Introduction
Primary gastrointestinal (GI) follicular lymphoma
(FL) is a rare malignancy accounting for only 1–3.6% of primary
non-Hodgkin lymphomas of the GI tract (1). GI-FL is most commonly found within the
second portion of the duodenum, but may also involve any part of
the small intestine, colon, rectum and stomach (2,3). GI-FL
is a relatively new clinical entity and is classified as a distinct
variant of systemic FL with a favorable clinical course (4). As several treatment strategies have
been proposed, the standard therapy remains to be determined. In
the largest case series of GI-FL, rituximab monotherapy was
successfully used in 5 patients, resulting in long-term complete
remission or stable disease for a median follow-up of 36 months
(5). We herein report the case of a
51-year-old female patient with duodenal GI-FL who experienced
complete remission after treatment with single-agent rituximab
followed by nodal relapse 5 years after treatment completion. The
aim of the present case was to add to the limited available data on
long-term outcomes of GI-FL and the long-term efficacy of rituximab
during initial treatment and relapse.
Case report
A 51-year-old female patient with a past medical
history significant for hypertension presented with history of
dyspepsia over several months, despite proton pump inhibitor
therapy. The patient reported no fevers, weight loss, decreased
appetite, melena or night sweats. Physical examination revealed no
abnormalities such as lymphadenopathy, hepatomegaly or
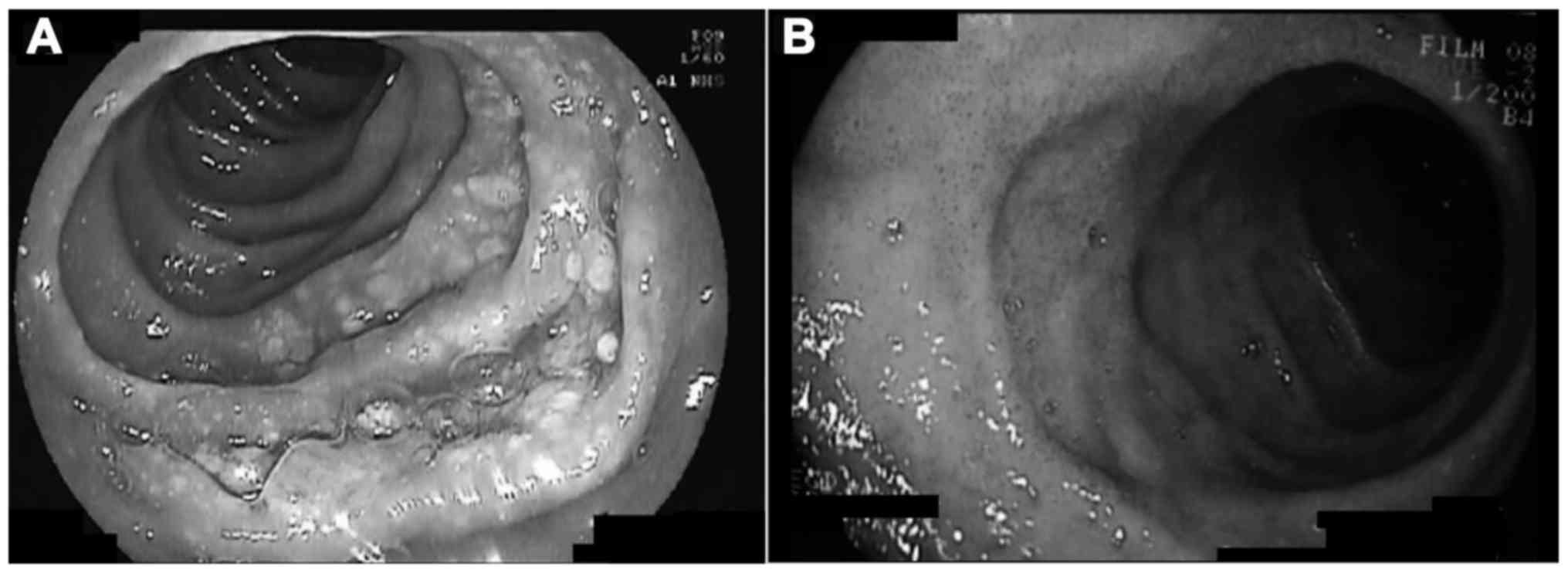
splenomegaly. Esophagogastroduodenoscopy (EGD) revealed irregular
patchy thickening of the mucosa along the second portion of the
duodenum with multiple small white nodular formations (Fig. 1A). Biopsy revealed atypical lymphoid
cells with immunostaining positive for CD10, CD20 and B-cell
lymphoma 2 (BCL2), consistent with FL. Furthermore, IgH/BCL2
rearrangement was detected on polymerase chain reaction analysis.
