Introduction
Oesophagogastric adenocarcinoma (OAC), including
adenocarcinoma of the gastro-oesophageal junction and stomach, is a
major health concern, particularly in elderly patients (1). A recent study from the USA reported
that approximately two-thirds of OACs were diagnosed at an advanced
stage, with regional lymph node invasion or distant metastases
(2). In cases with advanced T stage
or regional lymph node involvement (T3/4 or N+), without
evidence of distant metastases, surgical resection with D2 lymph
node dissection is indicated. The MAGIC trial in 2006 first
demonstrated an improvement in 5-year survival from 23 to 36% in
patients with resectable stage II and III OAC treated with six
cycles of perioperative chemotherapy with 5-fluorouracil (5-FU),
cisplatin and epirubicin (ECF regimen) compared with surgery alone,
establishing perioperative cytostatic treatment as the new standard
of care (3).
Since then, it has been demonstrated that epirubicin
does not confer any additional benefit in terms of overall survival
(OS) in patients undergoing preoperative chemotherapy for OAC
(4). The European Society for
Medical Oncology guidelines currently state that ‘it may be
reasonable to use any fluoropyrimidine-platinum doublet or triplet’
(5). The treatment suggested herein,
although not explicitly mentioned, includes cisplatin and S-1
(Cis/S-1).
The fluoropyrimidine S-1 contains tegafur (an
inactive 5-FU prodrug) and the two enzyme inhibitors gimeracil and
oteracil. These components improve the efficacy and safety of the
cytostatic agent (6) namely tegafur,
gimeracil, and oteracil. In Europe, S-1 has been approved in
combination with cisplatin for the palliative treatment of advanced
OAC. Furthermore, in Japan, S-1 monotherapy represents the standard
of care in the adjuvant setting following OAC resection (7). In the FLAGS trial, including 1,053
patients with metastatic OAC, Cis/S-1 did not prolong OS, but
exhibited a significantly improved safety profile compared with
cisplatin/infusional 5-FU (8,9). The
same favourable side effect profile of Cis/S-1 may also be expected
in the perioperative setting. Cis/S-1 has been proven to be
feasible and effective for the perioperative therapy of Asian OAC
patients, but experience with perioperative Cis/S-1 in Caucasian
OAC patients has not been reported thus far (10,11).
Case reports
Case 1
A 75-year-old male patient with an Eastern
Cooperative Oncology Group (ECOG) performance status score of 1
presented at the Department of Gastroenterology, Hepatology and
Infectious Diseases (Otto-von-Guericke University Hospital,
Magdeburg, Germany) in July 2016 with appetite loss and
postprandial pain in the upper abdomen. Ambulatory
oesophagogastroduodenoscopy (OGD) revealed a tumour at the
gastro-oesophageal junction (Siewert type III). Repeated endoscopy
with biopsy at our department confirmed the clinical suspicion.
Histological examination revealed intestinal type adenocarcinoma
according to the Laurén classification (12). The human epidermal growth factor
receptor 2 (HER2) status was negative. There was no evidence of
Helicobacter pylori infection. Staging computed tomography (CT)
scan and endosonography revealed stage III disease (uT4uN3cM0)
based on the 7th edition of the American Joint Committee on Cancer
Staging Manual (2010) (13).
