Introduction
Cancer of unknown primary (CUP) is a heterogeneous
group of malignancies that are defined as the presence of
metastases, without identifying a primary tumor following an
extensive evaluation of the patient (1). The identification of the primary tumor
represents a diagnostic and therapeutic challenge: the antemortem
frequency of detection of the primary site is <20–30% (2), meanwhile CUP represents between 2.3 and
4.2% of adult cancers (3). In
Mexico, 4,223 new cases of CUP were diagnosed in 2001, representing
~4% of cancer cases during that year (4). Unfortunately, the median survival rate,
even in patients treated with cytotoxic agents, was <1 year
(5). Chemotherapy has been the
cornerstone in the treatment of CUP; however, establishment of the
results has been difficult due to the heterogeneity of patients in
the series. CUP treatment must be individualized according to the
clinical setting, considering the favorable or unfavorable group
that the patient belonged to prior to the therapeutic decision.
However, the benefits of chemotherapy compared with best supportive
care in the subgroups of poor prognosis have yet to be fully
elucidated, and the optimal treatment regimen has not been
determined (6). Several chemotherapy
schemes have been successful in groups of patients with favorable
clinical characteristics. However, most patients with CUP are in
the unfavorable group, and this exhibits low rates of response to
systemic treatment, which is decided empirically according to
clinical and functional status. On the other hand, it has been
proposed that clinicopathological features, including age, gender,
functional status, weight loss, histology, tumor location, number
of metastases and the levels of tumor markers, may represent
relevant prognostic variables (7–14). These
variables have not been obtained consistently, and so larger
studies are required to validate specific clinical, pathological
and molecular profiles in order to differentiate patients that are
likely to benefit from treatment from those who would be likely to
experience only deterioration in their quality of life. There are
no well-established clinical and molecular markers for CUP, and
therefore recognition of such markers is of vital importance in
determining the best treatment option. The aim of the present study
was to determine whether clinicopathological parameters were
prognostic factors for the response to chemotherapy in patients
with CUP. Overall survival, progression-free survival and response
rates to chemotherapy were investigated in the present study.
Patients and methods
Patients
A total of 149 patients with CUP treated at the
Oncology Hospital, National Medical Center ‘Century XXI’, IMSS,
Mexico City, Mexico between January 2002 and December 2009 were
retrospectively analyzed. Patients >18 years of age diagnosed
with CUP, who were histologically confirmed and with any
histological subtype, were carefully selected. Patients previously
treated in other units, those with hematological, renal or liver
failure at the time of inclusion, or those with the presence of a
second neoplasm were excluded. The clinicopathological factors
analyzed were: Age, gender, functional status, histology, tumor
location, number of metastases, and the levels of the tumor
markers, lactate dehydrogenase (LDH) and albumin.
Statistical analysis
Overall survival (OS) was defined as the lifetime in
months from the start of treatment until the patient succumbed to
mortality. Progression-free survival (PFS) was determined from the
start of the treatment to the date on which the disease progressed,
determined clinically or by imaging, either by increasing tumor
volume or development of new lesions. Response criteria were as
follows: Complete response (CR) indicated no measurable tumor by
clinical analysis and/or by imaging; partial response (PR) referred
to a reduction of ≥30% in the largest diameter of one of the target
lesions compared with the baseline study; and stable disease (SD)
referred to a measurable reduction in tumor volume of <30% in
maximum diameter, with no appearance of new lesions. Toxicity to
treatment was determined according to the National Cancer Institute
(NCI) Common Terminology Criteria for Adverse Events (CTCAE,
https://ctep.cancer.gov). For the statistical
analysis, comparison between subgroups was performed using the
Chi-square test for quantitative variables, and Fisher's exact test
for qualitative variables. The analysis of OS and PFS was performed
using the Kaplan-Meier method with confidence intervals (CIs) of
95%. Statistical analysis was performed using SPSS software,
version 17 (SPSS, Inc., Chicago, IL, USA). For univariate analysis,
a statistical comparison of median survival with the t-test was
used, and multivariate analysis was performed using the Cox model.
Only the variables with P<0.05, on performing a univariate
analysis, were included in the present study. Proportional hazards
were analyzed using graphical and statistical methods. P<0.05
was considered to indicate a statistically significant value.
Results
Patient characteristics
A cohort of 149 patients diagnosed with CUP treated
between January 2002 and December 2009 were carefully selected for
the present study. Table I shows the
clinicopathological characteristics of the patients involved in
this study. Of the patients, 60% received only one line of
chemotherapy. The mean age was 56.9 years (range, 25–90) and the
numbers of patients according to gender (51.67% male, 48.32%
female) were similar. A total of 65.7% of subjects had ECOG-1,
whereas adenocarcinoma and squamous cell carcinoma accounted for
85.57% of the histologies. A total of 75% of the tumors had a poor
degree of differentiation tumor activity, which was confirmed in
2–3 sites in 53.69% of the cases. Molecular analysis revealed that
there was an elevation in the levels of the tumor marker, cancer
antigen 125 (CA125), in 34.22% of cases, being the most frequent
biomarker (16.77%). A significant increase in the expression of
lactate dehydrogenase (LDH) was identified in 41.6% of the
patients, and the level of albumin decreased in 12.1% of the
individuals.
 | Table I.Characteristics of patients with CUP
from January 2002 to December 2009. |
Table I.
