Introduction
Much of the morbidity in elderly men involves organs
of the pelvic region. These include lower urinary tract symptoms,
incontinence and erectile dysfunction. The functions of these
organs are regulated by the regional autonomic ganglia, primarily
the pelvic ganglia. The pathological changes that produce autonomic
dysfunction in human aging are largely unknown; however, in
experimental animal models specific pathological changes have been
identified in selected sympathetic ganglia (1) Alterations in the pelvic ganglia and
their function may also be modified by nutritional and
environmental factors (2).
Sympathetic ganglia are infrequently biopsied or
removed surgically, so the majority of the human studies have been
performed using autopsy material (3,4).
Therefore, these studies have unavoidable histological artifacts
and inherent difficulty in retrospectively establishing the
normality of sympathetic ganglions cells. In order to avoid these
difficulties, the present study examined pelvic sympathetic ganglia
derived from surgical pathology. Tissue obtained from total
prostatectomy and radical cystectomy and prostatectomy were handled
immediately following excision in order to avoid the artifacts of
autolysis found in post mortem material. The aim of the present
study was to examine the histopathological changes in the pelvic
ganglia, in the region surrounding the prostate, in surgical
specimens in benign and malignant disease.
Materials and methods
Patients
Two groups of patients were selected for this study,
and each group underwent prostatectomy in the Urology Department
(Hsharon Hospital, Petach Tikva, Israel) between the March 1993 and
November 2000. The patients received either radical prostatectomy
due to carcinoma of the prostate (group A) or radical cystectomy
and prostatectomy, due to urothelial carcinoma of the urinary
bladder (group B).
Group A
Group A consisted of 26 cases of prostatectomy
performed due to prostatic carcinoma. The age of the patients
ranged between 54–74 years (mean, 62.7). Eight patients were known
to be smokers and one was a heavy alcohol user. In total, 16
patients (51.6%) suffered from hypertension, which was controlled
by therapy. Five patients (16%) had ischemic heart disease and 3
(9.7%) had a history of stroke. The follow up period ranged between
6 months and 216 months (mean, 156 months).
Group B
Group B consisted of 10 cases of radical cystectomy
due to urothelial carcinoma of the bladder. In all the cases there
was no invasion of the urothelial carcinoma into the prostate. The
histopathological examination of all prostate tissue in this group
revealed benign prostatic hyperplasia (BPH). The age of the
patients ranged between 56–88 years (mean, 69.3 years). Four
(28.5%) patients suffered from ischemic heart disease, 6 (42.8) had
controlled hypertension, 7 (50%) were known to be smokers. 1 (7%)
had colon cancer and 1 (7%) suffered from lung cancer and underwent
irradiation therapy. The follow-up period ranged between 11 and 144
months (mean, 69.2 months).
Pathological examination
All specimens were sent to the pathology department
as fresh tissues. The external surface was stained with Indian ink
to mark the surgical margins. Total sampling of the prostates was
performed (5) by sectioning the
prostate tissues (thickness, 2–3 mm), which were formalin-fixed,
paraffin-embedded and stained with hematoxylin and eosin (H&E).
Slices of the posterior areas of the prostate were examined, the
sympathetic ganglia were identified and counted, and the
histopathological changes were determined.
Statistical analysis
Data were analyzed using two-way analysis of
variance. The group of prostates with adenocarcinoma compared with
those with BPH as the first between subjects factor and age as the
second factor. In order to further explore the main effects, a post
hoc Tukey test was performed when comparing age or Student t-test
when comparing between the groups A and B. To examine the
association between variables, Pearson's correlation analysis was
conducted. In order to overcome small sample bias, bootstrapping
analysis was performed. All tests were calculated as two-tailed
with SPSS 21.0 (IBM SPSS, Armonk, NY, USA). Results are presented
as mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
Group A, adenocarcinoma of the
prostate
All patients suffered Gleason's grade 6 primary
adenocarcinoma of the prostate (6).
The percentage of involvement of the prostate tissue by carcinoma
ranged between 10 and 75% (mean, 34%). In four (12.9%) patients,
the surgical margins were involved, while in the rest the margins
were free of tumor. In one (3.2%) of these tissue samples,
peri-neural invasion was present. Lymphatic invasion was identified
in one (3.2%) patient, and three patients (9.7%) had lymphatic and
vascular invasion of the tumor. In total, 3 patients (9.7%) were in
stage T1B, 4 patients (13%) were at stage T2A, 18 patients (58%)
were stage T2B, 2 patients (6.4%) were stage T3A, 3 patients (9.7%)
were stage T3C and one patient (3.2%) was stage T4A (7). PSA levels in the blood prior to surgery
ranged between 5.3 and 44 ng/ml (mean, 11.35 ng/ml). In the
majority of laboratories a PSA serum level of 4 ng/ml is reported
as the cut-off between normal and abnormal (6). PSA levels in the blood following
surgery ranged between 0 and 4.2 ng/ml (mean, 0.23 ng/ml). Two
(6.4%) of these patients suffered from local recurrence of the
tumors and one (3.2%) developed distant metastasis to the bone. At
the end of this study 12 patients (38.7%) had succumbed to the
disease.
