Introduction
The significance of the breast cancer 1
(BRCA1) and BRCA2 mutations in familial breast and
ovarian cancer has been well established (1,2).
However, the mutations of these genes are estimated to cause, at
most, 20–30% of cases of hereditary breast cancer (3). The present authors studied the
BRCA1/2 mutations in 191 patients in a previous study, but
the prevalence was shown to be unexpectedly low (4,5). In
fact, it was only 7% among the analyzed patients who had a family
history of breast cancers.
Partner and localizer of BRCA2 (PALB2) was
identified as a moderate-risk gene in breast and pancreas cancer
(6). PALB2 is located on
chromosome 16p12.2 containing 13 exons and 12 introns, and is
involved in BRCA2-associated pathways (6). Recently, Antoniou et al
(7) reported that PALB2
carriers have a high risk of developing breast cancer, and
concluded that the cumulative risk of mutation carrier was 34% by
the age of 70 in their prospective follow-up study on 154
families.
The prevalence of the PALB2 mutation was
reported to be 1.2–3.4% in European countries, whereas it is very
rare in Asian countries (8–18). To the best of our knowledge, no study
has been performed that has identified the PALB2 deleterious
mutation in Japanese patients with breast cancer. From our first
cohort data, no deleterious PALB2 mutations were identified