Characteristics of patients with CUP
from January 2002 to December 2009.
| Characteristic | Number of
patients | (%) |
|---|
| Gender |
|
|
|
Male | 77 | 51.67 |
|
Female | 72 | 48.32 |
| Age (years) |
|
|
| Median
± SD | 56.94±12.69 | – |
|
Range | (25–90) |
|
| ECOG performance
status |
|
|
| 0 | 0 | 0 |
| 1 | 98 | 65.77 |
| 2 | 49 | 32.88 |
| 3 | 2 | 1.34 |
| Histology |
|
|
|
Squamous cell carcinoma | 18 | 12.08 |
|
Adenocarcinoma | 72 | 48.32 |
|
Neuroendocrine tumor | 2 | 1.34 |
|
Carcinoma | 57 | 38.25 |
| Differentiation
grade |
|
|
| Well
differentiated | 4 | 2.68 |
|
Moderately differentiated | 34 | 22.81 |
| Poorly
differentiated | 111 | 74.49 |
| Number sites of
disease |
|
|
| 1 | 49 | 0.67 |
|
2–3 | 80 | 53.69 |
|
>3 | 20 | 13.42 |
| Elevated tumor
marker | 51 | 34.22 |
|
CEA | 23 | 15.43 |
|
AFP | 4 | 2.68 |
|
bHGC | 0 | 0 |
|
PSA | 2 | 1.34 |
|
CA125 | 25 | 16.77 |
|
CA19–9 | 6 | 4.02 |
| LDH (>340
IU/l) | 62 | 41.60 |
| Albumin <3.4
g/dl | 18 | 12.10 |
| Number of
chemotherapy |
|
|
| schemes |
|
|
| 1 | 90 | 60.40 |
| 2 | 42 | 28.18 |
| 3 | 13 | 8.72 |
|
>3 | 4 | 2.68 |
Location of metastases
Table II describes
the location of the various sites of metastases in patients. The
most frequently observed locations were the liver (33.5% of
patients), neck (30.2%), lung (24.8%), supraclavicular (18.1%),
bone (16.7%), axillar (15.4%), peritoneum (14.0%), mediastinum and
retroperitoneum (13.4%). Other less frequent locations (<10%)
were localized in the pleura, skin, groin, pelvis, central nervous
system, small intestine, colon, pancreas, parotid, pericardium and
adrenal. Tumor activity was reported in the spleen, stomach, breast
and bone marrow in 1% of the patients.
 | Table II.Location of tumor activity in
patients with CUP (n=149). |
Table II.
Location of tumor activity in
patients with CUP (n=149).
| Site of
location | Number of
cases | (%) |
|---|
| Liver | 50 | 33.5 |
| Cervical | 45 | 30.2 |
| Lung | 37 | 24.8 |
|
Supraclavicular | 27 | 18.1 |
| Bone | 25 | 16.7 |
| Axilla | 23 | 15.4 |
| Peritoneum | 21 | 14.0 |
| Mediastinum | 20 | 13.4 |
|
Retroperitoneum | 20 | 13.4 |
| Pleura | 11 |
7.3 |
| Skin | 9 |
6.0 |
| Groin | 8 |
5.3 |
| Pelvis | 6 |
4.0 |
| Central nervous
system | 5 |
3.3 |
| Small
intestine | 3 |
2.0 |
| Colon | 2 |
1.3 |
| Pancreas | 2 |
1.3 |
| Parotid | 2 |
1.3 |
| Pericardium | 2 |
1.3 |
| Adrenal | 2 |
1.3 |
| Spleen | 1 | 0.67 |
| Gastric | 1 | 0.67 |
| Breast | 1 | 0.67 |
| Bone marrow | 1 | 0.67 |
Response rates
A total of 45 patients (30.2%) demonstrated a
response to chemotherapy, of whom 12 patients (8.1%) presented with
CR, and 33 patients (22.1%) exhibited PR. SD was observed in 17
patients (11.4%). Eighty-three patients (55.7%) progressed during
treatment, and 4 patients (2.7%) did not exhibit any response. A
total of 21 cases of mortality (14.1%) were associated with a
diagnostic confirmatory note in the record (Table III). Notably, univariate analysis
showed that ECOG (P=0.004), elevated levels of LDH (P=0.03) and
histology (P=0.031) were prognostic factors for the response to
chemotherapy (Table IV).
Subsequently, a multivariate analysis of prognostic factors of the
response to chemotherapy was performed using a logistic regression
model. Notably, the results demonstrated that the ECOG was
significantly associated (P=0.008) with the chemotherapy response
(Table V).
 | Table III.Response rates to chemotherapy of
patients with CUP (n=149). |
Table III.
Response rates to chemotherapy of
patients with CUP (n=149).
| Type of
response | Number | (%) |
|---|
| Response |
|
|
|
Complete | 12 | 8.1 |
|
Parcial | 33 | 22.1 |
|
Global | 45 | 30.2 |
| Progression | 83 | 55.7 |
| No response | 4 | 2.7 |
| Stable disease | 17 | 11.4 |
| Mortalities | 21 | 14.1 |
 | Table IV.Univariate analysis of prognostic
factors of response to chemotherapy. |
Table IV.