Perioperative treatment with Cis/S-1 was initiated. Two
preoperative 4-week cycles of intravenous cisplatin 75
mg/m2 on day 1 and oral S-1 25 mg/m2 twice
daily on days 1–21 were administered. Apart from mild
thrombocytopenia [grade I according to the Common Terminology
Criteria for Adverse Events (CTCAE) v4.0 (14)] and a mild exanthema of the chest
region (CTCAE v4.0 grade II), no further adverse events were
observed. There was no treatment delay. The body weight remained
stable during the entire course of the cytostatic treatment, and no
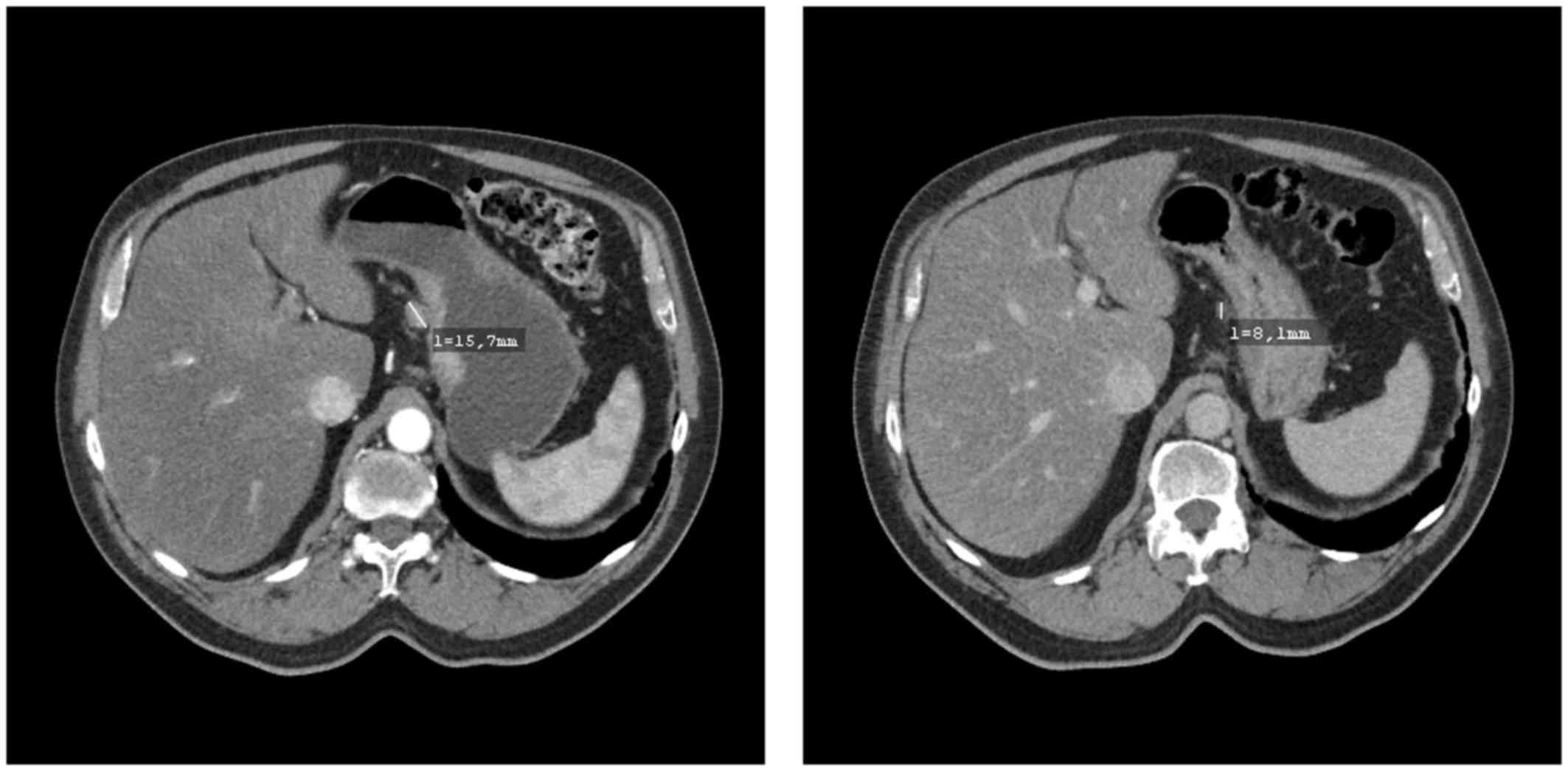
deterioration of the ECOG score was observed. A preoperative CT
scan revealed partial remission of the perigastric lymph node
metastases and excluded distant metastases (Fig. 1). In early December 2016, radical
gastrectomy with D2 lymph node dissection was performed. No
complications occurred after surgery and the patient was discharged
on the 11th postoperative day. The histopathological assessment of
the resected tissue confirmed complete resection (R0) and proved
partial regression (grade II according to Becker et al) with
20% residual tumour cells (15).
After surgery, a further two cycles of cisplatin/S-1 were
administered uneventfully. The last postoperative follow-up (June
2017) revealed no signs of tumour recurrence.