in 155 patients with breast and/or ovarian cancer who were
estimated to be at risk of hereditary cancer according to the
National Comprehensive Cancer Network (NCCN) criteria (19). In the present case study, an
additional 128 cases having breast and/or ovarian cancer were
studied, and the case of a patient with bilateral breast cancer is
presented who harbors the deleterious mutation in PALB2.
Factoring in the first cohort of 155 cases, the frequency of the
PALB2 mutation is now estimated at 0.35 % (1/283) in the
Japanese population.
Case report
A 63-year-old female was referred to our hospital
(Department of Breast Surgery, Yamanashi Prefectural Central
Hospital, Kofu, Japan) due to the presence of a lump in her left
breast and bloody discharge from the right-side nipple. The patient
had no personal history of other cancers or diseases. Her family
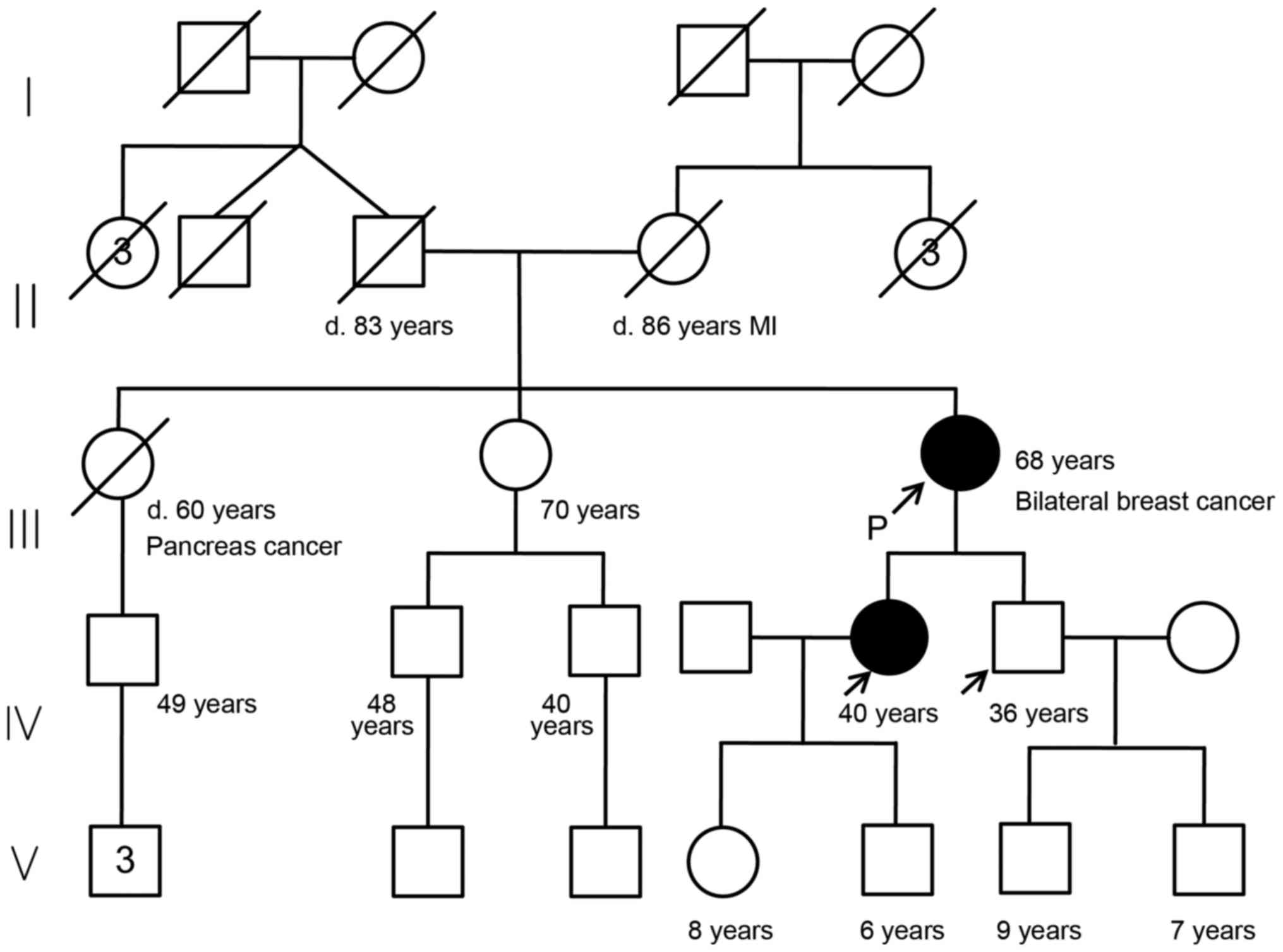
history is shown in the pedigree chart (Fig. 1). The patient had two gravidas and
two parities.
The cytology of nipple discharge was performed by
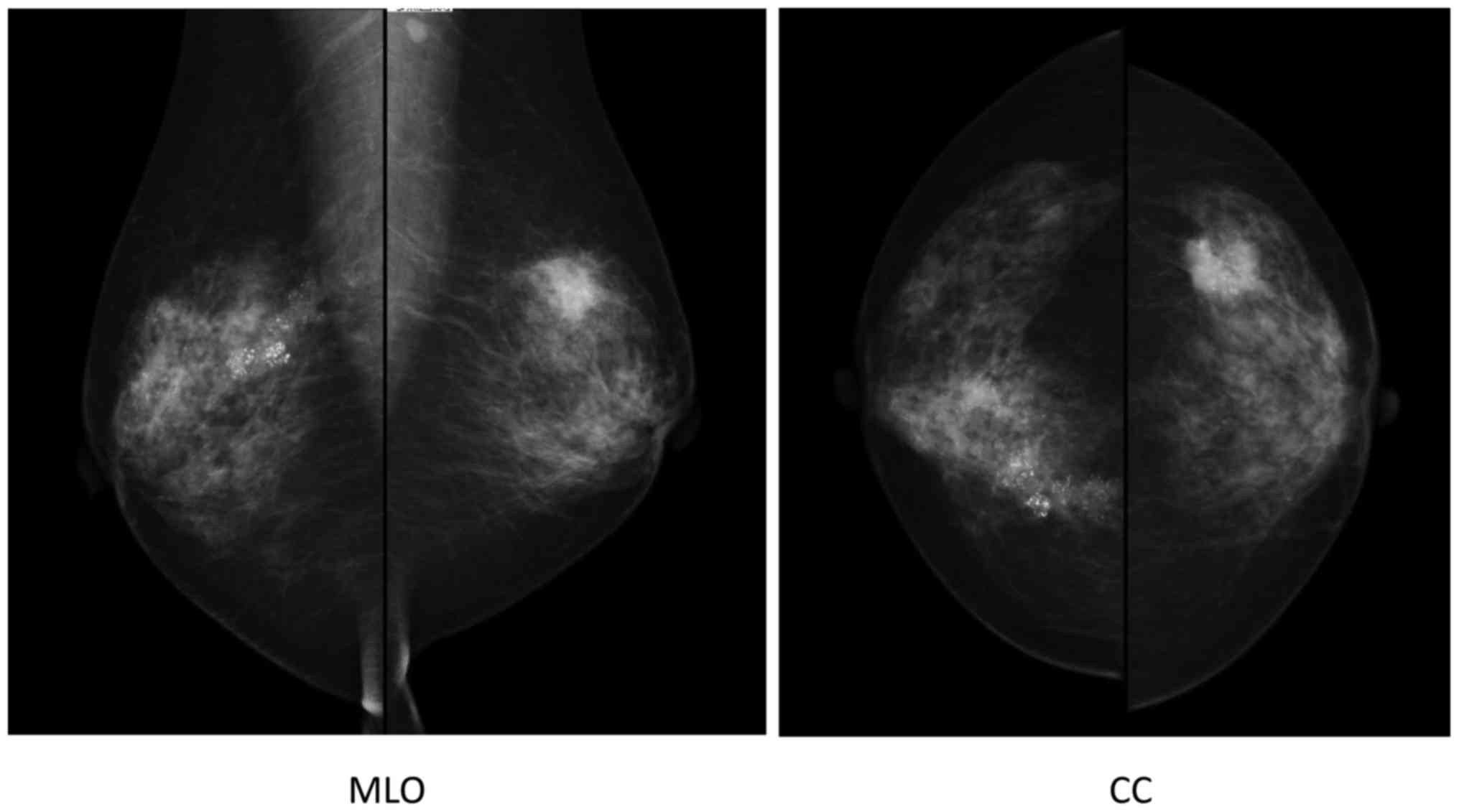
the clinic, revealing the presence of malignant cells. Mammography
indicated segmental pleomorphic calcification in the right breast,
and a spiculated polygonal tumor measuring 2 cm in diameter with