Group A histopathological changes
In group A 157 ganglions were identified, of which
91 were found in the right posterior region, 6 in the mid posterior
region and 60 in the left posterior region with a total count of
3,835 ganglion cells (Table I). On
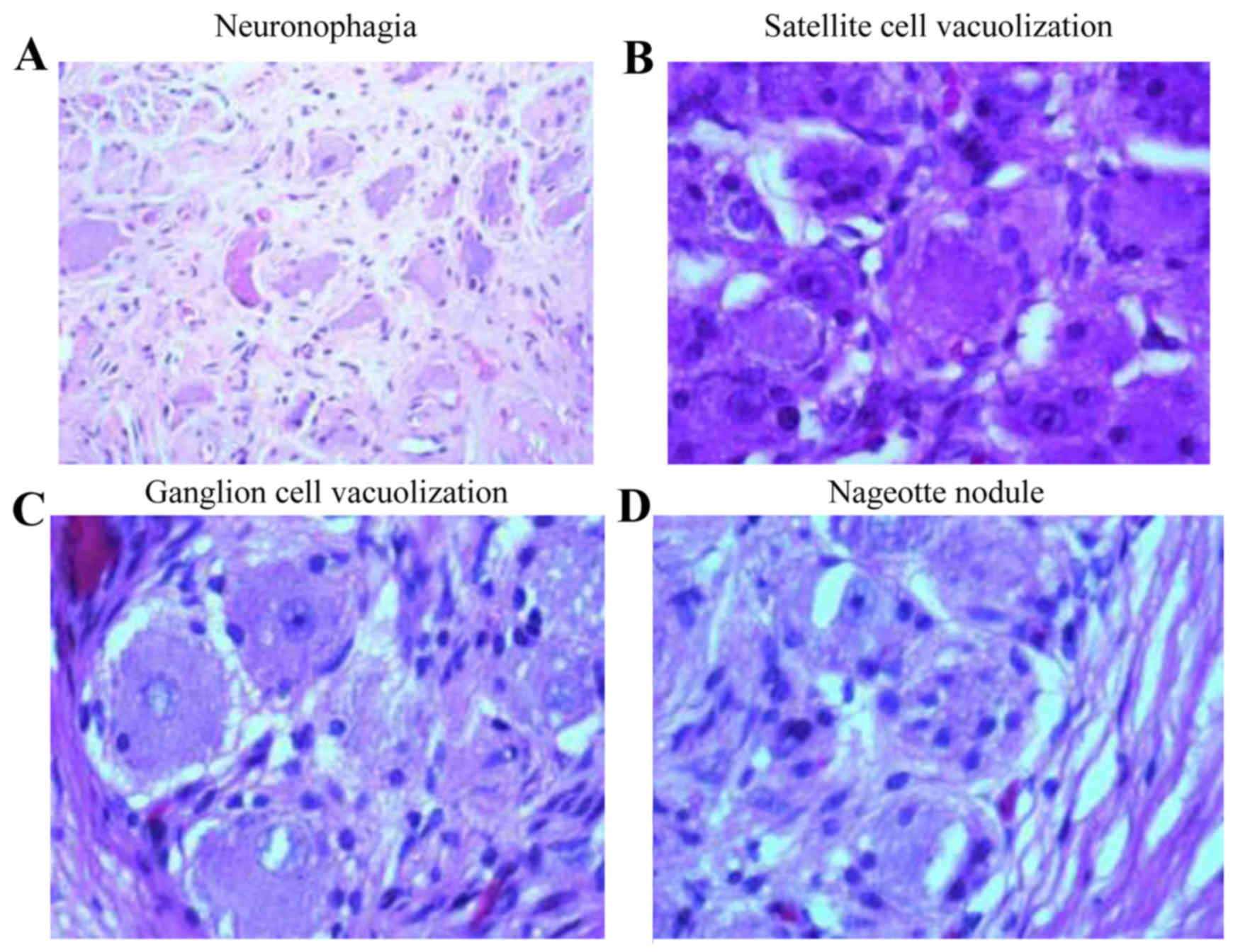
histological examination the prevalent changes were as follows:
Neuron vacuolization in 283 ganglion cells, pyknosis in 135
ganglion cells, satellite cell vacuolization in 351 ganglion cells,
neuronophagia was in 634 ganglion cells and nageotte nodules in 40
ganglion cells. Pyknosis is a condensation and reduction in the
size of cells and nuclei (Fig. 1A).
