Prostatic disease is a common health problem among
males in Western countries and has a marked impact on the quality
of life of aging males (1). Prostate
cancer (PCa) is the most prevalent cancer type in males >50
years of age and the second most common cause of cancer-associated
mortality in males (2). Benign
prostatic hyperplasia (BPH) represents the most common urologic
disorder among elderly men, with ~40% of males >60 years
affected (3). Chronic
prostatitis/chronic pelvic pain syndromes (CP/CPPS) are other
challenging urology disorders responsible for considerable
disability in affected patients (4).
The majority of these prostatic disorders are age-associated and
epidemiologically linked. The presence of functioning Leydig cells
of the testes and good 5-α-reductase activity are essential for the
development of these conditions (5).
Their pathogenesis is multifactorial, involving several factors,
including genetic instability, inflammation, endocrine disruptors,
atherosclerosis, hormones and oxidative stress (5). The integration of these factors in a
well-defined pathogenic process from the initiation, development
and progression of the abovementioned prostatic conditions has not
been attained to date. Oxidative stress is among the possible
mechanisms that may account for chronic prostatic disorders. In
this paper, a systematic review of the role of oxidative stress in
chronic prostatic disorders was conducted and the emerging
treatments that target this pathogenesis are discussed.
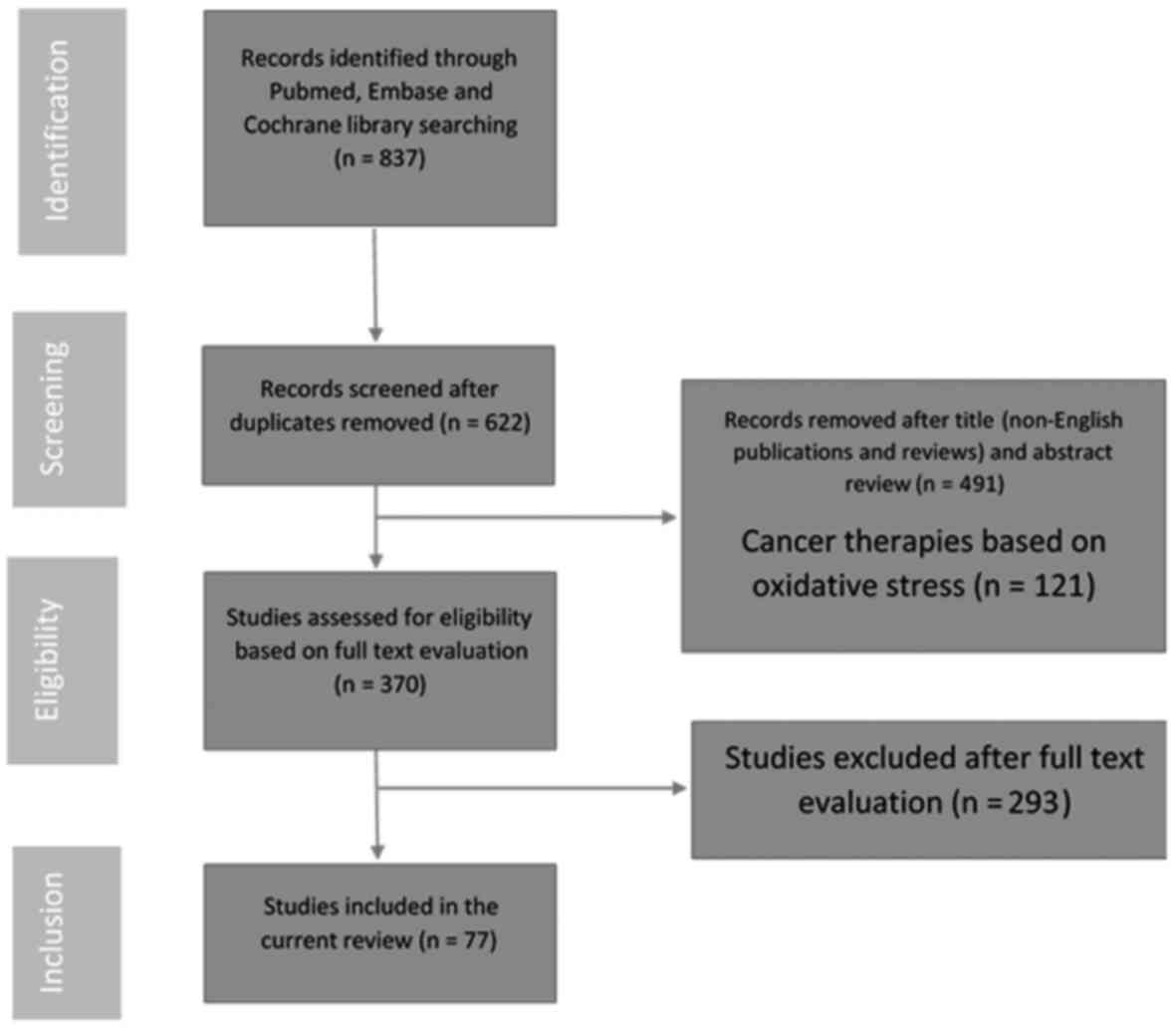
A systematic review of the literature available
online was conducted in March 2016 using the following keywords:
‘Oxidative stress’ and ‘prostate’. Searches for relevant original
articles, clinical studies and research papers published since
January 2000 were performed using the Cochrane Library Database,
PubMed and MEDLINE. The authors manually reviewed the significant
results and citations. The Preferred Reporting Items for Systematic
Reviews and Meta-Analyses process for reporting included and
excluded studies was followed; the flowchart in Fig. 1 lists the numbers of papers
identified and then included or excluded at each stage. Studies
reported in English and presenting data regarding the measurement
of oxidative stress in patients with BPH, PCa or CP/CPPS were
included. Animal studies and in vitro models were also
included. Review articles and articles published in languages other
than English were excluded.