Univariate analysis of prognostic
factors of response to chemotherapy.
| Variable | CR (%) n=12 | PR (%) n=33 | P-value |
|---|
| Gender |
|
| 0.75 |
|
Male | 5 (41.7) | 18 (54.5) |
|
|
Female | 7 (58.3) | 15 (45.5) |
|
| ECOG |
|
| 0.004 |
| 1 | 11 (91.7) | 26 (78.8) |
|
| 2 | 1 (8.3) | 7 (21.2) |
|
| 3 | 0 (0) | 0 (0) |
|
| Histology |
|
| 0.031 |
|
NET | 0 (0) | 0 (0) |
|
|
Squamous cell carcinoma | 3 (25.0) | 7 (21.2) |
|
|
Carcinoma | 4 (33.3) | 10 (30.3) |
|
|
Adenocarcinoma | 5 (41.7) | 16 (48.5) |
|
| Differentiation
grade |
|
| 0.46 |
| Well
differentiated | 0 (0) | 1 (3.0) |
|
|
Moderately differentiated | 4 (33.3) | 11 (33.3) |
|
| Poorly
differentiated | 8 (66.7) | 21 (63.7) |
|
| Tumor marker |
|
| 0.33 |
|
Normal | 10 (83.3) | 23 (69.7) |
|
|
Elevated | 2 (16.7) | 10 (30.3) |
|
| LDH |
|
| 0.03 |
|
Normal | 11 (91.7) | 18 (54.5) |
|
|
Elevated >340 IU/l | 1 (8.3) | 15 (45.5) |
|
| Albumin |
|
| 0.43 |
|
Normal | 12 (100) | 29 (87.8) |
|
|
Decreased (<3.4 g/dl) | 0 (0) | 4 (12.2) |
|
 | Table V.Multivariate logistic regression
analysis of prognostic factors of response to chemotherapy. |
Table V.
Multivariate logistic regression
analysis of prognostic factors of response to chemotherapy.
| Variable | β-value | OR | CI (95%) | P-value |
|---|
| ECOG | −1.13 | 0.42 | 0.13- 0.74 | 0.008 |
Survival analysis
The PFS was 7.1±9.9 months (range, 1–57 months)
(Table VI). Survival curves for PFS