During neuronophagy, activated microglial cells surround and ingest
a dead neuron (Fig. 1B). In
satellite cell vacuolization, cytoplasmic vacuolization is visible
in the satellite cells (Fig. 1C).
Nageotte nodules are compact areas of satellite cell proliferation
(Fig. 1D). In neuron vacuolization
there are clear vacuoles in the cytoplasm of the ganglion
cells.
 | Table I.The histopathological changes in
sympathetic ganglion cells in adenocarcinoma and BPH prostate
tissues, according to patient age. |
Table I.
The histopathological changes in
sympathetic ganglion cells in adenocarcinoma and BPH prostate
tissues, according to patient age.
| No. of neurons |
|---|
|
|
|
|
|
|---|
| Age of patients | N | Total no. of
neurons | Neuron
vacuolization | Pyknosis | Satellite
vacuolization | Neuronophagy | Nageotte nodules |
|---|
| Group A |
|
|
|
|
|
|
|
|
50–59 | 4 | 453 | 61 | 25 | 50 | 62 | 2 |
|
60–69 | 17 | 2,425 | 148 | 95 | 171 | 402 | 29 |
|
70–89 | 5 | 957 | 74 | 15 | 130 | 170 | 9 |
| Group B |
|
50–59 | 2 | 149 | 3 | 0 | 10 | 11 | 2 |
|
60–69 | 6 | 1,038 | 33 | 9 | 55 | 92 | 6 |
|
70–89 | 2 | 53 | 11 | 4 | 7 | 9 | 1 |
Group B, radical cystectomy and
prostatectomy
Pathological examination of the urinary bladder
revealed low-grade urothelial carcinoma in 2 patients and
high-grade urothelial carcinoma in 8 patients. The stage of the
disease (8) was A in 2 patients, B
in 3 patients and C in 5 patients. The prostate exhibited BPH in
all patients in group B. Of the 10 tissues in this group, 28
ganglion were identified, of which 16 were found in the right
posterior region and 12 in the left posterior region with a total
count of 1,240 ganglion cells.
Group B histopathological changes
The histopathological examination of the ganglion
cells revealed: Neuron vacuolization in 47 ganglion cells, pyknosis
in 13 ganglion cells, satellite cell vacuolization in 72 ganglion
cells, neuronophagia in 112 ganglion cells and 9 nageotte nodules
(Table I).
Statistical analysis
Two way ANOVA reveled significant differences in
neuron vacuolization between the ganglion cells in the prostatic
carcinoma group compared with those with BPH
[F(1,29)=4.602, P<0.05]. This difference was found to
be significant only between ages 50–59 (P<0.05). Pyknosis and
neuronophagia revealed significant differences in the ganglion
cells of the prostatic carcinoma group compared with those with BPH
[F(1,28)=4.185, P<0.05] and
[F(1,28)=4.469, P<0.05] accordingly. However, no
significant difference was found between age groups
[F(2,28)<1]. Age was significantly correlated with
increase in satellite cell vacuolization (RP=0.349,
P<0.044) in the patients with prostate adenocarcinoma. No
significant differences according to age were identified within the
BPH group. A tendency toward significance was found between age and
the presence of nageotte nodules in the peri-prostatic ganglion
cells (RP=0.316, P=0.062) among patients diagnosed with
prostate adenocarcinoma. No significant differences were found
within the BPH group according to age and the presence of nageotte
nodules.
Discussion
The present study identified several changes in the
peri-prostatic ganglion cells. These consisted of neuron
vacuolization, pyknosis, satellite vacuolization, neuronophagia and
nageotte nodules. All these findings were statistically significant
other than the nageotte nodules, which exhibited a tendency toward
significance. Significant differences in neuron vacuolization in
ganglion cells between the peri-prostatic tissue with
adenocarcinoma of the prostate and patients with BPH were only
identified in the patients aged 50–59 years. In all the other
groups this difference had no statistical significance. This trend
may be explained by the fact that all the cases examined were
>50 years, when degenerative changes start. All these changes
were more marked in the peri-prostatic ganglion cells of group A
(prostatic adenocarcinoma), which may be due to environmental
changes associated with the local presence of malignancy. A
significant portion of morbidity in elderly males involves the
pelvic organs and their autonomic neural regulation. These include
lower urinary tract symptoms, incontinence and impairment of bowel
activity and motility. All these functions are regulated by the
regional autonomic ganglia, primarily the pelvic ganglia, and may
be caused by the degenerative changes described.