pleomorphic calcification in the left breast. Furthermore, an
irregularly shaped axillar lymph node was observed on the left side
(Fig. 2).
Fine-needle aspiration cytology for the left-sided
breast tumor also revealed the presence of malignant cells. The
patient was diagnosed with bilateral breast cancer, and underwent a
right-sided mastectomy and breast reconstruction, and left-sided
breast-conserving therapy. Pathological findings revealed that the
right-sided breast cancer was ductal carcinoma in situ
(DCIS), with no lymph node metastasis, grade 2, estrogen receptor
(ER) (7+) and progesterone receptor (PR) (3+) according to the
Allred Score (20), and human
epidermal growth factor 2 (HER2) (1+) according to the American
Society of Clinical Oncology (ASCO)/College of American
Pathologists (CAP) criteria (21). The left-sided breast cancer was
invasive ductal carcinoma (non-specific type) with lymph node
metastases (2/12), grade 2, ER (8+), PR (6+), and HER2 (1+).
Epirubicin-cyclophosphamide (EC) adjuvant chemotherapy (epirubicin,
90 mg/m2, and cyclophosphamide, 600 mg/m2, 3
times a week for 4 cycles, followed by docetaxel, 75
mg/m2, 3 times a week for 4 cycles) was administered,
and subsequently, radiation therapy (50 Gray) for the left-side
breast was performed. The patient received oral hormone therapy
with toremifen (40 mg/day) for 5 years.