Case 2
A 71-year-old male patient in good general condition
(ECOG performance status score 1) developed weakness and
non-specific thoracic pain. The past medical history was remarkable
for coronary heart disease, and the patient had already received
two coronary stents. A recent percutaneous coronary angiography
excluded significant stenoses. The laboratory tests revealed mild
iron deficiency anaemia. The next diagnostic step was an OGD with
biopsies, revealing adenocarcinoma of intestinal type (according to
the Laurén classification) in the gastric antrum. HER2 status and
Helicobacter pylori serology were negative. Staging CT scan and
endosonography revealed locoregional disease without distant
metastases (uT3uN1cM0). Perioperative cytostatic therapy with two
4-week cycles of Cis/S-1 was administered. The side effects
included dysgeusia, appetite loss and mild recurring episodes of
vomiting (CTCAE v4.0 grade II), which were successfully controlled
with antiemetics. During the treatment, a weight loss of 5 kg
(CTCAE v4.0 grade I) was observed. Therefore, dietary
supplementation with high-calorie sip feed nutrition products was
prescribed. In addition, a clinically non-relevant thrombocytopenia
was observed (CTCAE v4.0 grade I). No treatment delay was deemed
necessary. During neoadjuvant therapy, no deterioration of the ECOG
score was observed. A preoperative CT scan revealed considerable
shrinking of both the primary cancer and the regional lymph node
metastases (partial response).
In December 2016, a minimally invasive gastrectomy
with D2 lymphadenectomy was performed uneventfully. The
postoperative course was uncomplicated and the patient was
discharged on the 13th postoperative day. Histopathological
examination revealed negative resection margins (R0). The
histological tumour regression grade according to Becker et
al was 3 (15). Between February
and March 2017, two cycles of adjuvant cisplatin/S-1 were
administered; however, a dose reduction was required (cisplatin 60
mg/m2, S-1 20 mg/m2 twice daily) due to
nausea (CTCAE v4.0 grade III). The last follow-up CT scan (July
2017) revealed no signs of tumour recurrence.
Discussion
We herein report the first two cases of Caucasian
OAC patients receiving neoadjuvant Cis/S-1. Preoperative Cis/S-1
has been already investigated in Japanese OAC patients (10,11).
However, the efficacy and side effect profile of S-1 is different
between Asian and Caucasian subjects due to the differences in
metabolism (16). Thus, the results
of those studies may not be transferable to Caucasian patients. In
both reported cases, no serious adverse events (CTCAE v4.0 grade
III/IV) were observed preoperatively, and no therapy delay or dose
reduction was required. Both patients were in a good preoperative
condition and the staging revealed considerable tumour shrinkage.
Surgery was performed without any complications, and tumour
resection with negative margins (R0) was histologically confirmed
in both cases.
The only chance for cure of non-metastatic OAC is
complete resection. Perioperative chemotherapy improves OS in OAC
patients with locoregional disease (3,5,17,18).
However, as gastrectomy and particularly oesophagectomy are
high-risk procedures, the patient's preoperative general condition
is crucial for the success of the interdisciplinary therapy
approach. This is relevant, as OAC mostly occurs in elderly
patients, and chronological age is a marker for increased physical
frailty.
Whether elderly OAC patients with locoregional
disease should receive perioperative triplet or a doublet
chemotherapy has been investigated in recent trials. In a subgroup
analysis of the FLOT65+ trial [5-FU, leucovorin and oxaliplatin
with (FLOT) or without (FLO) docetaxel], the FLOT group exhibited
increased chemotherapy-related toxicity and deterioration of
quality of life global health status scores during the first 8
weeks of treatment compared with the FLO group (19). Another randomized study, which
compared the triplet epirubicin, cisplatin and capecitabine (ECX)
with the doublet CX (i.e., without epirubicin), yielded comparable
efficacy results for both regimens (20). It should be noted that, in that
trial, no patients in the CX and 12% of the patients in the ECX arm
discontinued treatment due to toxicity. In summary, due to its
inferior safety profile and potential deterioration of the
preoperative general condition, neoadjuvant triplet chemotherapy
should be discouraged in elderly OAC patients.
Although a platinum/fluoropyrimidine doublet regimen
represents the standard of care in the perioperative therapy of OAC
patients with locoregional disease, it is debatable whether
perioperative regimens should be cisplatin- or oxaliplatin-based.