The diagnosis of GI-FL was confirmed. According to the World Health
Organization classification, the pathological grade was 1 (6). Complete staging work-up was performed,
including colonoscopy with ileoscopy, computed tomography (CT) scan
of the neck, chest, abdomen and pelvis, bone marrow aspirate and
biopsy, and positron emission tomography (PET) scan, and the
findings were unremarkable. The tumor was stage I according to the
Lugano staging classification (7).
The Follicular Lymphoma International Prognostic Index 2 (FLIPI2)
was 0, corresponding to a 3-year survival rate of 99% (8). The patient was started on single-agent
rituximab (375 mg/m2 IV per week for a total of 4
weeks). A repeat EGD 3 months after treatment initiation revealed
complete resolution of the duodenal lesions (Fig. 1B), and biopsies confirmed normal
histology. The patient remained in complete remission on further
follow-up studies consisting of yearly endoscopic evaluation of the
GI tract and CT scans of the chest, abdomen and pelvis. After 62
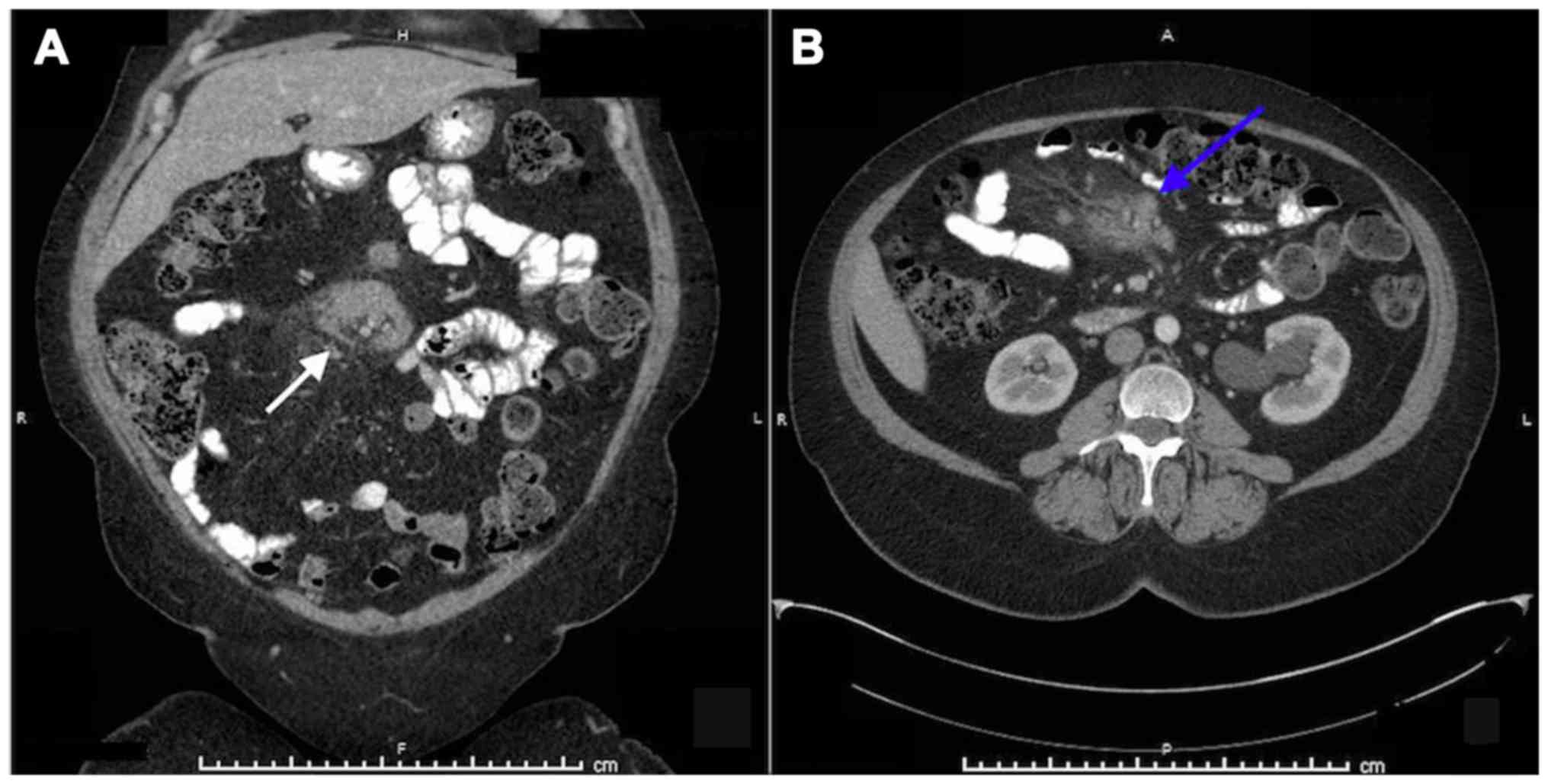
months, a follow-up CT scan of the abdomen reported several
mesenteric lymphadenopathies, with the largest lymph nodes
measuring 5.4×1.7 and 5.1×2.1 cm (Fig.
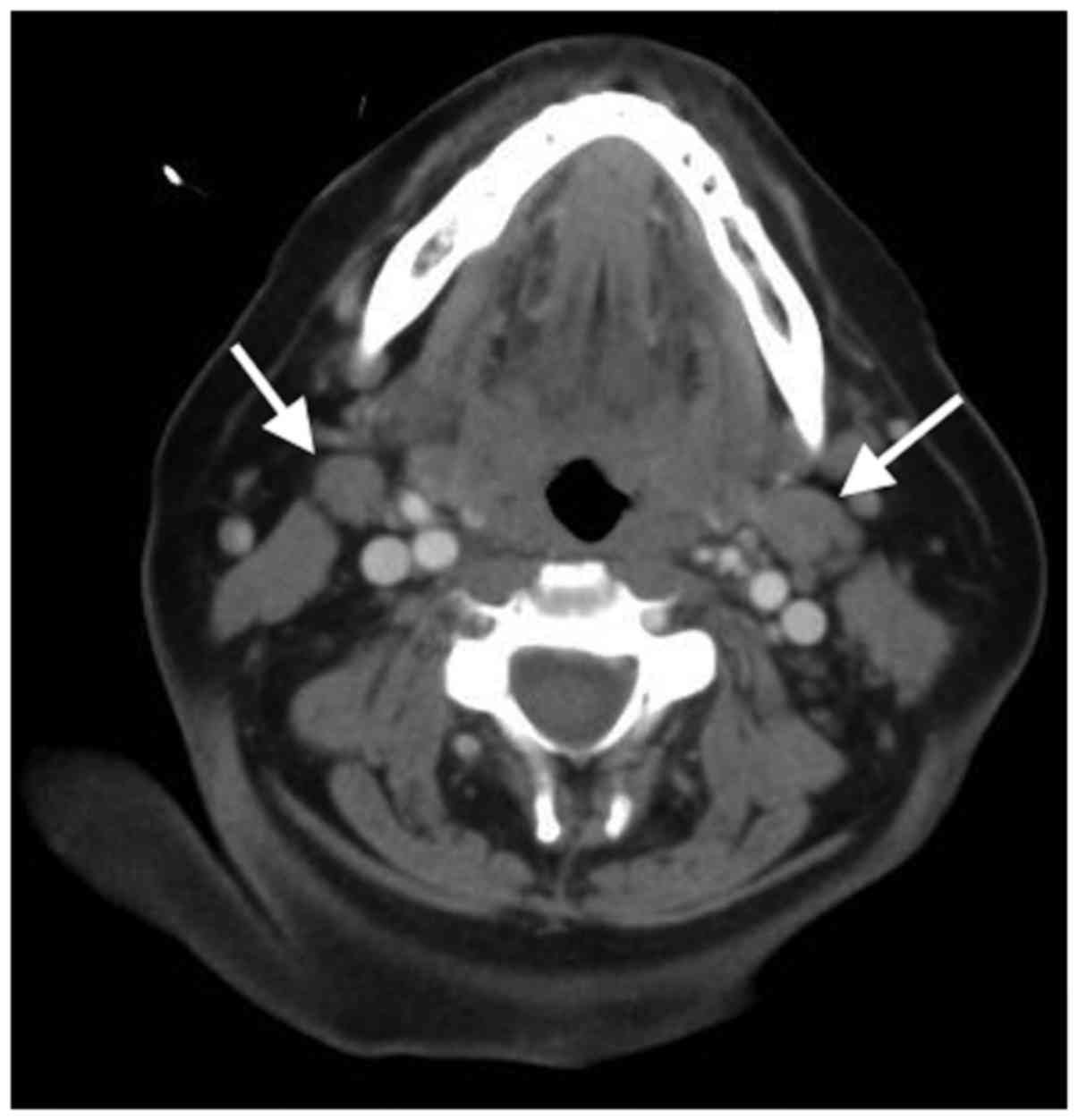
2). A CT of the neck also revealed multiple enlarged cervical
lymph nodes bilaterally (Fig. 3).
EGD and colonoscopy with biopsies of the duodenal mucosa were
normal. PET scanning revealed no additional organ involvement.