The benefits and disadvantages of knowing the
results of genetic testing were explained to the patient. Added to
the explanation was the possibility that there could be uncertain
results that would need to be clarified in future investigations.
The patient and her family (40-year-old daughter and 36-year-old
son) were referred to genetic counseling (S.N. and T.K.). Written
informed consent was obtained from the patient and from her
daughter and son.
Germline mutations for BRCA1/2 and
PALB2 were analyzed using targeted sequencing, as previously
reported (4,19,22).
Briefly, the Ion AmpliSeq™ BRCA1 and BRCA2 and the Ion AmpliSeq™
BRCA Reflex Hereditary Cancer Research panels (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) were used, targeting the whole
exons of the BRCA1/2 genes and an additional 25 hereditary
cancer-associated genes (22,23).
Buffy coat DNA was used as a template, and the sequencing library
was generated using an AmpliSeq Library kit 2.0 (Thermo Fisher
Scientific, Inc.) (24–31). Next-generation sequencing analysis
was subsequently performed on an Ion PGM or Ion Proton platform
(Thermo Fisher Scientific, Inc.) (24–31).
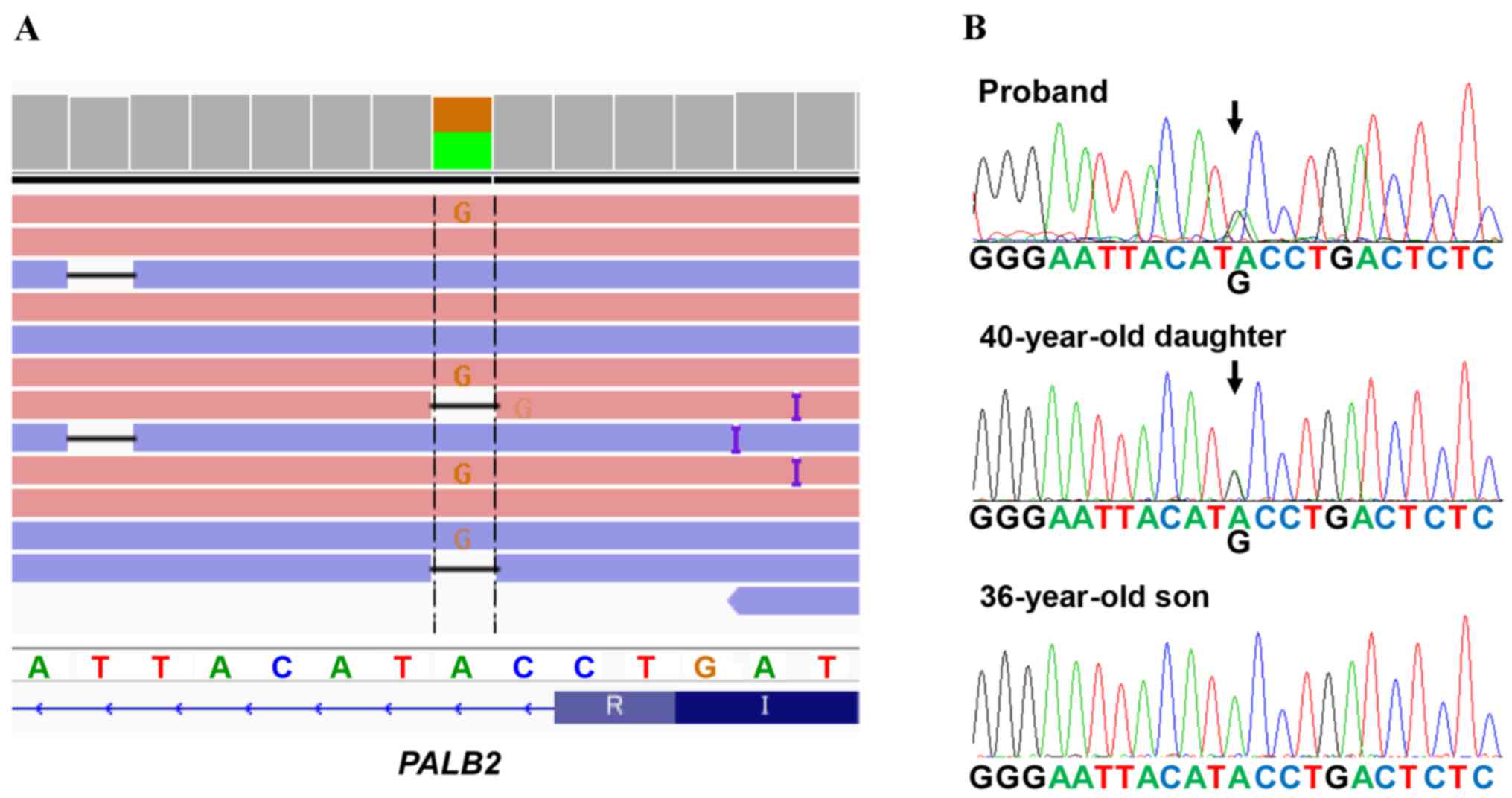
A deleterious mutation of PALB2 (chr16:
23635328, c. 2834+2 T>C) was identified (Fig. 3A), which is the first case in 283
analyzed patients in our hospital during the period between 2013
and 2016, i.e., 0.35% or 1/283 of Japanese patients were revealed
to have the PALB2 deleterious mutation. Furthermore the
splice-site mutation in PALB2 was not identified in the
Exome Aggregation Consortium (ExAC), the Human Genetic Variation
Database (HGVD), the Integrative Japanese Genome Variation (iJGVD)
or the Catalogue Of Somatic Mutations In Cancer (COSMIC) databases.
To the best of our knowledge, this variant has therefore not been
reported previously, suggesting that our identified variant is
novel one.