The etiology and pathogenesis of BPH, a common
disease in aging males, remain to be elucidated. Several parameters
and signaling pathways have been suggested to serve a role in
prostatic growth, but there is no integrative theory to explain the
interaction among all these mediators. Recently, it has been
revealed that CP may directly induce prostatic proliferation of
stromal and glandular cells via the production of reactive oxygen
species (ROS), leading to prostatic tissue damage and vascular
injury (6,7). Oxidative stress damage to the prostatic
tissue is not only limited to the structure and function of the
proteins, but also to alterations in the DNA repair machinery and
post-translational modifications (8).
Oxidative DNA damage results in point mutations,
deletions or rearrangements and contributes to a change in the
normal regulation of programmed cell death, thereby leading to
hyperplastic or precancerous transformation (9). The rapid prostatic cell turnover in
human prostate tissue and the paucity of DNA repair enzymes
contribute to the particular vulnerability of the prostatic gland
to oxidative stress (9). However,
the molecular mechanisms leading to chronic inflammation, BPH and
malignant transformation are not well understood. NF-κB, a
transcriptional factor that regulates inflammation, immune
response, cell proliferation, cell migration and apoptosis, has
been recently studied (10). ROS
have been revealed to stimulate NF-κB by activating the
NF-κB-inducing kinase (NIK) and TNF-α/AP-1 transduction pathways,
thus leading to the occurrence of CP. NF-κB can also induce loss of
imprinting of insulin-like growth factor 2 in both cancerous and
noncancerous human prostate cells. Consequently, it was proposed
that NF-κB modulation may prevent oxidative stress-induced
alterations in the epigenome.
Apart from the direct effect of oxidative stress on
DNA, ROS can also be indirectly genotoxic (11). ROS can initiate autocatalytic lipid
peroxidation that generates several genotoxic breakdown products,
including peroxyl radicals, alkoxyl radicals and aldehydes, such as
malondialdehyde (11). In contrast
to free radicals, these aldehydes can diffuse out of the cell and
reach distant targets. The measurement of circulating
malondialdehyde levels in plasma or serum provides a non-invasive
method of estimating the oxidative stress level and can be used as
a biomarker to examine lipid peroxidation-mediated disorders.
Plasma peroxide levels and lipid peroxidation were significantly
increased in patients with BPH compared with controls (12–14).
However, studies of circulating malondialdehyde levels in
associated with BPH have produced contradictory results (12,15).
In BPH, 8-OH deoxyguanosine (dG) is a marker of
oxidative DNA damage. Quantitative analysis of this marker revealed
increased levels in the epithelial cells of BPH, as compared with
in the normal transition zone (15).
NADPH oxidase 4 (NOX4) has also been studied as a member of a
family of proteins that generate ROS in mice; the NOX4 transgenic
mice exhibited increased levels of 8-OH dG (15). During the course of the study,
increased prostate weight was noted through epithelial
proliferation and stromal alterations, such as fibrosis (16).
Oxidative stress in BPH is primarily modulated by
angiotensin II (Ang II) and myeloperoxidase (MPO). Ang II is the
main effector peptide of the renin angiotensin system (RAS) and
exerts a variety of biological actions, including NOX activation,
stimulation of cell growth and migration, and the inflammation of
smooth muscle cells and fibroblasts (17,18).
Increased Ang II specific activity has been reported in patients
suffering from BPH (19).
MPO is a member of the peroxidase superfamily that
is stored within the azurophilic granules of neutrophils and
monocytes (20). MPO is responsible
for the formation of hypochlorous acid, a strong oxidant agent that
generates modified oxidized lipoproteins (Mox-LDL) (20). MPO is released in the extracellular
medium in response to high levels of cytokines, promotes oxidative
damage to the host tissue and activates procarcinogens (21). MPO is present in prostatic epithelial
cells; however, its role in the various chronic prostatic disorders
has not yet been elucidated (22).
MPO and Ang II interact in the bloodstream to produce Mox-LDLs,
which then promote the release of IL-8 and TNF-α by endothelial
cells and monocytes, respectively (23).
Oxidative stress contributes to epigenetic
alterations that may ultimately lead to the development of cancer.