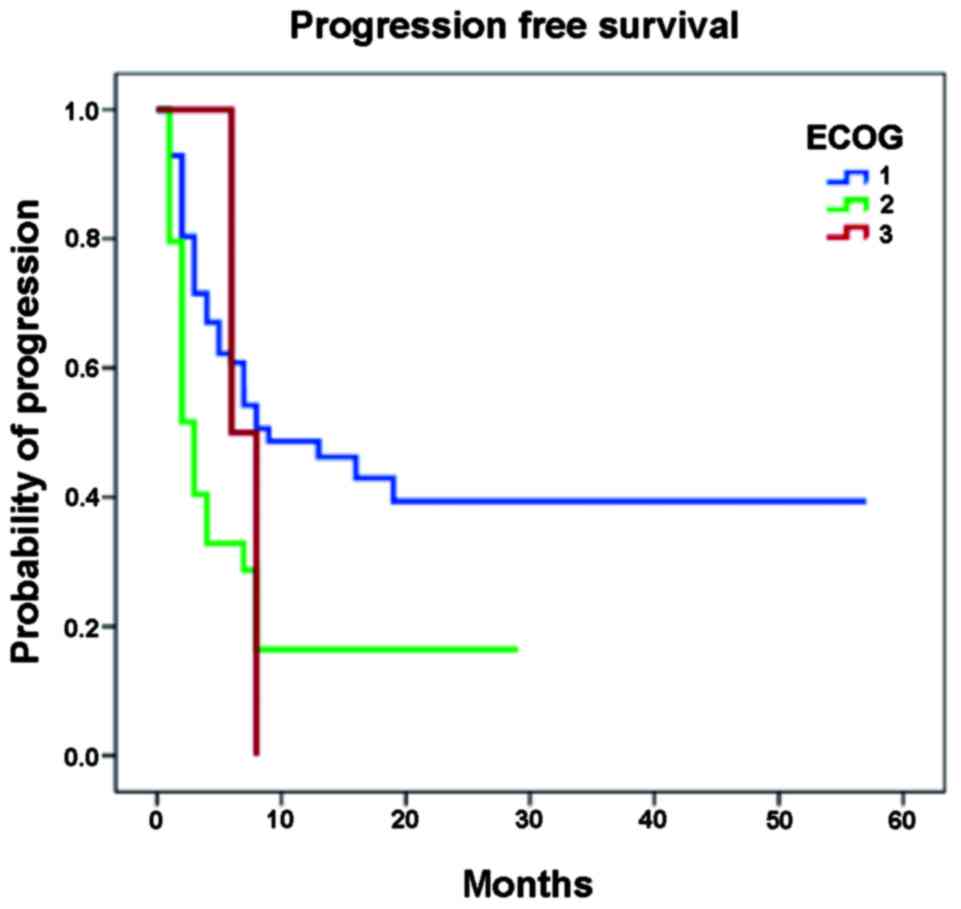
derived using the Kaplan-Meier method are shown in Fig. 1. Notably, in the univariate analysis,
the ECOG and LDH had high statistical significance, as predictive
of PFS (Table VII). On performing
a multivariate logistic regression, only ECOG was observed as an
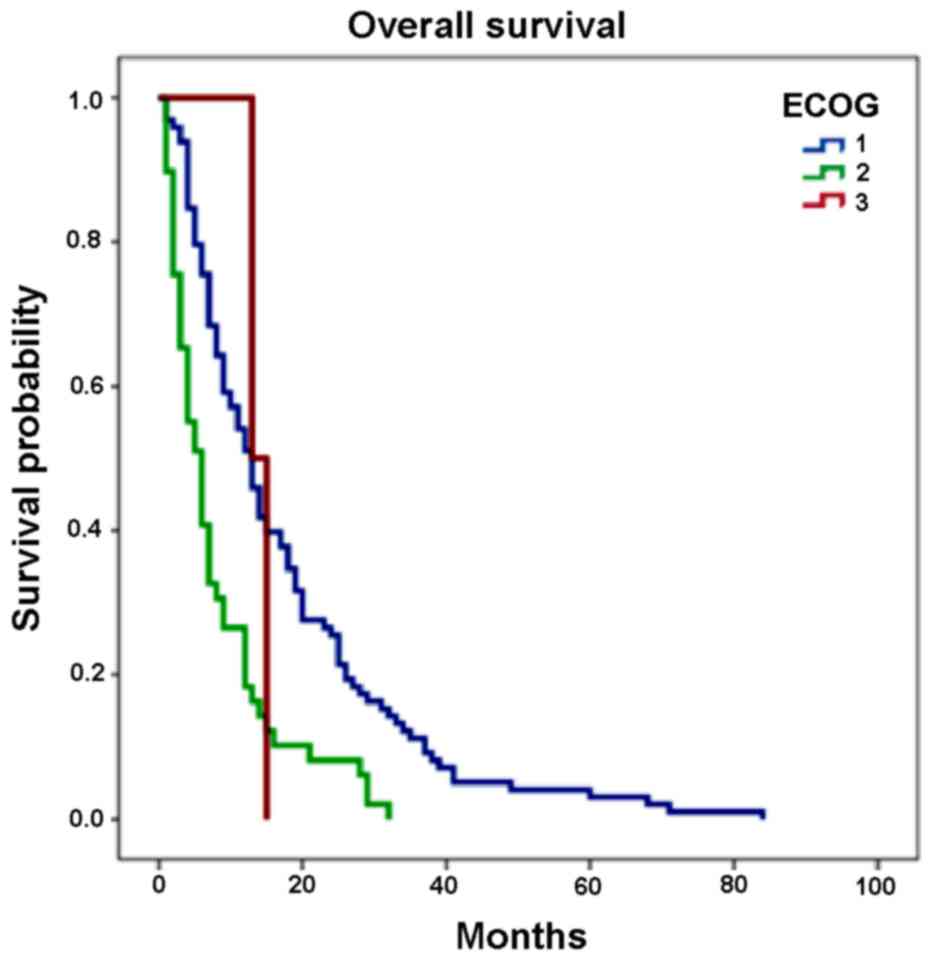
independent factor of progression (P<0.0001; Table VIII). In addition, OS was
14.2±14.1 months (range, 1–84 months) as shown in Table IV. Survival curves derived using the
Kaplan-Meier method for OS are shown in Fig. 2. The only independent predictor of
mortality was the ECOG (P<0.0001); additional analysis revealed
that there were no other clinical and pathological factors
predictive of mortality.
 | Table VI.Global survival and progression-free
survival in patients with no known primary tumor (n=149). |
Table VI.
Global survival and progression-free
survival in patients with no known primary tumor (n=149).
| Survival | Months | ± SD |
|---|
| Overall
survival |
|
Median | 14.2 | 14.1 |
|
Range | 1–84 |
|
| Progression free
survival |
| 9.09 |
|
Median | 7.1 |
|
|
Range | 1–57 |
|
| ECOG |
| 1 | 25.9 (CI 95%,
19.5–32.4) |
|
| 2 | 7.4 (CI 95%,
4.1–10.7) |
|
| 3 | 7.0 (CI 95%,
5.0–8.9) |
|
 | Table VII.Univariate analysis of prognostic
factors of progression to chemotherapy. |
Table VII.
Univariate analysis of prognostic
factors of progression to chemotherapy.