The pedigree chart of the patient is shown in
Fig. 1. The patient had two elder
sisters, the eldest of whom succumbed to pancreatic cancer at 60
years of age, whereas the other sister is alive and well at 70
years of age. The patients' parents died from causes unrelated to
cancer. To the best of the patient's knowledge, no other family
members (7 uncles or aunts, and 3 nephews and their descendants)
have experienced cancer. The patient's 40-year-old daughter and
36-year-old son underwent gene informed consent to have genetic
testing for the PALB2 mutation. It was revealed that the
daughter was affected, whereas the son was not (Fig. 3B). The mutation was also confirmed
using Sanger sequencing (Fig. 3B).
The 40-year-old daughter is now receiving regular check-ups for
malignancies, including those of the breast and pancreas.
Discussion
PALB2 serves a crucial role in the
localization and stabilization of BRCA2 in nuclear
chromatin, which is essential for BRCA2 to function in
double-strand-break DNA repair by homologous recombination.
PALB2 mono-allelic mutations result in cancer development,
and bi-allelic mutations lead to a type of Fanconi anemia (6).
Recently, Antoniou et al (7) reported that PALB2 carriers have
a high risk of developing breast cancer, and determined that the
cumulative risk of mutation carrier was 34% by the age of 70 in
their prospective follow-up study on 154 families. In the USA,
Canada, and Europe, the frequency of PALB2 deleterious
mutations was revealed to vary from 1.1 to 3.4% (8–15). A
total of 4 previous studies have arisen from Asia. One study by Cao
et al (16) from China
revealed 3 cases out of 360 (0.8%) with the deleterious mutations,
although there were none from Korea (300 cases) or from Malaysia
(122 cases) (17,18). The previous study by the present
authors on Japanese patients (n=155) revealed that none of them had
the deleterious mutation (19).