Oxidative stress has been demonstrated to increase during
carcinogenesis, and the activity of certain genes that serve an
antioxidant role also increases. NAD(P)H:quinoneoxidoreductase 1
(NQO1) is a cytoprotective enzyme that acts as a genome protector
of DNA alterations secondary to oxidative stress (24). NQO1 knockdown increases IL-8, which
in turn decreases p53 and activates NF-κB to mediate cell survival
(25). NF-κB promotes cancer
progression in castration-resistant PCa (CRPC) through
spermidine/spermine N-acetyltransferase (SSAT) via the effect of
androgens (26). NF-κB also alters
the regulation of IGF2 imprinting by downregulating CCCTC-binding
factor (CTCF) (27).
Tumorigenesis may also result from the deregulation
of oxidative stress responses (28).
Oxidative stress may contribute to an increase in cell viability
and resistance to stress by upregulating ERp57 (29). Oxidative stress increases the
transcription factors for certain cancer survival proteins,
including Hsp27 and PRDX6, thus protecting PCa cells from necrosis
induced by oxidative stress (29).
ROS induce carcinogenesis by increasing STAMP2 expression via the
ATF4 gene. STAMP2 subsequently increases ROS through its iron
reductase activity (30).
Oxidative stress-induced DNA mutations with
secondary genomic instability causing carcinogenesis have been
attributed in part to the epigenetic silencing of glutathione
S-transferase π (GSTP1) and catalase deficiency (31,32).
Other mutations have also been associated with cancer progression.
Testicular nuclear receptor 4 (TR4) serves protective roles against
oxidative stress and DNA damage; however, the association between
TR4 and tumor progression remains unclear. TR4 is associated with
the Gleason score of PCa via the promotion of CCL2/CCR2 signaling,
thus promoting the deregulation of oxidative scavengers under
chronic inflammation (33,34). In human PCa, post-translational
modifications are induced by redox imbalances, including the
oxidation of thioredoxin 1, sulfinylation of peroxiredoxins, and
the nitration and methylation of manganese superoxide dismutase
(35).
Several studies have suggested the role of oxidative
stress in causing mitochondrial DNA mutations, which may lead to
genetic and epigenetic alterations in the nucleus and are thus
involved in carcinogenesis (36,37). The
glutathione S-transferase (GST) gene superfamily (A, M, T and P
gene classes) encodes a group of enzymes that protect the DNA from
oxidative damage. Polymorphisms in these genes have been thoroughly
studied, and the current evidence does not suggest any effect on
the risk for PCa (38). This finding
may be explained by the fact that carcinogens whose effect is
modifiable by GST minimally contribute to carcinogenesis (38).
GPx4 has been suggested to be one of the
selenoproteins that serve a role against ROS-induced lipid
peroxidation. Knock-out mice for the gene producing GPx4 express
high levels of the proliferation indicator Ki-67 and specific
markers of pro-oncogenic pathway activation, such as pS6 and pMAPK
(39). Jones et al (40) investigated the role of the chemokine
receptor Cysteine-X-C Receptor 4 (CXCR4) and NOX during oxidative
stress in PCa cells. The interaction between CXCR4 and its ligand,
stromal cell-derived factor-1 α (SDF-1α), was revealed to regulate
the activity of NOX2, which leads to ROS production through the
PI3K/AKT pathway. This interaction was also determined to be
involved in PCa progression as it promotes cell migration that is
associated with metastasis (40).
Oxidative stress acts on the tumor microenvironment
and ROS have an important role in this pathway via the mTORC1/c-Myc
cascade, which is mainly controlled by p62. In the context of p62
deficiency, the stroma of the tumor is metabolically reprogrammed
into a pro-tumorigenic milieu (41).
Chronic prostatitis is a frequently diagnosed
disease with a poorly established pathophysiology (5). The generic name encompasses various
clinical entities, but the most frequent and most challenging is
chronic prostatitis class NIH III, which is also known as chronic
pelvic pain syndrome (5). This
condition is characterized by the absence of infective etiology
(43). Inflammation serves a
principal role in this disease, and multiple agents of the
inflammatory pathway have been studied and targeted (44). To date, oxidative stress is
considered to be a significant factor in the inflammatory cascade
of chronic prostatitis (4,45). Only a few in vivo studies on
the subject have been performed due to the highly variable patient
population. The majority of studies have been conducted in
vitro, with no considerable impact on clinical decision making
(46–48). The available data indicate that the
concentration of 8-isoprostanes, lipid-derived mediators of
oxidative stress, in prostatic secretions and urine is increased
among males with CP (4). Future
studies exploring oxidative stress in CP are awaited; however, the
clinical heterogeneity of these disorders renders trial design
notably complicated.
As growing evidence supports the role of oxidative
stress in chronic prostate diseases, investigators are struggling
to explore possible therapeutic agents that can target oxidative
stress (49). One must not forget
that many of the foods normally consumed in the diet contain
natural antioxidants. For example, blackberries, walnuts,
strawberries, artichokes, cranberries, brewed coffee, raspberries,
pecans, and grape juice all contain high concentrations of
antioxidants per quantity served (50). Various substances have been tested
for reducing the formation of ROS or increasing ROS levels enough
to induce cellular apoptosis in prostatic cancerous cells (51–57).
Further discussed here are the phenolic molecules and the other
synthetic or natural non-phenolic options.