Age-associated degenerative changes have been
extensively reported in the literature, mainly in rats. Warburton
and Santer (1) revealed that with
aging a definite decrease in the number of sympathetic, but not
parasympathetic, nerve cells innervating the bladder, lower ureters
and urethra occurs. In the prostate itself the variations are
primarily related to a lack of balance in sex hormones (9). Investigations of the changes in the
sympathetic ganglion cells of rats induced by citral, which
simulates sex hormone functions, were performed by Golomb et
al (10). These authors found
changes that were detected only in the older rat group (age, 18
months). These changes included accumulation of lipofuscin pigment
in the ganglion cells, local aggregation of satellite cells nodules
of nageotte and neuronal vacuolar degeneration (10). The effects of X-irradiation on
ganglion cells of the lower back in rats were investigated by
Masurovsky et al (11). In
the first days, a sharp degeneration in the satellite cells was
noted and later sequestration, vacuolization and autolysis were
detected. All these changes were possibly linked to metabolic and
membrane permeability (11).
Acquired immunodeficiency syndrome-associated changes were
evaluated by Burdo et al (12) through examination of the dorsal root
ganglia of CD8-depleted simian immunodeficiency virus-infected
rhesus macaques. The authors identified satellitosis, the presence
of nageotte nodules, neuronophagy as well as increased numbers of
CD68+ macrophages and abundant viral replication.
Kuntz (13) noted
that age and environment affect the sympathetic ganglia. This
previous study was performed on autopsy material of 50 patients
aged 6–71 years old. The changes included hyalinization of the
cytoplasm, hydropic enlargement or edema of ganglion cells,
vacuolization of the cytoplasm, neuro-fibrillary changes and
destruction of the cytoplasm by phagocytic cells. These changes
appeared at 30–35 years and increased with age (13).
The effects of Acrolein on various cells including
the sympathetic ganglia were examined by Liu-Snyder et al
(14). They found cell death was
induced by Acrolein 12 h after its application. There are very few
studies on human surgical material. Hannani et al (15) found a statistically significant
increase in the proportion of myenteric ganglia with cavities with
increasing age. In the present study the sympathetic ganglia in the
peripheral-posterior zone of the prostate were examined in surgical
specimens obtained from total prostatectomy and total cystectomy.
The following histological changes were found: Neuronophagia,
neuron vacuolization, satellite vacuolization, pyknosis and
nageotte nodule formation. Certain histological changes were
significantly associated with age.
Previous studies have examined the influence of
diabetes on the nervous system. Histological changes in the dorsal
root and sympathetic trunk were identified (16). These included degeneration of
ganglion cells, a decrease in their number and the formation of
nageotte nodules in a patient with fulminant type 1 diabetes
mellitus (16). Schmidt et al
(2) performed quantitative studies
on a series of adult autopsied diabetic and non-diabetic patients
of various ages, demonstrating that neuron-axonal dystrophy
increased with age in patients with diabetes, and males were more
severely affected than females. A decrease in the number of
neurons, primarily in the lumbar area, accompanied by satellite
cell proliferation in patients with diabetes has been demonstrated
in a number of studies (17–20). In 1909, Gomez and Pike (21) published data regarding the
histopathological changes in neurons in anemia. To the best of our
knowledge, this previous research was the first controlled study on
this subject and definitively the first review of the literature.
These authors reported the appearance of vacuoles in the cytoplasm
of the cells in prolonged anemia. In contagious disease, Fratkin
et al (22) reported the
appearance of nodules of nageotte and lymphocytic aggregation in
the presence of the West Nile virus. Nageotte nodules and
mononuclear accumulation in the sympathetic ganglion cells in
Guillen-Barre syndrome were identified by Patel et al
(23).
In conclusion, in the present study, morphological
changes in the sympathetic ganglia were identified in surgical
specimens obtained from total prostatectomy and total cystectomy in
elderly males. There were statistical differences between the
changes in the two groups. All changes were more marked in the
ganglion cells of prostatic adenocarcinoma compared with BPH, which
may be due to environmental changes occurring due to the local
presence of malignancy. Certain changes increased with patient age.
To the best of our knowledge, there are no studies in the
literature on the pelvic sympathetic ganglia in human surgical
material. The number of cases in the present study is limited and
all the patients were elderly, therefore larger scale studies may
be required to obtain more significant conclusions.