| Variable | Progression (%)
n=83 | RR | CI, 95% | P-value |
|---|
| Gender |
|
|
| 0.44 |
|
Male | 41 (49.4) |
|
|
|
|
Female | 42 (50.6) | 0.95 | 0.71–1.27 |
|
| ECOG |
|
|
|
|
| 1 | 46 (55.4) |
|
|
|
| 2 | 35 (42.2) | 0.65 | 0.49–0.86 | 0.004 |
| 3 | 2 (2.4) | 0.71 | 0.59–0.85 | 0.002 |
| Histology |
|
|
| 0.61 |
|
NET | 0 (0) |
|
|
|
|
Squamous cell carcinoma | 8 (9.7) |
|
|
|
|
Carcinoma | 33 (39.7) |
|
|
|
|
Adenocarcinoma | 42 (50.6) |
|
|
|
| Differentiation
grade |
|
|
| 0.15 |
| Well
differentiated | 2 (2.5) |
|
|
|
|
Moderately differentiated | 15 (18.0) |
|
|
|
| Poorly
differentiated | 66 (79.5) |
|
|
|
| Number of sites of
disease |
|
|
| 0.29 |
| 1 | 24 (28.9) |
|
|
|
|
2–3 | 47 (56.7) |
|
|
|
|
>3 | 12 (14.4) |
|
|
|
| Location of
disease |
|
|
| 0.122 |
|
Peritoneum | 8 (9.6) | 0.67 | 0.47- 0.95 | 0.1 |
| Lung,
pleura | 14 (16.9) | 0.80 | 0.57–1.13 | 0.27 |
|
Cervical | 19 (22.9) | 0.88 | 0.63–1.22 | 0.48 |
| Axilla,
SCV | 8 (9.6) | 0.97 | 0.60–1.56 | 0.90 |
|
Liver | 21 (25.3) | 1.13 | 0.81–1.56 | 0.43 |
|
Bone | 5 (5.8) | 1.42 | 0.84–2.40 | 0.13 |
|
Mediastinum | 4 (4.8) | 2.26 | 0.41–12.4 | 0.21 |
|
Retroperitoneum | 4 (5.1) | 2.31 | 0.52–12.7 | 0.23 |
| Tumor marker |
|
|
| 0.52 |
|
Normal | 49 (59.0) |
|
|
|
|
Elevated | 34 (41.0) | 0.75 | 0.56–0.99 |
|
| LDH |
|
|
| 0.031 |
|
Normal | 42 (50.6) |
|
|
|
|
Elevated (>340 IU/l) | 41 (49.4) | 0.73 | 0.55–0.96 |
|
| Albumin |
|
|
| 0.13 |
|
Normal | 70 (84.3) |
|
|
|
|
Decreased (<3.4 g/dl) | 13 (15.7) | 0.74 | 0.53–1.02 |
|
 | Table VIII.Multivariate logistic regression
analysis of prognostic factors of progression. |
Table VIII.
Multivariate logistic regression
analysis of prognostic factors of progression.
| Variable | β-value | OR | CI, 95% | P-value |
|---|
| ECOG | −1.226 | 0.37 | 1.4–6.08 | <0.0001 |
Toxicity evaluation
Hematological toxicity, including anemia,
thrombocytopenia, leukopenia and neutropenia of any grade, occurred
in 43.6% of patients; grade 3 to 4 was observed in 21.5% of the
patients. Gastrointestinal toxicity (nausea, vomiting, mucositis,
diarrhea, constipation, anorexia) in any degree was observed in
67.8% of the patients, documented at grades 3 to 4 in 20.2% of
cases. Dermatological toxicity was reported in 53.02% of the
patients, with alopecia being the most common cause (48.32%), and
one case (0.7%) documented severe dermatological toxicity secondary
to hand-foot syndrome. In addition, 16.8% of the patients reported
neurological toxicity (sensory neuropathy and/or motor), and 5
cases (3.4%) reached grade 3 to 4. Almost two-thirds of the
patients (64.4%) expressed a specific degree of constitutional
symptoms, and 12.7% of the cases exhibited severely limiting or
incapacitating conditions.
Discussion
The present study is a retrospective analysis of 7
years' experience in the treatment of patients with CUP in our
institution. The primary endpoint was to determine
clinicopathological factors that may confer lower response rates
and decreased survival rates in patients with CUP, in order to
establish subgroups of high and low risk, and identify those in
whom chemotherapy did not yield any clinical benefits, but only
toxic effects. In the present analysis, objective response rates to
treatment were 30.2%, which was similar to those observed in the
literature with platinum schemes (15–22). In
schemes based on platinum and taxane, the response rates were
30–50% (23–34), and reported response rates were
>50% (up to 79%) in Phase II trials, which included a
considerable number of patients at low risk (35–40), who
were present in the minority in the present study. Of the study
subjects, >85% received treatment regimens based on platinum. It
is important to note that, prior to 2004, the use of taxanes was
not common, and several of the schemes that were in use prior to
this date are now useless. The median OS was 14.2 months, whereas
the PFS was 7.1 months, also consistent with the trials.
Approximately 40% of the patients received more than one line of
treatment. At present, there is no set pattern of second-line
chemotherapy in CUP. The use of multiple lines of treatment is
subject to an appropriate assessment being made of the patient, and
its recommendation is questionable; therefore, it was reserved for
patients who had a good response rate with a previous scheme, and
who were of excellent functional status. The weighting of
risk-benefit and economic impact were not objectives of the present
study. Regarding the clinicopathological factors of poor response
to treatment, age, gender, ECOG, histology, grade of
differentiation, number and location of metastases, elevation of
tumor markers, elevated LDH and decreased albumin were analyzed.