Phenolic molecules may act on the various processes
of carcinogenesis by inducing cell apoptosis via the formation of
ROS (58). Phellinus linteus
is a mushroom that reduces cancer growth by increasing the toxicity
of oxidative stress (53).
Silibinin, a natural derivate of flavanone, reduced cell motility
and invasiveness in Du145 PCa cells (54). Other phenolic molecules act by
reducing the formation of ROS. Apigenin, a dietary plant flavone,
is taken up by healthy prostatic cells and quickly intercalated
into the DNA, thus providing protective mechanisms by reducing the
formation of ROS (57). Similarly,
pomegranate juice reduced PCa tumor proliferation in animal models
(59). Numerous other drugs and
phenolic compounds have been tested, including N-acetylalaninate
prodrugs, quercetin, Crataeva nurvala bark and seaweeds.
These compounds were all observed to produce significant cancer
cell death in in vitro and/or in vivo experiments
(60–63).
Non-phenolic molecules include natural and synthetic
options. Regucalcin is an intracellular calcium sensor that
counteracts the aging of cells and increases the activity of
apoptosis regulators in the prostate in animal models (51). Melatonin is another natural protein
that increases the activity of two major intracellular enzymes,
prostatic glutathione-S-transferase and glutathione peroxidase,
which are characterized by their antioxidant properties (55). Omega-3 and omega-6 fatty acids act
synergistically on tumor cell survival, proliferation and
angiogenesis by increasing lipid peroxidation (63). However, these biological actions may
not translate into clinical significance, and require confirmation
in interventional trials or observational studies with a long
follow-up duration (64).
Selenium and vitamin E are the most studied
antioxidants for reducing the risk of PCa by modifying the
intracellular redox state, as previously described in LNCaP cell
lines treated with selenium (65).
The Selenium and Vitamin E Cancer Prevention Trial is one of the
largest and most important studies in the chemoprevention of PCa to
date. Male patients were enrolled and randomized into groups set to
receive selenium, vitamin E, a combination of the two or a placebo.
Patients were followed for a median of 5.5 years, and the risk of
developing PCa was evaluated. The investigators observed no
significant difference in PCa risk across the four risk groups,
with 4.43% of patients developing PCa in the placebo group, 4.56%
in the selenium, 4.9% in the vitamin E and 4.56% in the combination
arm (66). After 18 months of
follow-up, the vitamin E arm exhibited a significantly increased
risk of PCa by 17% (67).
Researchers have suggested that selenium-enriched yeast, but not
selenomethionine, may be effective in reducing oxidative stress.
Richie et al (68) assessed
the differences between the two molecules in 69 healthy males.
After 9 months, levels of the oxidative stress biomarkers
8-isoprostaglandin F2α and 8OH-deoxyguanosine were reduced by 66
and 72%, respectively, in patients receiving selenium-enriched
yeast but not in those receiving selenomethionine. Furthermore, a
key point in using selenium as a chemopreventive agent is the
variability in individual responses to selenium on oxidative stress
and DNA damage (69). Investigators
followed 95 adults treated with selenium and noted inconsistent
changes in the oxidative stress response. In addition, DNA damage
was not significantly influenced by selenium treatment (69). APC-100 is the antioxidant moiety of
vitamin E (α-tocopherol). APC-100 exhibits anti-androgenic
properties, and preclinical studies have observed potent androgen
receptor (AR) signaling modulation and anti-cancer activity against
PCa cell lines (70). Recently,
Kyriakopoulos et al (71)
tested APC-100 in 20 patients with CRPC and determined that the
drug was safe. PSA stabilization in these patients was a sign of
drug activity despite suboptimal (71).
Other nutrients with antioxidative activity include
carotenoids, ginger and curcumin compounds, and zinc (58). Lycopene is a highly unsaturated
acyclic isomer of β-carotene present in red vegetables and fruits.
Lycopene's effect on preventing PCa is debatable, with conflicting
evidence (72–74). A notable prior study reported that
vitamin A from animal sources increases the risk of PCa, whereas
that from plant sources decreases the risk (75). Ginger compounds suppress growth and
induce apoptosis in LNCaP cells by inhibiting cyclooxygenase
activity the androgen receptor; pStat3 and pPKC (α/β) pathways and
the activation of the p21 pathway (76).
Recent studies in lipidomics and metabolomics have
demonstrated that omega-3 poly-unsaturated fatty acids inhibit
tumor growth directly and by modulating the immune system. These
compounds mainly act by regulating multiple complex metabolic
processes of oxidative stress pathways, including β-oxidation,
lipid release, cellular signaling, eicosanoid synthesis, direction
activation of nuclear receptors and gene transcription (77). Effectively, arachidonic acid may
potentiate the risk of metastatic migration. In addition, secondary
implantation may be potentiated by arachidonic acid but may also be
reduced by omega-3 poly-unsaturated fatty acids (78).
The synthetic non-phenolic molecules that were
tested for antioxidative effect in prostate diseases are rare. The
ability of allopurinol to reduce oxidative stress has been assessed
in humans; in a randomized, double-blinded study, 50 males affected
with metabolic syndrome received allopurinol or placebo. A
significant reduction of malondialdehyde and MPO was reported in
the treatment arm (56).
Oxidative stress is a physiological phenomenon, but
it is not yet clear which trigger mechanisms make it pathological.