The PALB2 mutation has been reported to be
associated with the development of pancreatic cancer. The
prevalence of the PALB2 mutation among familial pancreatic
cancer was reported to be ~3–4% in the USA and European countries
(32,33). In Japan, Takai et al (34) recently reported that two deleterious
PALB2 mutations were detected in 54 familial pancreas cancer
families, as well as three BRCA2 and two ATM
deleterious mutations. However, the association between
PALB2 mutations and the risk of pancreatic cancer has yet to
be fully elucidated among the Japanese population.
In the present case study, a 60-year-old elder
sister was known to have had pancreatic cancer. However, it was
impossible to examine the PALB2 germline mutations, since a
DNA sample was not available from the sister. To reveal whether the
identified PALB2 splice-site mutation has affected tumor
development, it will be better to perform segregation analysis in
this family. As a minimum at the present time, the proband's
daughter, who has the PALB2 mutation, should continue to
have regular check-ups assessing the risk of developing pancreatic
cancer, as well as breast cancer.
Compared with the USA and European countries,
analysis of BRCA1/2 for the detection of hereditary breast
and/or ovarian cancer has not been widely accepted in Japan.
Reports originating from Japan remain few in number (5,35,36).
Further investigations are required to reveal the genetic features
of Japanese patients with breast and/or other cancers (ovary,
pancreas, prostate, and so forth).
It is important to understand the association
between carcinogenesis and the dysfunction of DNA-repair genes in
Japanese patients due to the up-and-coming therapeutic strategies
that employ poly(ADP-ribose) polymerase (PARP) inhibitors, such as
Orapalib (37,38). Recently, multi-gene assays for
hereditary cancer have been developed (23,39), and
other genes associated with double-strand DNA repair, such as
PALB2, ATM, BARD1, and RAD51, will be
analyzed for patients with hereditary cancer. These analyses are
expected to reveal the association between DNA-repair genes and
carcinogenesis with various types of cancer.
In conclusion, to the best of our knowledge, this is
the first identified case of PALB2 mutations in a Japanese
patient with breast cancer. The present study therefore suggests
that the PALB2 mutation is associated with the development
of breast and pancreas cancer, even in Japanese patients. At
present, the frequency of the germline mutation in PALB2 is
0.35% (1/283 cases).
Acknowledgements
We thank Takuro Uchida, Yumi Kubota and Shino Kirito
for their assistance. This study was approved by the institutional
review board at Yamanashi Prefectural Central Hospital, and funded
by a Grant-in-aid for Genome Research program from Yamanashi
Prefecture.
References
|
1
|
Collaborative Group on Hormonal Factors in
Breast Cancer, . Familial breast cancer: Collaborative reanalysis
of individual data from 52 epidemiological studies including 58,209
women with breast cancer and 101,986 women without the disease.
Lancet. 358:1389–1399. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim H and Choi DH: Distribution of BRCA1
and BRCA2 mutations in Asian patients with breast cancer. J Breast
Cancer. 16:357–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melchor L and Benítez J: The complex
genetic landscape of familial breast cancer. Hum Genet.
132:845–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya
K, Mochizuki H and Omata M: Detection of BRCA1 and BRCA2 germline
mutations in Japanese population using next-generation sequencing.