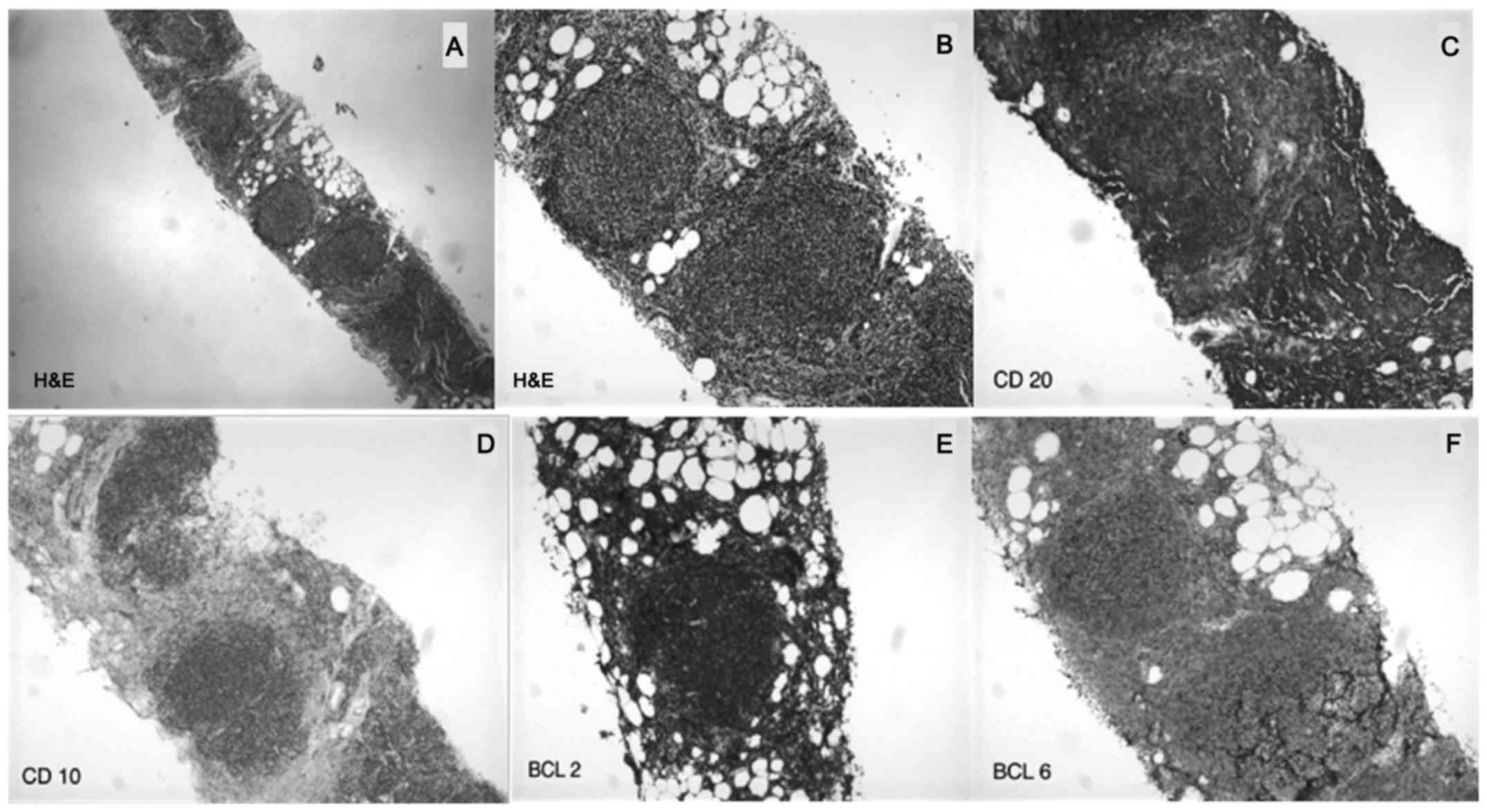
Biopsy of an enlarged mesenteric lymph node demonstrated recurrent
FL (Fig. 4). The patient's FLIPI2
score on recurrence remained 0, given the absence of bone marrow
involvement, normal hemoglobin level, largest involved node sized
<6 cm and a normal serum β-2 microglobulin level. The patient
was restarted on the same course of rituximab monotherapy as
previously described. A follow-up CT scan 2 months after treatment
demonstrated complete resolution of the cervical and mesenteric
lymphadenopathies. No recurrence has been detected up to 3 years
after completion of the second course of rituximab monotherapy
(last follow-up, April 2017).
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Discussion
FL, defined as a neoplasm of follicular center B
cells, is a frequent indolent lymphoma most commonly of nodal
origin (4). Despite the majority of
the patients being diagnosed at stage III or IV, the median
survival is 8–12 years, leading to the designation of FL as an
indolent malignancy (9). Extranodal
tissue involvement usually occurs in the setting of disseminated
nodal disease. The majority of GI non-Hodgkin lymphomas are either
mucosa-associated lymphoid tissue lymphomas or diffuse large B-cell
lymphomas (2). GI-FL is a relatively
new clinical entity first reported in 1997 (10); since then, it has been reported with
increasing frequency and was recently classified as a distinct
variant of systemic FL (4).