Evidence suggests that ROS from chronic inflammation serve a role
in the pathogenesis of prostatic diseases. Due to the large number
of patients who have inflammatory processes in the prostate
regardless of whether the condition is symptomatic, this link is
intriguing, and well-designed long-duration studies examining the
effects of supplemental or dietary intake, as aforementioned, on
prostate pathology, incidence, treatment and progression are
required.
|
1
|
Lebret T, Culine S, Davin JL, Hennequin C,
Mignard JP, Moreau JL, Rossi D, Zerbib M, Mahmoudi A and Latorzeff
I: Quality of life of 1276 elderly patients with prostate cancer,
starting treatment with a gonadotropin-releasing hormone agonist:
Results of a French observational study. Aging Male. 17:87–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aoun F, Marcelis Q and Roumeguère T:
Minimally invasive devices for treating lower urinary tract
symptoms in benign prostate hyperplasia: Technology update. Res Rep
Urol. 7:125–136. 2015.PubMed/NCBI
|
|
4
|
Kullisaar T, Türk S, Punab M and Mändar R:
Oxidative stress-cause or consequence of male genital tract
disorders? Prostate. 72:977–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoun F, Albisinni S, Chemaly AK, Zanaty M
and Roumeguère T: In search for a common pathway for health issues
in men-the sign of a holmesian deduction. Asian Pac J Cancer Prev.
17:1–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bostanci Y, Kazzazi A, Momtahen S, Laze J
and Djavan B: Correlation between benign prostatic hyperplasia and
inflammation. Curr Opin Urol. 23:5–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chughtai B, Lee R, Te A and Kaplan S: Role
of inflammation in benign prostatic hyperplasia. Rev Urol.
13:147–150. 2011.PubMed/NCBI
|
|
8
|
Sciarra A, Mariotti G, Salciccia S, Gomez
Autran A, Monti S, Toscano V and Di Silverio F: Prostate growth and
inflammation. J Steroid Biochem Mol Biol. 108:254–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamid AR, Umbas R and Mochtar CA: Recent
role of inflammation in prostate diseases: Chemoprevention
development opportunity. Acta Med Indones. 43:59–65.
2011.PubMed/NCBI
|
|
10
|
Wong CP, Bray TM and Ho E: Induction of
proinflammatory response in prostate cancer epithelial cells by
activated macrophages. Cancer Lett. 276:38–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meagher EA and FitzGerald GA: Indices of
lipid peroxidation in vivo: Strengths and limitations. Free Radic
Biol Med. 28:1745–1750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merendino RA, Salvo F, Saija A, Di
Pasquale G, Tomaino A, Minciullo PL, Fraccica G and Gangemi S:
Malondialdehyde in benign prostate hypertrophy: A useful marker?
Mediators Inflamm. 12:127–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pace G, Di Massimo C, De Amicis D,
Corbacelli C, Di Renzo L, Vicentini C, Miano L and Ciancarelli
Tozzi MG: Oxidative stress in benign prostatic hyperplasia and
prostate cancer. Urol Int. 85:328–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arsova-Sarafinovska Z, Eken A, Matevska N,
Erdem O, Sayal A, Savaser A, Banev S, Petrovski D, Dzikova S,
Georgiev V, et al: Increased oxidative/nitrosative stress and
decreased antioxidant enzyme activities in prostate cancer. Clin
Biochem. 42:1228–1235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almushatat AS, Talwar D, McArdle PA,
Williamson C, Sattar N, O'Reilly DS, Underwood MA and McMillan DC:
Vitamin antioxidants, lipid peroxidation and the systemic
inflammatory response in patients with prostate cancer. Int J
Cancer. 118:1051–1053. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vital P, Castro P and Ittmann M: Oxidative
stress promotes benign prostatic hyperplasia. Prostate. 76:58–67.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massey KJ, Hong NJ and Garvin JL:
Angiotensin II stimulates superoxide production in the thick
ascending limb by activating NOX4. Am J Physiol Cell Physiol.
303:C781–C789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verbon EH, Post JA and Boonstra J: The
influence of reactive oxygen species on cell cycle progression in
mammalian cells. Gene. 511:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nassis L, Frauman AG, Ohishi M, Zhuo J,
Casley DJ, Johnston CI and Fabiani ME: Localization of
angiotensin-converting enzyme in the human prostate: Pathological
expression in benign prostatic hyperplasia. J Pathol. 195:571–579.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klebanoff SJ: Myeloperoxidase: Friend and
foe. J Leukoc Biol. 77:598–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boudjeltia KZ, Legssyer I, Van Antwerpen
P, Kisoka RL, Babar S, Moguilevsky N, Delree P, Ducobu J, Remacle
C, Vanhaeverbeek M and Brohee D: Triggering of inflammatory
response by myeloperoxidase-oxidized LDL. Biochem Cell Biol.