Mol Genet Genomic Med. 3:121–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi
H, Ikegami A, Teramoto K, Amemiya K, Mochizuki H and Omata M: BRCA1
and BRCA2 mutations in Japanese patients with ovarian, fallopian
tube, and primary peritoneal cancer. Cancer. 122:84–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tischkowitz M and Xia B: PALB2/FANCN:
Recombining cancer and Fanconi anemia. Cancer Res. 70:7353–7359.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antoniou AC, Casadei S, Heikkinen T,
Barrowdale D, Pylkäs K, Roberts J, Lee A, Subramanian D, De Leeneer
K, Fostira F, et al: Breast-cancer risk in families with mutations
in PALB2. N Engl J Med. 371:497–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casadei S, Norquist BM, Walsh T, Stray S,
Mandell JB, Lee MK, Stamatoyannopoulos JA and King MC: Contribution
of inherited mutations in the BRCA2-interacting protein PALB2 to
familial breast cancer. Cancer Res. 71:2222–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng Y, Zhang J, Niu Q, Huo D and Olopade
OI: Novel germline PALB2 truncating mutations in African American
breast cancer patients. Cancer. 118:1362–1370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hartley T, Cavallone L, Sabbaghian N,
Silva-Smith R, Hamel N, Aleynikova O, Smith E, Hastings V, Pinto P,
Tischkowitz M, et al: Mutation analysis of PALB2 in BRCA1 and
BRCA2-negative breast and/or ovarian cancer families from Eastern
Ontario, Canada. Hered Cancer Clin Pract. 12:192014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rahman N, Seal S, Thompson D, Kelly P,
Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T, et
al: PALB2, which encodes a BRCA2-interacting protein, is a breast
cancer susceptibility gene. Nat Genet. 39:165–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bogdanova N, Sokolenko AP, Iyevleva AG,
Abysheva SN, Blaut M, Bremer M, Christiansen H, Rave-Fränk M, Dörk
T and Imyanitov EN: PALB2 mutations in German and Russian patients
with bilateral breast cancer. Breast Cancer Res Treat. 126:545–550.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Catucci I, Peterlongo P, Ciceri S, Colombo
M, Pasquini G, Barile M, Bonanni B, Verderio P, Pizzamiglio S,
Foglia C, et al: PALB2 sequencing in Italian familial breast cancer
cases reveals a high-risk mutation recurrent in the province of
Bergamo. Genet Med. 16:688–694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erkko H, Xia B, Nikkilä J, Schleutker J,
Syrjäkoski K, Mannermaa A, Kallioniemi A, Pylkäs K, Karppinen SM,
Rapakko K, et al: A recurrent mutation in PALB2 in Finnish cancer
families. Nature. 446:316–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blanco A, de la Hoya M, Osorio A, Diez O,
Miramar MD, Infante M, Martinez-Bouzas C, Torres A, Lasa A, Llort
G, et al: Analysis of PALB2 gene in BRCA1/BRCA2 negative Spanish
hereditary breast/ovarian cancer families with pancreatic cancer
cases. PLoS One. 8:e675382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao W, Wang X and Li JC: Hereditary breast
cancer in the Han Chinese population. J Epidemiol. 23:75–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH, Choi DH, Cho DY, Ahn SH, Son BH
and Haffty BG: PALB2 mutations 1592delT and 229delT are not present
in Korean breast cancer patients negative for BRCA1 and BRCA2
mutations. Breast Cancer Res Treat. 122:303–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phuah SY, Lee SY, Kang P, Kang IN, Yoon
SY, Thong MK, Hartman M, Sng JH, Yip CH, Taib NA and Teo SH:
Prevalence of PALB2 mutations in breast cancer patients in
multi-ethnic Asian population in Malaysia and Singapore. PLoS One.
8:e736382013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagomi H, Sakamoto I, Hirotsu Y, Amemiya
K, Mochiduki H and Omata M: Analysis of PALB2 mutations in 155
Japanese patients with breast and/or ovarian cancer. Int J Clin
Oncol. 21:270–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daltoé RD, Madeira KP, de Carvalho AA, de
Rezende LC, Silva IV and Rangel LB: Evaluation of the progesterone
receptor status in breast cancer using three different antibodies:
A comparison by Allred score system. Int J Clin Exp Pathol.
7:331–339. 2014.PubMed/NCBI
|
|
21
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya
K, Oyama T, Mochizuki H and Omata M: Multigene panel analysis
identified germline mutations of DNA repair genes in breast and
ovarian cancer. Mol Genet Genomic Med. 3:459–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kean S: Breast cancer. The ‘other’ breast
cancer genes. Science. 343:1457–1459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirotsu Y, Zheng TH, Amemiya K, Mochizuki
H, Guleng B and Omata M: Targeted and exome sequencing identified
somatic mutations in hepatocellular carcinoma. Hepatol Res.