Furthermore, no studies comparing perioperative Cis/S-1 and FLO are
available. However, cisplatin and oxaliplatin have been compared as
first-line treatment of advanced oesophagogastric cancer (21–23). In
a German study comparing FLO to infusional 5-FU plus cisplatin (FLP
regimen), oxaliplatin was safer with respect to haematological and
non-haematological toxicity (i.e. nausea, vomiting and renal
toxicity), but was associated with a significantly higher rate of
peripheral polyneuropathy (22). In
a recent phase III study comparing S-1/oxaliplatin (SOX regimen)
and Cis/S-1 in Japanese OAC patients, similar results were obtained
with respect to the safety issues, whereas no significant
difference in terms of progression-free survival (PFS) and OS were
observed between the two regimens (24,25).
Haematological toxicity is reversible, nausea and vomiting are
preventable, whereas renal toxicity can be monitored. On the
contrary, oxaliplatin-induced polyneuropathy is frequently
irreversible and may even worsen after withdrawal of the drug,
consistently compromising the quality of life in OAC survivors
(26). In our experience,
oxaliplatin-induced peripheral polyneuropathy occurs early in the
adjuvant (postoperative) phase of perioperative treatment, leading
to withdrawal of the drug. In view of the long-term neurotoxic
sequelae of oxaliplatin and the lack of effective treatment options
for this side effect, cisplatin-based chemotherapy may be preferred
in the perioperative setting.
The fluoropyrimidine S-1 has shown favourable safety
and efficacy data as palliative treatment of OAC. The FLAGS trial
demonstrated significant improvements of tolerability due to the
treatment with Cis/S-1 compared with Cis/5-FU, whereas OS and PFS
did not differ significantly (8,27). At
least one treatment-related serious adverse event (CTCAE grade
>II) was observed in 29.7% in the Cis/5-FU arm compared with
20.5% in the Cis/S-1 arm. Treatment-related deaths were also
significantly more common in the Cis/5-FU group (4.9 vs. 2.5%). In
addition to the favourable side effect profile, other positive
aspects of the Cis/S1 therapy should be highlighted. Due to the
4-week cycles, only one cisplatin infusion per month is necessary.
This may result in i) improved quality of life, ii) reduced
frequency of visits to the oncology department, and iii) reduced
disease perception. The gained time may be invested in physical
exercise and other coping strategies for a further improvement of
the outcome, provided the patient's compliance is ensured.
In our experience, neoadjuvant and possibly
perioperative Cis/S-1 represents a feasible, effective and
well-tolerated treatment option for elderly Caucasian OAC patients
with locoregional disease.
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1964 and later versions. Informed consent or
substitute for it was obtained from both patients.
Acknowledgements
Dr Marino Venerito received honoraria from Merck
Serono and Bayer Vital and is a member of the of advisory boards of
Amgen, Lilly and Nordic Pharma.
References
|
1
|
Robert Koch Institute the Association of
Population-based Cancer Registries in Germany: Cancer in Germany
2009/2010. 2014.
|
|
2
|
Jin H, Pinheiro PS, Callahan KE and
Altekruse SF: Examining the gastric cancer survival gap between
Asians and whites in the United States. Gastric Cancer. 20:1–10.
2016.PubMed/NCBI
|
|
3
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: MAGIC Trial Participants: Perioperative
chemotherapy versus surgery alone for resectable gastroesophageal
cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alderson D, Langley RE, Nankivell MG,
Blazeby JM, Griffi M, Crellin A and Cunningham D: Neoadjuvant
chemotherapy for resectable oesophageal and junctional
adenocarcinoma: results from the UK Medical Research Council
randomised OEO5 trial (ISRCTN 01852072). J Clin Oncol. 33
suppl:abstr 4002. 2015.
|
|
5
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: ESMO Guidelines Committee: Gastric
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 suppl 5:v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayakawa M and Kojima Y:
Tegafur/gimeracil/oteracil (S-1) approved for the treatment of
advanced gastric cancer in adults when given in combination with
cisplatin: A review comparing it with other fluoropyrimidine-based
therapies. Onco Targets Ther. 4:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ajani JA, Rodriguez W, Bodoky G,
Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A,
Lang I and Falcon S: Multicenter phase III comparison of
cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced
gastric or gastroesophageal adenocarcinoma study: The FLAGS trial.
