–44. 2014. View Article : Google Scholar : PubMed/NCBI
|