84:805–812. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roumeguère T, Delree P, Van Antwerpen P,
Rorive S, Vanhamme L, de Ryhove Lde L, Serteyn D, Wespes E,
Vanhaerverbeek M and Boudjeltia KZ: Intriguing location of
myeloperoxidase in the prostate: A preliminary immunohistochemical
study. Prostate. 72:507–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boudjeltia KZ, Delporte C, Van Antwerpen
P, Franck T, Serteyn D, Moguilevsky N, Raes M, Vanhamme L,
Vanhaeverbeek M, Van Meerhaeghe A and Roumeguère T:
Myeloperoxidase-dependent LDL modifications in bloodstream are
mainly predicted by angiotensin II, adiponectin, and
myeloperoxidase activity: A cross-sectional study in men. Mediators
Inflamm. 2013:7507422013.PubMed/NCBI
|
|
24
|
Kurfurstova D, Bartkova J, Vrtel R,
Mickova A, Burdova A, Majera D, Mistrik M, Kral M, Santer FR,
Bouchal J and Bartek J: DNA damage signalling barrier, oxidative
stress and treatment-relevant DNA repair factor alterations during
progression of human prostate cancer. Mol Oncol. 10:879–894. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thapa D, Meng P, Bedolla RG, Reddick RL,
Kumar AP and Ghosh R: NQO1 suppresses NF-κB-p300 interaction to
regulate inflammatory mediators associated with prostate
tumorigenesis. Cancer Res. 74:5644–5655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang W, Eickhoff JC, Mehraein-Ghomi F,
Church DR, Wilding G and Basu HS: Expression of spermidine/spermine
N(1)-acetyl transferase (SSAT) in human prostate tissues is related
to prostate cancer progression and metastasis. Prostate.
75:1150–1159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang B, Wagner J, Damaschke N, Yao T,
Wuerzberger-Davis SM, Lee MH, Svaren J, Miyamoto S and Jarrard DF:
A novel pathway links oxidative stress to loss of insulin growth
factor-2 (IGF2) imprinting through NF-κB activation. PloS One.
9:e880522014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Labanca E, De Luca P, Gueron G, Paez A,
Moiola CP, Massillo C, Porretti J, Giudice J, Zalazar F, Navone N,
et al: Association of HO-1 and BRCA1 is critical for the
maintenance of cellular homeostasis in prostate cancer. Mol Cancer
Res. 13:1455–1464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basu A, Ross Cajigas-Du CK, Rios-Colon L,
Mediavilla-Varela M, Daniels-Wells TR, Leoh LS, Rojas H, Banerjee
H, Martinez SR, Acevedo-Martinez S and Casiano CA: LEDGF/p75
overexpression attenuates oxidative stress-induced necrosis and
upregulates the oxidoreductase ERP57/PDIA3/GRP58 in prostate
cancer. PloS One. 11:e01465492016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Wang L, Qu S, Sheng X, Kristian A,
Mælandsmo GM, Pällmann N, Yuca E, Tekedereli I, Gorgulu K, et al:
STAMP2 increases oxidative stress and is critical for prostate
cancer. EMBO Mol Med. 7:315–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mian OY, Khattab MH, Hedayati M, Coulter
J, Abubaker-Sharif B, Schwaninger JM, Veeraswamy RK, Brooks JD,
Hopkins L, Shinohara DB, et al: GSTP1 loss results in accumulation
of oxidative DNA base damage and promotes prostate cancer cell
survival following exposure to protracted oxidative stress.
Prostate. 76:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geybels MS, van den Brandt PA, van
Schooten FJ and Verhage BA: Oxidative stress-related genetic
variants, pro- and antioxidant intake and status, and advanced
prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 24:178–186.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding X, Yang DR, Lee SO, Chen YL, Xia L,
Lin SJ, Yu S, Niu YJ, Li G and Chang C: TR4 nuclear receptor
promotes prostate cancer metastasis via upregulation of CCL2/CCR2
signaling. Int J Cancer. 136:955–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Debelec-Butuner B, Ertunc N and Korkmaz
KS: Inflammation contributes to NKX3.1 loss and augments DNA damage
but does not alter the DNA damage response via increased SIRT1
expression. J Inflamm (Lond). 12:122015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chaiswing L, Zhong W and Oberley TD:
Increasing discordant antioxidant protein levels and enzymatic
activities contribute to increasing redox imbalance observed during
human prostate cancer progression. Free Radic Biol Med. 67:342–352.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yakes FM and Van Houten B: Mitochondrial
DNA damage is more extensive and persists longer than nuclear DNA
damage in human cells following oxidative stress. Proc Natl Acad
Sci USA. 94:514–519. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ntais C, Polycarpou A and Ioannidis JP:
Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the
risk of prostate cancer: A meta-analysis. Cancer Epidemiol
Biomarkers Prev. 14:176–181. 2005.PubMed/NCBI
|
|
39
|
Luchman HA, Villemaire ML, Bismar TA,
Carlson BA and Jirik FR: Prostate epithelium-specific deletion of
the selenocysteine tRNA gene Trsp leads to early onset
intraepithelial neoplasia. Am J Pathol. 184:871–877. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jones KJ, Chetram MA, Bethea DA, Bryant
LK, Odero-Marah V and Hinton CV: Cysteine (C)-X-C receptor 4
regulates NADPH oxidase-2 during oxidative stress in prostate
cancer cells. Cancer Microenviron. Sep 28–2013.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valencia T, Kim JY, Abu-Baker S,
Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA,
Metallo CM, Diaz-Meco MT and Moscat J: Metabolic reprogramming of
stromal fibroblasts through p62-mTORC1 signaling promotes
inflammation and tumorigenesis. Cancer Cell. 26:121–135. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao ZG, Xu X, Xue YM and Zhao SL:
Comparison of 4-hydroxynonenal-induced p53-mediated apoptosis in
prostate cancer cells LNCaP and DU145. Contemp Oncol (Pozn).