46:1145–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goto T, Hirotsu Y, Oyama T, Amemiya K and
Omata M: Analysis of tumor-derived DNA in plasma and bone marrow
fluid in lung cancer patients. Med Oncol. 33:292016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakada H, Nakagomi H, Hirotsu Y, Amemiya
K, Mochizuki H, Inoue M, Oyama T and Omata M: A study of tumor
heterogeneity in a case with breast cancer. Breast Cancer.
29–Sep;2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amemiya K, Hirotsu Y, Goto T, Nakagomi H,
Mochizuki H, Oyama T and Omata M: Touch imprint cytology with
massively parallel sequencing (TIC-seq): A simple and rapid method
to snapshot genetic alterations in tumors. Cancer Med. 5:3426–3436.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirotsu Y, Kojima Y, Okimoto K, Amemiya K,
Mochizuki H and Omata M: Comparison between two amplicon-based
sequencing panels of different scales in the detection of somatic
mutations associated with gastric cancer. BMC Genomics. 17:8332016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirotsu Y, Nakagomi H, Amemiya K, Oyama T,
Inoue M, Mochizuki H and Omata M: Intrinsic HER2 V777L mutation
mediates resistance to trastuzumab in a breast cancer patient. Med
Oncol. 34:32017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakagomi H, Hirotsu Y, Amemiya K, Nakada
H, Inoue M, Mochizuki H, Oyama T and Omata M: Rapid changes in
circulating tumor DNA in serially sampled plasma during treatment
of breast cancer: A case report. Am J Case Rep. 18:26–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goto T, Hirotsu Y, Mochizuki H, Nakagomi
T, Oyama T, Amemiya K and Omata M: Stepwise addition of genetic
changes correlated with histological change from
‘well-differentiated’ to ‘sarcomatoid’ phenotypes: A case report.
BMC Cancer. 17:652017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slater EP, Langer P, Niemczyk E, Strauch
K, Butler J, Habbe N, Neoptolemos JP, Greenhalf W and Bartsch DK:
PALB2 mutations in European familial pancreatic cancer families.
Clin Genet. 78:490–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hofstatter EW, Domchek SM, Miron A, Garber
J, Wang M, Componeschi K, Boghossian L, Miron PL, Nathanson KL and
Tung N: PALB2 mutations in familial breast and pancreatic cancer.
Fam Cancer. 10:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takai E, Yachida S, Shimizu K, Furuse J,
Kubo E, Ohmoto A, Suzuki M, Hruban RH, Okusaka T, Morizane C and
Furukawa T: Germline mutations in Japanese familial pancreatic
cancer patients. Oncotarget. 7:74227–74235. 2016.PubMed/NCBI
|
|
35
|
Sugano K, Nakamura S, Ando J, Takayama S,
Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, et
al: Cross-sectional analysis of germline BRCA1 and BRCA2 mutations
in Japanese patients suspected to have hereditary breast/ovarian
cancer. Cancer Sci. 99:1967–1976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakamura S, Takahashi M, Tozaki M,
Nakayama T, Nomizu T, Miki Y, Murakami Y, Aoki D, Iwase T,
Nishimura S, et al: Prevalence and differentiation of hereditary
breast and ovarian cancers in Japan. Breast Cancer. 22:462–468.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JM, Ledermann JA and Kohn EC: PARP
Inhibitors for BRCA1/2 mutation-associated and BRCA-like
malignancies. Ann Oncol. 25:32–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomised phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wooster R, Bignell G, Lancaster J, Swift
S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C and Micklem G:
Identification of the breast cancer susceptibility gene BRCA2.
Nature. 378:789–792. 1995. View Article : Google Scholar : PubMed/NCBI
|