Therefore, data regarding long-term follow-up are scarce. While the
majority of patients with nodal FL have systemic disease on
presentation, GI-FL rarely disseminates outside of the GI tract, as
93% of cases are stage I or II (11). Other distinguishing characteristics
include the absence of marginal zone or plasmacytic differentiation
and a higher frequency of grade 1 histological grading in GI-FL
(11).
Endoscopically, duodenal FL presents with multiple
white granules, as shown in the present case (2). Given the potential multifocal
involvement of the GI tract, endoscopic evaluation throughout the
whole GI tract is recommended. As in nodal FL, the tumor cells are
positive for pan B-cell antigens and negative for CD5 and cyclin D1
(12).
Several treatment options for GI-FL have been
proposed; however, standard therapy remains to be determined. The
currently available options include watchful waiting, radiotherapy,
single-agent rituximab, chemotherapy with cyclophosphamide,
doxorubicin, vincristine and prednisone (CHOP), rituximab plus
CHOP, and radiotherapy plus rituximab (5). However, there are limited data on the
effectiveness of each treatment strategy in GI-FL. In general, only
~6% of all patients with GI-FL relapse after initial treatment. It
is noteworthy, however, that 50% of recurrences relapse outside of
the GI tract (11). Hence, follow-up
evaluation after remission should include whole-body evaluation
with PET scan or gallium scintigram. A recent analysis of the
Surveillance, Epidemiology, and End Results database in the United
States revealed that small intestinal involvement is an independent
predictor of longer survival in GI-FL (13).
Optimal management of GI-FL has not been
established. Damaj et al (14) reported no significant difference in
the prognosis between patients with and those without treatment.
Therefore, the watchful waiting approach has been adopted in
several asymptomatic cases (15,16).
Given that our patient reported dyspepsia, it was decided to
proceed with therapy rather than the watchful waiting approach.
After the introduction of rituximab-containing regimens, several
randomized controlled trials reported improved complete response
rate, duration of the response and overall survival in nodal FL
(17–19).
In the largest case series of GI-FL published to
date, 5 of 63 patients were treated with rituximab monotherapy.
Complete regression occurred in 4 patients, and the fifth patient
exhibited stable disease at 118 months of follow-up. However,
follow-up was limited to a median of 36 months in this treatment
group and the long-term efficacy of rituximab could not be
determined (5). A review of the
literature by Yamamoto et al in 2010 reported 8 patients
treated with rituximab monotherapy as having partial or complete
response with no relapse (11).
Although prospective studies must be conducted to determine the
usefulness of rituximab monotherapy for GI-FL, the previous results
indicate that rituximab monotherapy may be an effective therapeutic
option. Furthermore, data regarding the optimal dose and duration
of rituximab therapy are scarce, as most of the data are
extrapolated from standard therapeutic regimens for nodal FL.
Several studies demonstrated the long-term efficacy of rituximab
monotherapy in nodal FL. Colombat et al reported that 80% of
patients with low tumor burden FL responded to rituximab
monotherapy and 48% achieved complete remission after once-weekly
rituximab infusions for a total of 4 doses. Regarding long-term
prognosis, the progression-free survival was 23.5 months and the
overall survival rate was 91.7% (20). An analysis of the National LymphoCare
Database of stage I FL revealed that diverse treatment approaches,
including rituximab monotherapy, radiation therapy, or a
combination of radiation therapy and chemotherapy, resulted in
similar excellent outcomes (21). In
general, there appears to be no difference between rituximab and
other conventional therapies for GI-FL, possibly due to the
favorable prognosis of the disease. In addition, irradiation of the
small bowel is associated with chronic radiation enteritis, leading
to malabsorption and diarrhea. Therefore, given the potential
toxicity of radiation therapy, the effectiveness of rituximab
monotherapy, and the indolent nature of GI-FL, single-agent
rituximab was selected in this case.