18:22–28. 2014.PubMed/NCBI
|
|
43
|
Hu C, Yang H, Zhao Y, Chen X, Dong Y, Li
L, Dong Y, Cui J, Zhu T, Zheng P, et al: The role of inflammatory
cytokines and ERK1/2 signaling in chronic prostatitis/chronic
pelvic pain syndrome with related mental health disorders. Sci Rep.
6:286082016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Strauss AC and Dimitrakov JD: New
treatments for chronic prostatitis/chronic pelvic pain syndrome.
Nat Rev Urol. 7:127–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Polackwich AS and Shoskes DA: Chronic
prostatitis/chronic pelvic pain syndrome: A review of evaluation
and therapy. Prostate Cancer Prostatic Dis. 19:132–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mosli HA, Al-Abd AM, El-Shaer MA, Khedr A,
Gazzaz FS and Abdel-Naim AB: Local inflammation influences
oestrogen metabolism in prostatic tissue. BJU Int. 110:274–282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang LL, Huang YH, Yan CY, Wei XD, Hou JQ,
Pu JX and Lv JX: N-acetylcysteine ameliorates prostatitis via
miR-141 regulating Keap1/Nrf2 signaling. Inflammation. 39:938–947.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pontari MA and Ruggieri MR: Mechanisms in
prostatitis/chronic pelvic pain syndrome. J Urol. 172:839–845.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang X, Yuan L, Xiong C, Yin C and Ruan J:
Abacopteris penangiana exerts testosterone-induced benign prostatic
hyperplasia protective effect through regulating inflammatory
responses, reducing oxidative stress and anti-proliferative. J
Ethnopharmacol. 157:105–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Halvorsen BL, Carlsen MH, Phillips KM,
Bøhn SK, Holte K, Jacobs DR Jr and Blomhoff R: Content of
redox-active compounds (ie, antioxidants) in foods consumed in the
United States. Am J Clin Nutr. 84:95–135. 2006.PubMed/NCBI
|
|
51
|
Vaz CV, Marques R, Maia CJ and Socorro S:
Aging-associated changes in oxidative stress, cell proliferation,
and apoptosis are prevented in the prostate of transgenic rats
overexpressing regucalcin. Transl Res. 166:693–705. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bae WJ, Ha US, Kim S, Kim SJ, Hong SH, Lee
JY, Hwang TK, Hwang SY, Kim HJ and Kim SW: Reduction of oxidative
stress may play a role in the anti-inflammatory effect of the novel
herbal formulation in a rat model of hydrochloric acid-induced
cystitis. Neurourol Urodyn. 34:86–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Konno S, Chu K, Feuer N, Phillips J and
Choudhury M: Potent anticancer effects of bioactive mushroom
extracts (Phellinus linteus) on a variety of human cancer cells. J
Clin Med Res. 7:76–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Prajapati V, Kale RK and Singh RP:
Silibinin combination with arsenic strongly inhibits survival and
invasiveness of human prostate carcinoma cells. Nutr Cancer.
67:647–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gobbo MG, Costa CF, Silva DG, de Almeida
EA and Góes RM: Effect of melatonin intake on oxidative stress
biomarkers in male reproductive organs of rats under experimental
diabetes. Oxid Med Cell Longev. 2015:6145792015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yiginer O, Ozcelik F, Inanc T, Aparci M,
Ozmen N, Cingozbay BY, Kardesoglu E, Suleymanoglu S, Sener G and
Cebeci BS: Allopurinol improves endothelial function and reduces
oxidant-inflammatory enzyme of myeloperoxidase in metabolic
syndrome. Clin Res Cardiol. 97:334–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sharma H, Kanwal R, Bhaskaran N and Gupta
S: Plant flavone apigenin binds to nucleic acid bases and reduces
oxidative DNA damage in prostate epithelial cells. PloS One.