The results demonstrated that, in univariate analysis of response
to treatment, the significant factors were ECOG-1, normal LDH and
adenocarcinoma histology for a greater response to treatment;
however, when performing multivariate logistic regression analysis,
only ECOG proved to be an independent predictor of the response to
treatment. Similarly, when analyzing the prognostic factors for OS
and PFS, the ECOG was the only independent factor for these two
characteristics. The other variables analyzed did not reach a
statistically significant P-value. It should be noted that,
according to the multivariate logistic regression analysis, the
level of LDH was identified at the limit of statistical
significance (P=0.054), and this may be due to the fact that
patients were not stratified according to elevated levels of this
protein. The ECOG as a predictor of a poor response to cytotoxic
therapy in patients with CUP has been referred to in numerous
studies that had similar aims (8,10,12,22,41–44).
Several studies have identified prognostic factors
associated with survival in patients with unknown primary cancer.
However, there is, thus far, a solid classification system in place
that enables the stratification of patients according to these
characteristics in risk groups, since the groups of patients
studied tend to be heterogeneous, and therefore the factors
mentioned are inconsistent. The present study has revealed specific
aspects of heterogeneity of the patients, including multiple
histologies, and the grade of differentiation and application of
various treatments. Adenocarcinoma and squamous cell histologies
yielded higher rates of CR and PR for adenocarcinoma (41.7 and
48.5%, respectively) compared with a CR of 33.3% and a PR of 30.3%
for squamous cell carcinoma. Similarly, a poor differentiation
grade represented >60% of cases of objective responses to
treatment. However, on performing the univariate analysis, none of
these variables were revealed to be statistically significant. The
data collection in retrospective studies, such as the present
example, has a number of disadvantages: Usually, there is bias in
the catch; there are not properly specified degrees of toxicity in
all cases; and there is the possibility of errors emerging as a
consequence of subjective assessment.
It would be imperative in subsequent prospective
analyses to reduce the heterogeneity of the study population,
excluding patients from well-defined subgroups with good prognosis
with specific treatment indications, and yet without losing sight
of those patients from established groups of potentially curable
disease or under good control, as lymphomas, germ cell tumors,
breast cancer or neuroendocrine tumors (45). At present, there are no Phase III
studies comparing systemic treatment with best supportive care in
patients with unfavorable risk factors. Prospective clinical trials
are required to establish the optimal treatment for each patient,
and to clearly define the group of patients who will benefit from
cytotoxic treatment.
Treatment of patients with CUP remains a challenge
for oncology, and requires a multidisciplinary approach. The
objective should be focused on preventing the requirement for
empirical management, in this context, with the advent of molecular
and genetic profiles that are currently under study for this
complex neoplasm (46–51) and the development of therapeutics
based on a combination of molecular biology, microarray and
immunohistochemistry approaches, and therefore clinical and
pathological factors will have an essential role in the management
of these patients.
Taken together, the ECOG performance status is an
independent predictor of poor response to chemotherapy, and lower
OS and PFS in patients with CUP.
Acknowledgements
The authors acknowledge the National Medical Center
‘Century XXI’, IMSS, México for their support.
References
|
1
|
Pavlidis N and Fizazi K: Carcinoma of
unknown primary (CUP). Crit Rev Oncol Hematol. 69:271–278. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlidis N, Briasoulis E, Hainsworth J and
Greco F: Diagnostic and therapeutic management of cancer of an
unknown primary. Eur J Cancer. 39:1990–2005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krementz ET, Cerise EJ, Foster DS and
Morgan L Jr: Metastases of undetermined source. Curr Pobl Cancer.
4:4–37. 1979.
|
|
4
|
Bosetti C, Rodríguez T, Chatenoud L,
Bertuccio P, Levi F, Negri E and La Vecchia C: Trends in cancer
mortality in Mexico, 1981–2007. Eur J Cancer Prev. 20:355–363.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pentheroukadis G, Briasoulis E and
Pavlidis N: Cancer of unknown primary site: Missing primary or
missing biology? Oncologist. 12:418–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sporn JR and Greenberg BR: Empirical
chemotherapy for adenocarcinoma of unknown primary site. Semin
Oncol. 20:261–267. 1993.PubMed/NCBI
|
|
7
|
Culine S: Prognostic factors in unknown
primary cancer. Semin Oncol. 36:60–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbruzzese JL, Abbruzzese MC, Hess KR,
Raber MN, Lenzi R and Frost P: Unknown primary carcinoma: Natural
history and prognostic factors in 657 consecutive patients. J Clin
Oncol. 12:1272–1280. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hess KR, Abbruzzese MC, Lenzi R, Raber MN
and Abbruzzese JL: Classification and regression tree analysis of
1,000 consecutive patients with unknown primary carcinoma. Clin
Cancer Res. 5:3403–3410. 1999.PubMed/NCBI
|
|
10
|
Lortholary A, Abadie-Lacourtoisie S,
Guérin O, Mege M, Rauglaudre GD and Gamelin E: Cancers of unknown
origin: 311 cases. Bull Cancer. 88:619–627. 2001.(In French).