In the present case, single-agent rituximab was used
as intravenous infusion once weekly for a total of 4 doses,
resulting in complete remission for 62 months. Re-treatment with
rituximab was proven to be safe and effective in nodal FL during
relapse after initial rituximab exposure (22). Therefore, the same treatment regimen
was used during nodal relapse, leading to stable disease up to 3
years after completion of therapy. The efficacy of this regimen in
nodal FL was demonstrated in the RESORT trial. In low tumor burden
nodal FL, a re-treatment strategy with rituximab 375
mg/m2 for 4 weekly doses upon relapse provides disease
control comparable to a rituximab maintenance strategy (23). The present case demonstrated the
potential benefit of this protocol in GI-FL during initial
treatment and nodal relapse.
In conclusion, despite being considered a rare
entity, the reported cases of GI-FL are increasing as is our
understanding of its diagnosis, prognosis and treatment. However, a
consensus regarding the management of this disease has not been
established. GI-FL appears to have a good prognosis, but little is
known on the effect of rituximab on the clinical course of GI-FL.
The majority of the reported cases responded to rituximab, yet
follow-up was limited. The present case demonstrated that rituximab
monotherapy is effective in the initial treatment of GI-FL, as well
as during systemic relapse. However, further studies are required
to determine the optimal treatment regimen.
References
|
1
|
Anderson JR, Armitage JO and Weisenburger
DD: Epidemiology of the non-Hodgkin's lymphomas: Distributions of
the major subtypes differ by geographic locations. Non-Hodgkin's
Lymphoma Classification Project. Ann Oncol. 9:717–720. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takata K, Okada H, Ohmiya N, Nakamura S,
Kitadai Y, Tari A, Akamatsu T, Kawai H, Tanaka S, Araki H, et al:
Primary gastrointestinal follicular lymphoma involving the duodenal
second portion is a distinct entity: A multicenter, retrospective
analysis in Japan. Cancer Sci. 102:1532–1536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sentani K, Maeshima AM, Nomoto J, Maruyama
D, Kim SW, Watanabe T, Kobayashi Y, Tobinai K and Matsuno Y:
Follicular lymphoma of the duodenum: A clinicopathologic analysis
of 26 cases. Jpn J Clin Oncol. 38:547–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmatz A-I, Streubel B, Kretschmer-Chott
E, Püspök A, Jäger U, Mannhalter C, Tiemann M, Ott G, Fischbach W,
Herzog P, et al: Primary follicular lymphoma of the duodenum is a
distinct mucosal/submucosal variant of follicular lymphoma: A
retrospective study of 63 cases. J Clin Oncol. 29:1445–1451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakić-Razumović J and Aurer I: The World
Health Organization classification of lymphomas. Croat Med J.
43:527–534. 2002.PubMed/NCBI
|
|
7
|
Rohatiner A, d'Amore F, Coiffier B,
Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem
P, Shipp M, et al: Report on a workshop convened to discuss the
pathological and staging classifications of gastrointestinal tract
lymphoma. Ann Oncol. 5:397–400. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Federico M, Bellei M, Marcheselli L,
Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni
A, Soubeyran P, et al: Follicular lymphoma international prognostic
index 2: A new prognostic index for follicular lymphoma developed
by the international follicular lymphoma prognostic factor project.
J Clin Oncol. 27:4555–4562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Armitage JO and Weisenburger DD: New
approach to classifying non-Hodgkin's lymphomas: Clinical features
of the major histologic subtypes. Non-Hodgkin's Lymphoma
Classification Project. J Clin Oncol. 16:2780–2795. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Misdraji J, del Castillo Fernandez C and
Ferry JA: Follicle center lymphoma of the ampulla of Vater
presenting with jaundice: Report of a case. Am J Surg Pathol.
21:484–488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto S, Nakase H, Yamashita K,
Matsuura M, Takada M, Kawanami C and Chiba T: Gastrointestinal
follicular lymphoma: Review of the literature. J Gastroenterol.