Acknowledgements
The authors would like to thank Prof. Abramovici
Armand (deceased), Department of Pathology, Sackler Faculty of
Medicine, Tel Aviv University, Tel Aviv for his help in performing
the present study.
References
|
1
|
Warburton AL and Santer RM: Sympathetic
and sensory innervation of the urinary tract in young adult and
aged rats: A semi-quantitative histochemical and
immunohistochemical study. Histochem J. 26:127–133. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt RE, Plurad SB, Parvin CA and Roth
KA: Effect of diabetes and aging on human sympathetic autonomic
ganglia. Am J Pathol. 143:143–153. 1993.PubMed/NCBI
|
|
3
|
Schmidt RE, Chae HY, Parvin CA and Roth
KA: Neuroaxonal dystrophy in aging human sympathetic ganglia. Am J
Pathol. 136:1327–1338. 1990.PubMed/NCBI
|
|
4
|
Schmidt RE: Neuropathology of human
sympathetic autonomic ganglia. Microsc Res Tech. 35:107–121. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koren R, Gur U, Lask D, Livne MP, Shvero
A, Neumann G and Rath-Wolfson L: Histopathological findings in the
prostatic urethra evaluated by a new technique for processing
radical prostatectomy specimens. Con Med. 5:8–16. 2010.
|
|
6
|
Robbins and CotranPathologic basis of
disease. 9th. Saunders; pp. 9882015
|
|
7
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016.PubMed/NCBI
|
|
8
|
Cheng L, Montironi R, Davidson DD and
Lopez-Beltran A: Staging and reporting of urothelial carcinoma of
the urinary bladder. Mod Pathol. 22:(Suppl 2). S70–S95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keast JR and Saunders RJ: Testosterone has
potent, selective effects on the morphology of pelvic autonomic
neurons which control the bladder, lower bowel and internal
reproductive organs of the male rat. Neuroscience. 85:543–556.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Golomb E, Scolnik M, Koren R, Servadio C,
Sandbank U and Abramovici A: Effects of senescence and citral on
neuronal vacuolar degeneration in rat pelvic ganglia.
Neurotoxicology. 22:73–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masurovsky EB, Bunge MB and Bunge RP:
Cytological studies of organotypic cultures of rat dorsal root
ganglia following X-irradiation in vitro. I. Changes in neurons and
satellite cells. J Cell Biol. 32:467–496. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burdo TH, Orzechowski K, Knight HL, Miller
AD and Williams K: Dorsal root ganglia damage in SIV-infected
rhesus macaques: An animal model of HIV-induced sensory neuropathy.
Am J Pathol. 180:1362–1369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuntz A: Histological variations in
autonomic ganglia and ganglion cells associated with age and
disease. Am J Pathol. 14:783–796. 1938.PubMed/NCBI
|
|
14
|
Liu-Snyder P, McNally H, Shi R and Borgens
RB: Acrolein-mediated mechanisms of neuronal death. J Neurosci Res.
84:209–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanani M, Fellig Y, Udassin R and Freund
HR: Age-related changes in the morphology of the myenteric plexus
of the human colon. Auton Neurosci. 113:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makino M, Hiwatashi D, Minemura K and
Kawaguchi K: Autonomic and sensory ganglionopathy occurring in a
patient with fulminant type 1 diabetes mellitus. Pathol Int.
66:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dolman CL: The morbid anatomy of diabetic
neuropathy. Neurology. 13:135–142. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenbaum D, Richardson PC, Salmon MV and
Urich H: Pathological observations on six cases of diabetic
neuropathy. Brain. 87:201–214. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olsson Y, Säve-Söderbergh J, Sourander P
and Angervall L: A patho-anatomical study of the central and
peripheral nervous system in diabetes of early onset and long
duration. Pathol Eur. 3:62–79. 1968.PubMed/NCBI
|
|
20
|
Russell JW, Sullivan KA, Windebank AJ,
Herrmann DN and Feldman EL: Neurons undergo apoptosis in animal and
cell culture models of diabetes. Neurobiol Dis. 6:347–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gomez L and Pike FH: The histological
changes in nerve cells due to total temporary anemia of the central
nervous system. J Exp Med. 11:257–265. 1909. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fratkin JD, Leis AA, Stokic DS, Slavinski
SA and Geiss RW: Spinal cord neuropathology in human west nile
virus infection. Arch Pathol Lab Med. 128:533–537. 2004.PubMed/NCBI
|
|
23
|
Patel MB, Goyal SK, Punnam SR, Pandya K,
Khetarpal V and Thakur RK: Guillain-Barré Syndrome with asystole
requiring permanent pacemaker: A case report. J Med Case Rep.
3:52009. View Article : Google Scholar : PubMed/NCBI
|