PubMed/NCBI
|
|
11
|
Culine S, Kramar A, Saghatchian M, Bugat
R, Lesimple T, Lortholary A, Merrouche Y, Laplanche A and Fizazi K:
French Study Group on Carcinomas of Unknown Primary: Development
and validation of a prognostic model to predict the length of
survival in patients with carcinomas of an unknown primary site. J
Clin Oncol. 20:4679–4683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seve P, Ray-Coquard I, Trillet-Lenoir V,
Sawyer M, Hanson J, Broussolle C, Negrier S, Dumontet C and Mackey
JR: Low serum albumin levels and liver metastases are powerful
prognostic markers for survival in patients with carcinomas of
unknown primary site. Cancer. 107:2698–2705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lenzi R, Abbruzzese J, Amato R, et al:
Cisplatin, 5-fluorouracil and follinic acid for the treatment of
carcinoma of unknown primary: A phase II study. Proc Am Soc Clin
Oncol. 10:3011991.
|
|
14
|
Farrugia DC, Norman AR, Nicolson MC, Gore
M, Bolodeoku ED, Webb A and Cunningham D: Unknown primary
carcinoma: Randomized studies are needed to identify optimal
treatments and their benefits. Eur J Cancer. 32A:2256–2261. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rigg A, Cunningham D, Gore M, Hill M,
O'Brien M, Nicolson M, Chang J, Watson M, Norman A, Hill A, Oates
J, et al: A phase I/II study of leucovorin, carboplatin and
5-fluorouracil (LCF) in patients with carcinoma of unknown primary
site or advanced oesophagogastric/pancreatic adenocarcinomas. Br J
Cancer. 75:101–105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pavlidis N, Kosmidis P, Skarlos D,
Brissoulis E, Beer M, Theoharis D, Bafaloukos D, Maraveyas A and
Fountzilas G: Subsets of tumors responsive to cisplatin or
carboplatin combinatios in patients with carcinoma of unknown
primary site. A hellenic cooperative oncology group study. Ann
Oncol. 3:631–634. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Briasoulis E, Tsavaris N, Fountzilas G,
Athanasiadis A, Kosmidis P, Bafaloukos D, Skarlos D, Samantas E and
Pavlidis N: Combination regimen with carboplatin, epirubicin and
etoposide in metastatic carcinomas of unknown primary site: A
Hellenic Co-oncology group phase II study. Oncology. 55:426–430.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warner E, Goel R, Chang J, Chow W, Verma
S, Dancey J, Franssen E, Dulude H, Girouard M, Correia J and
Gallant G: A mulicenter phase II study of carboplatin and prolonged
oral etoposide in the treatment of cancer of unknown primary site
(CUPS). Br J Cancer. 77:2376–2380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voog E, Merrouche Y, Trillet-Lenoir V,
Lasset C, Peaud PY, Rebattu P and Negrier S: Multicentric phase II
study of cisplatin and etoposide in patients with metastatic
carcinoma of unknown primary. Am J Clin Oncol. 23:614–616. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Macdonald AG, Nicolson MC, Samuel LM,
Hutcheon AW and Ahmed FY: A phase II study of mitomycin C,
cisplatin and continuous infusion 5-fluorouracil (MCF) in the
treatment of patients with carcinoma of unknown primary site. Br J
Cancer. 86:1238–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piga A, Gesuita R, Catalano V, Nortilli R,
Getto G, Cardillo F, Giorgi F, Riva N, Porfiri E, Montironi R, et
al: Identification of clinical prognostic factors in patients with
unknown primary tumors treated with a platinum-based combination.
Oncology. 69:135–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pittman KB, Olver IN, Koczwara B, Kotasek
D, Patterson WK, Keefe DM, Karapetis CS, Parnis FX, Moldovan S,
Yeend SJ, et al: Gemcitabine and carboplatin in carcinoma of
unknown primary site: A phase II Adelaide cancer trials and
education collaborative study. Br J Cancer. 95:1309–1313. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hainsworth JD, Erland JB, Kalman LA,
Schreeder MT and Greco FA: Carcinoma of unknown primary site:
Treatment with 1-h paclitaxel, carboplatin, and extended-schedule
etoposide. J Clin Oncol. 15:2385–2393. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Briasoulis E, Kalofonos H, Bafaloukos D,
Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C,
Kosmidis P and Pavlidis N: Carboplatin plus paclitaxel in unknown
primary carcinoma: A phase II study. The Hellenic cooperative
oncology group study. J Clin Oncol. 18:3101–3117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greco FA, Burris HA III, Erland JB, Gray
JR, Kalman LA, Schreeder MT and Hainsworth JD: Carcinoma of unknown
primary site. Cancer. 89:2655–2660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouleuc C, Saghatchian M, Di Tullio L,
Louvet CH, Levy E, Di Palma M, et al: A multicenter phase II study
of docetaxel and cisplatin in the treatment of cancer of unknown
primary site. Proc Am Soc Clin Oncol. 137b:22982001.
|
|
27
|
Darby A, Richardson L, Nokes L, Harvey M,
Hassan A and Iveson T: Phase II study of single agent docetaxel in
carcinoma of unknown primary site. Proc Am Soc Clin Oncol.