45:370–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bende RJ, Smit LA, Bossenbroek JG, Aarts
WM, Spaargaren M, de Leval L, Boeckxstaens GE, Pals ST and van
Noesel CJ: Primary follicular lymphoma of the small intestine:
alpha4beta7 expression and immunoglobulin configuration suggest an
origin from local antigen-experienced B cells. Am J Pathol.
162:105–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chouhan J, Batra S, Gupta R and Guha S:
Gastrointestinal follicular lymphoma: Using primary site as a
predictor of survival. Cancer Med. 5:2669–2677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Damaj G, Verkarre V, Delmer A,
Solal-Celigny P, Yakoub-Agha I, Cellier C, Maurschhauser F,
Bouabdallah R, Leblond V, Lefrère F, et al: Primary follicular
lymphoma of the gastrointestinal tract: A study of 25 cases and a
literature review. Ann Oncol. 14:623–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kodama M, Kitadai Y, Shishido T, Shimamoto
M, Fukumoto A, Masuda H, Tanaka S, Yoshihara M, Sakai A, Nakayama H
and Chayama K: Primary follicular lymphoma of the gastrointestinal
tract: A retrospective case series. Endoscopy. 40:343–346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shia J, Teruya-Feldstein J, Pan D, Hegde
A, Klimstra DS, Chaganti RS, Qin J, Portlock CS and Filippa DA:
Primary follicular lymphoma of the gastrointestinal tract: A
clinical and pathologic study of 26 cases. Am J Surg Pathol.
26:216–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marcus R, Imrie K, Belch A, Cunningham D,
Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo
J, et al: CVP chemotherapy plus rituximab compared with CVP as
first-line treatment for advanced follicular lymphoma. Blood.
105:1417–1423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiddemann W, Kneba M, Dreyling M, Schmitz
N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H,
Hegewisch-Becker S, et al: Frontline therapy with rituximab added
to the combination of cyclophosphamide, doxorubicin, vincristine
and prednisone (CHOP) significantly improves the outcome for
patients with advanced-stage follicular lymphoma compared with
therapy with CHOP alone: Results of a prospective randomized study
of the German Low-Grade Lymphoma Study Group. Blood. 106:3725–3732.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herold M, Haas A, Srock S, Neser S, Al-Ali
KH, Neubauer A, Dölken G, Naumann R, Knauf W, Freund M, et al:
Rituximab added to first-line mitoxantrone, chlorambucil, and
prednisolone chemotherapy followed by interferon maintenance
prolongs survival in patients with advanced follicular lymphoma: An
east german study group hematology and oncology study. J Clin
Oncol. 25:1986–1992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colombat P, Brousse N, Salles G,
Morschhauser F, Brice P, Soubeyran P, Delwail V, Deconinck E,
Haioun C, Foussard C, et al: Rituximab induction immunotherapy for
first-line low-tumor-burden follicular lymphoma: Survival analyses
with 7-year follow-up. Ann Oncol. 23:2380–2385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Friedberg JW, Byrtek M, Link BK, Flowers
C, Taylor M, Hainsworth J, Cerhan JR, Zelenetz AD, Hirata J and
Miller TP: Effectiveness of first-line management strategies for
stage I follicular lymphoma: Analysis of the National LymphoCare
Study. J Clin Oncol. 30:3368–3375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis TA, Grillo-López AJ, White CA,
McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL,
Rosenberg J and Levy R: Rituximab anti-CD20 monoclonal antibody
therapy in non-Hodgkin's lymphoma: Safety and efficacy of
re-treatment. J Clin Oncol. 18:3135–3143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kahl BS, Hong F, Williams ME, Gascoyne RD,
Wagner LI, Krauss JC, Habermann TM, Swinnen LJ, Schuster SJ,
Peterson CG, et al: Rituximab extended schedule or re-treatment
trial for low-tumor burden follicular lymphoma: Eastern cooperative
oncology group protocol e4402. J Clin Oncol. 32:3096–3102. 2014.
View Article : Google Scholar : PubMed/NCBI
|