9:e915882014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Anantharaju PG, Gowda PC, Vimalambike MG
and Madhunapantula SV: An overview on the role of dietary phenolics
for the treatment of cancers. Nutr J. 15:992016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gueritat J, Lefeuvre-Orfila L, Vincent S,
Cretual A, Ravanat JL, Gratas-Delamarche A, Rannou-Bekono F and
Rebillard A: Exercise training combined with antioxidant
supplementation prevents the antiproliferative activity of their
single treatment in prostate cancer through inhibition of redox
adaptation. Free Radic Biol Med. 77:95–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
McGoldrick CA, Jiang YL, Brannon M,
Krishnan K and Stone WL: In vitro evaluation of novel
N-acetylalaninate prodrugs that selectively induce apoptosis in
prostate cancer cells. BMC Cancer. 14:6752014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kumar DG, Deepa P, Rathi MA, Meenakshi P
and Gopalakrishnan VK: Modulatory effects of Crataeva nurvala bark
against testosterone and N-methyl-N-nitrosourea-induced oxidative
damage in prostate of male albino rats. Pharmacogn Mag. 8:285–291.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ratnayake R, Liu Y, Paul VJ and Luesch H:
Cultivated sea lettuce is a multiorgan protector from oxidative and
inflammatory stress by enhancing the endogenous antioxidant defense
system. Cancer Prev Res (Phila). 6:989–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yonezawa Y, Hada T, Uryu K, Tsuzuki T,
Eitsuka T, Miyazawa T, Murakami-Nakai C, Yoshida H and Mizushina Y:
Inhibitory effect of conjugated eicosapentaenoic acid on mammalian
DNA polymerase and topoisomerase activities and human cancer cell
proliferation. Biochem Pharmacol. 70:453–460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lovegrove C, Ahmed K, Challacombe B, Khan
MS, Poper R and Dasgupta P: Systematic review of prostate cancer
risk and association with consumption of fish and fish-oils:
Analysis of 495,321 participants. Int J Clin Pract. 69:87–105.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Flis A, Suchocki P, Królikowska MA,
Suchocka Z, Remiszewska M, Śliwka L, Książek I, Sitarz K, Sochacka
M, Hoser G, et al: Selenitetriglycerides-Redox-active agents.
Pharmacol Rep. 67:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lippman SM, Klein EA, Goodman PJ, Lucia
MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM,
Hartline JA, et al: Effect of selenium and vitamin E on risk of
prostate cancer and other cancers: The selenium and vitamin e
cancer prevention trial (SELECT). JAMA. 301:39–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Klein EA, Thompson IM Jr, Tangen CM,
Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL,
Gaziano JM, et al: Vitamin E and the risk of prostate cancer: The
selenium and vitamin E cancer prevention trial (SELECT). JAMA.
306:1549–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Richie JP Jr, Das A, Calcagnotto AM, Sinha
R, Neidig W, Liao J, Lengerich EJ, Berg A, Hartman TJ, Ciccarella
A, et al: Comparative effects of two different forms of selenium on
oxidative stress biomarkers in healthy men: A randomized clinical
trial. Cancer Prev Res (Phila). 7:796–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jablonska E, Raimondi S, Gromadzinska J,
Reszka E, Wieczorek E, Krol MB, Smok-Pieniazek A, Nocun M, Stepnik
M, Socha K, et al: DNA damage and oxidative stress response to
selenium yeast in the non-smoking individuals: A short-term
supplementation trial with respect to GPX1 and SEPP1 polymorphism.
Eur J Nutr. 55:2469–2484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Thompson TA and Wilding G: Androgen
antagonist activity by the antioxidant moiety of vitamin E,
2,2,5,7,8-pentamethyl-6-chromanol in human prostate carcinoma
cells. Mol Cancer Ther. 2:797–803. 2003.PubMed/NCBI
|
|
71
|
Kyriakopoulos CE, Heath EI, Eickhoff JC,
Kolesar J, Yayehyirad M, Moll T, Wilding G and Liu G: A multicenter
phase 1/2a dose-escalation study of the antioxidant moiety of
vitamin E 2,2,5,7,8-pentamethyl-6-chromanol (APC-100) in men with
advanced prostate cancer. Invest New Drugs. 34:225–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Giovannucci E, Ascherio A, Rimm EB,
Stampfer MJ, Colditz GA and Willett WC: Intake of carotenoids and
retinol in relation to risk of prostate cancer. J Natl Cancer Inst.
87:1767–1776. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Albanes D, Heinonen OP, Huttunen JK,
Taylor PR, Virtamo J, Edwards BK, Haapakoski J, Rautalahti M,
Hartman AM, Palmgren J, et al: Effects of alpha-tocopherol and
beta-carotene supplements on cancer incidence in the
alpha-tocopherol beta-carotene cancer prevention study. Am J Clin
Nutr. 62 6 Suppl:1427S–1430S. 1995.PubMed/NCBI
|
|
74
|
Norrish AE, Jackson RT, Sharpe SJ and
Skeaff CM: Prostate cancer and dietary carotenoids. Am J Epidemiol.
151:119–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kolonel LN, Nomura AM and Cooney RV:
Dietary fat and prostate cancer: Current status. J Natl Cancer
Inst. 91:414–428. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bemis DL, Capodice JL, Anastasiadis AG,
Katz AE and Buttyan R: Zyflamend, a unique herbal preparation with
nonselective COX inhibitory activity, induces apoptosis of prostate
cancer cells that lack COX-2 expression. Nutr Cancer. 52:202–212.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gu Z, Suburu J, Chen H and Chen YQ:
Mechanisms of Omega-3 polyunsaturated fatty acids in prostate
cancer prevention. Biomed Res Int. 2013:8245632013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brown MD, Hart CA, Gazi E, Bagley S and
Clarke NW: Promotion of prostatic metastatic migration towards
human bone marrow stoma by Omega 6 and its inhibition by Omega 3
PUFAs. Br J Cancer. 94:842–853. 2006. View Article : Google Scholar : PubMed/NCBI
|