100b:21512001.
|
|
28
|
Gothelf A, Daugaard G and Nelausen K:
Paclitaxel, cisplatin and gemcitabine in the treatment of unknown
primary tumours, a phase II study. ESMO. 25:882002.
|
|
29
|
Greco FA, Burris HA III, Litchy S, Barton
JH, Bradof JE, Richards P, Scullin DC Jr, Erland JB, Morrissey LH
and Hainsworth JD: Gemcitabine, carboplatin, and paclitaxel for
patients with carcinoma of unknown primary site: A Minnie pearl
cancer research network study. J Clin Oncol. 20:1651–1656. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greco FA, Rodriguez GI, Shaffer DW,
Hermann R, Litchy S, Yardley DA, Burris HA III, Morrissey LH,
Erland JB and Hainsworth JD: Carcinoma of unknown primary site:
Sequential treatment with paclitaxel/carboplatin/etoposide and
gemcitabine/irinotecan: A Minnie pearl cancer research network
phase II trial. Oncologist. 9:644–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park YH, Ryoo BY, Choi SJ, Yang SH and Kim
HT: A phase II study of paclitaxel plus cisplatin chemotherapy in
an unfavourable group of patients with cancer of unknown primary
site. Jpn J Clin Oncol. 34:681–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pouessel D, Culine S, Becht C, Ychou M,
Romieu G, Fabbro M, Cupissol D and Pinguet F: Gemcitabine and
docetaxel as front-line chemotherapy in patients with carcinoma of
an unknown primary site. Cancer. 100:1257–1261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Rayes BF, Shields AF, Zalupski M,
Heilbrum LK, Jain V, Terry D, Ferris AM and Philip PA: A phase II
study of carboplatin and paclitaxel in adenocarcinoma of unknown
primary. Am J Clin Oncol. 28:152–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hainsworth JD, Spigel DR, Litchy S and
Greco FA: Phase II trial of paclitaxel, carboplatin, and etoposide
in advanced poorly differentiated neuroendocrine carcinoma: A
Minnie pearl cancer research network study. J Clin Oncol.
24:3548–3554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Gaast A, Verweij J, Henzen-Logmans
SC, Rodenburg CJ and Stoter G: Carcinoma of unknown primary:
Identification of a treatable subset? Ann Oncol. 1:119–122. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hainsworth JD, Johnson DH and Greco FA:
The role of etoposide in the treatment of poorly differenciated
carcinoma of unknown primary site. Cancer. 67:(Suppl 1). S310–S314.
1991. View Article : Google Scholar
|
|
37
|
Khansur T, Allred C, Little D and Anand V:
Cisplatin and 5-fluorouracil for metastatic squamous cell carcinoma
from unknown primary. Cancer Invest. 13:263–266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greco FA, Vaughn WK and Hainsworth JD:
Advanced poorly differenciated carcinoma of unknown primary site:
Recognition of a treatable syndrome. Ann Intern Med. 104:547–553.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Falkson CI and Cohen GL: Mitomycin,
epirubicin and cisplatin versus mitomycin-C alone as therapy for
carcinoma ok unknown primary origin. Oncology. 55:116–121. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guardiola E, Pivot X, Tchicknavorian X,
Magne N, Otto J, Thyss A and Schneider M: Combination of
cisplatin-doxorubicin-cyclophosphamide in adenocarcinoma of unknown
primary site: A phase II trial. Am J Clin Oncol. 24:372–375. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van der Gaast A, Verweij J, Planting AS,
Hop WC and Stoter G: Simple prognostic model to predict survival in
patients with undifferentiated carcinoma of unknown primary site. J
Clin Oncol. 13:1720–1725. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kambhu SA, Kelsen DP, Fiore J, Niedzwiecki
D, Chapman D, Vinciguerra V and Rosenbluth R: Metastatic
adenocarcinoma of unknown primary site. Prognostic variables and
treatment results. Am J Clin Oncol. 13:55–60. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seve P, Sawyer M, Hanson J, Broussolle C,
Dumontet C and Mackey JR: The influence of comorbidities, age, and
performance status on the prognosis and treatment of patients with
metastatic carcinoma of unknown primary site: A population-based
study. Cancer. 106:2058–2066. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pasterz R, Savaraj N and Burgess M:
Prognostic factors in metastatic carcinoma of unknown primary. J
Clin Oncol. 4:1652–1657. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lenzi R, Hess KR, Abbruzzese MC, Raber MN,
Ordoñez NG and Abbruzzese JL: Poorly differenciated carcinoma and
poorly differenciated adenocarcinoma of unknown origin: Favorable
subsets of patients with unknown-primary carcinoma? J Clin Oncol.
15:2056–2066. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Varadhachary GR, Talantov D, Raber MN,
Meng C, Hess KR, Jatkoe T, Lenzi R, Spigel DR, Wang Y, Greco FA, et
al: Molecular profiling of carcinoma of unknown primary and
correlation with clinical evaluation. J Clin Oncol. 26:4442–4448.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pentheroukadis G, Golfinopoulos V and
Pavlidis N: Swithing benchmarks in cancer of unknown primary: From
autopsy to microarray. Eur J Cancer. 43:2026–2036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fizazi K: Treatment of patients with
specific subsets of carcinoma of an unknown primary site. Ann
Oncol. 17:(Suppl 10). x177–x180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Varadhachary GR and Greco FA: Overview of
patient management and future directions in unknown primary
carcinoma. Semin Oncol. 36:75–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Viale G and Mastropasqua MG: Diagnostic
and therapeutic management of carcinoma of unknown primary:
Histopathological and molecular diagnosis. Ann Oncol. 17:(Suppl
10). x163–x167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van de Wouw AJ, Jansen RL, Speel EJ and
Hillen HF: The unknown biology of the unknown primary tumour: A
literature review. Ann Oncol. 14:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|