J Clin Oncol. 28:1547–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahlberg R, Lorenzen S, Thuss-Patience P,
Heinemann V, Pfeiffer P and Möhler M: New Perspectives in the
Treatment of Advanced Gastric Cancer: S-1 as a Novel Oral 5-FU
Therapy in Combination with Cisplatin. Chemotherapy. 62:62–70.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunisaki C, Makino H, Kimura J, Takagawa
R, Kanazawa A, Ota M, Kosaka T, Ono HA, Akiyama H and Endo I:
Impact of S-1 plus cisplatin neoadjuvant chemotherapy on scirrhous
gastric cancer. Oncology. 88:281–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okabe H, Hata H, Ueda S, Zaima M, Tokuka
A, Yoshimura T, Ota S, Kinjo Y, Yoshimura K and Sakai Y: Kyoto
University Surgical Oncology Group (KUSOG): A phase II study of
neoadjuvant chemotherapy with S-1 and cisplatin for stage III
gastric cancer: KUGC03. J Surg Oncol. 113:36–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; NY: 2010
|
|
14
|
Common Terminology Criteria for Adverse
Events (CTCAE). Version 4.0, published May. 2009.https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfApril
23–2017
|
|
15
|
Becker K, Mueller JD, Schulmacher C, Ott
K, Fink U, Busch R, Böttcher K, Siewert JR and Höfler H:
Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuah B, Goh BC, Lee SC, Soong R, Lau F,
Mulay M, Dinolfo M, Lim SE, Soo R, Furuie T, et al: Comparison of
the pharmacokinetics and pharmacodynamics of S-1 between Caucasian
and East Asian patients. Cancer Sci. 102:478–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric Cancer, Version 3.2016, NCCN Clinical Practice
Guidelines in Oncology. J Natl Compr Canc Netw. 14:1286–1312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moehler M, Al-Batran SE, Andus T, Anthuber
M, Arends J, Arnold D, Aust D, Baier P, Baretton G, Bernhardt J, et
al: AWMF: German S3-guideline ‘Diagnosis and treatment of
esophagogastric cancer’. Z Gastroenterol. 49:461–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorenzen S, Pauligk C, Homann N,
Schmalenberg H, Jäger E and Al-Batran S-E: Feasibility of
perioperative chemotherapy with infusional 5-FU, leucovorin, and
oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly
patients with locally advanced esophagogastric cancer. Br J Cancer.
108:519–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun J, Lee J, Park SH, Park JO, Park YS,
Lim HY and Kang WK: A randomised phase II study of combination
chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or
cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J
Cancer. 46:885–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Batran S-E, Hartmann JT, Probst S,
Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G,
Homann N, Wilhelm G, et al: Arbeitsgemeinschaft Internistische
Onkologie: Phase III trial in metastatic gastroesophageal
adenocarcinoma with fluorouracil, leucovorin plus either
oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft
Internistische Onkologie. J Clin Oncol. 26:1435–1442. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naïve patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bando H, Yamada Y, Tanabe S, Nishikawa K,
Gotoh M, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Efficacy and safety of S-1 and oxaliplatin combination therapy in
elderly patients with advanced gastric cancer. Gastric Cancer.
19:919–926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa K, Yamada Y, Ishido K, et al:
Impact of progression type on overall survival in patients with
advanced gastric cancer based on randomized phase III study of S-1
plus oxaliplatin versus S-1 plus cisplatin. Gastric Cancer.
2016.
|
|
26
|
Burakgazi AZ, Messersmith W, Vaidya D,
Hauer P, Hoke A and Polydefkis M: Longitudinal assessment of
oxaliplatin-induced neuropathy. Neurology. 77:980–986. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ajani JA, Buyse M, Lichinitser M,
Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha
R, Carrato A, et al: Combination of cisplatin/S-1 in the treatment
of patients with advanced gastric or gastroesophageal
adenocarcinoma: Results of noninferiority and safety analyses
compared with cisplatin/5-fluorouracil in the First-Line Advanced
Gastric Cancer Study. Eur J Cancer. 49:3616–3624. 2013. View Article : Google Scholar : PubMed/NCBI
|