Introduction
Gastric cancer is one of the leading causes of
cancer-related mortality worldwide. Despite a decrease in its
incidence in some regions of the world, gastric cancer remains a
major clinical challenge as the majority of cases are diagnosed in
an advanced stage, with a poor prognosis and limited treatment
options (1). Oxaliplatin, a
platinum-based antineoplastic agent, is used as an essential part
of chemotherapeutic regimens for colorectal liver metastasis
(2). Oxaliplatin-based regimens have
been associated with the development of injury to the hepatic
parenchyma in the form of hepatic sinusoidal obstruction syndrome
(HSOS) (3–6). Since oxaliplatin-induced severe hepatic
sinusoidal obstruction was first reported in 2004 (7), oxaliplatin-induced hepatic injury has
become a major concern in patients with metastatic colorectal
cancer (8–11). To the best of our knowledge, there
have been no previously published reports on oxaliplatin-induced
HSOS in other types of solid cancer. The present study described a
case of oxaliplatin-induced HSOS in a patient with gastric cancer
who received oxaliplatin-containing chemotherapy.
Case report
A 52-year-old man received gastroscopy because of
dysphagia at Union hospital, Tongji Medical College (Wuhan, China)
in Feb 2016. Gastroscopic examination indicated cardia-fundus
neoplasia and pathological examination demonstrated gastric
adnenocarcinoma. Subsequently, the patient underwent proximal
gastrectomy for cardia-fundus gastric carcinoma and the
pathological diagnosis was stage pT3N0M0 moderately and
well-differentiated tubular adenocarcinoma. For treatment, the
patient received five cycles of S-1 and oxaliplatin (SOX) regimen,
consisting of intravenous injection of 130 mg/m2
oxaliplatin (Sanofi Pharmaceutical, Gentilly, France) on day 1
followed by oral administration of 50 mg TGOP capsules (50 mg
tegafur, 14.5 mg gimeracil and 49 mg oteracil potassium; Qilu
Pharmaceutical Co., Ltd., Jinan, China) twice daily on days 1–14,
every 3 weeks. Following the five cycles of SOX, the patient
presented no abnormal symptoms, and the results of blood routine
examination and liver function tests were normal. The patient had
no previous history of liver disease and denied alcohol use.
The patient received a general check-up before the
sixth cycle of SOX on schedule. Peripheral blood routine
examination results were as follows: White blood cells,
3.79×109 cells/l; red blood cells, 3.38×1012
cells/l; and platelet count, 179×109 cells/l. Liver
function test results were as follows: Total bilirubin, 6.6 mmol/l;
direct bilirubin, 4.4 mmol/l; alanine aminotransferase, 34 U/l
(normal range, 21–72 U/l); aspartate aminotransferase, 55 U/l
(normal range, 17–59 U/l); alkaline phosphatase, 190 U/l (normal
range, 40–150 U/l); γ-glutamyl transferase, 58 U/l (normal range,
17–53 U/l); total protein, 64.9 g/l; and albumin, 32.1 g/l.
Non-invasive fibrosis scores were as follows: Aspartate
aminotransferase to platelet ratio index, 0.52; and fibrosis-4
score, 2.74. Tests for hepatitis A, B, C, and E, Epstein-Barr
virus, and cytomegalovirus infection were negative. Anti-nuclear
antibody was weak positive; other autoantibodies were negative;
immunoglobulin G level (18.3 g/l; normal range, 7.51–15.6 g/l) was
increased and other immunoglobulin levels were within the normal
range.
Enlargement of the lymph nodes was not observed in
the patient through physical examination and ultrasonography.
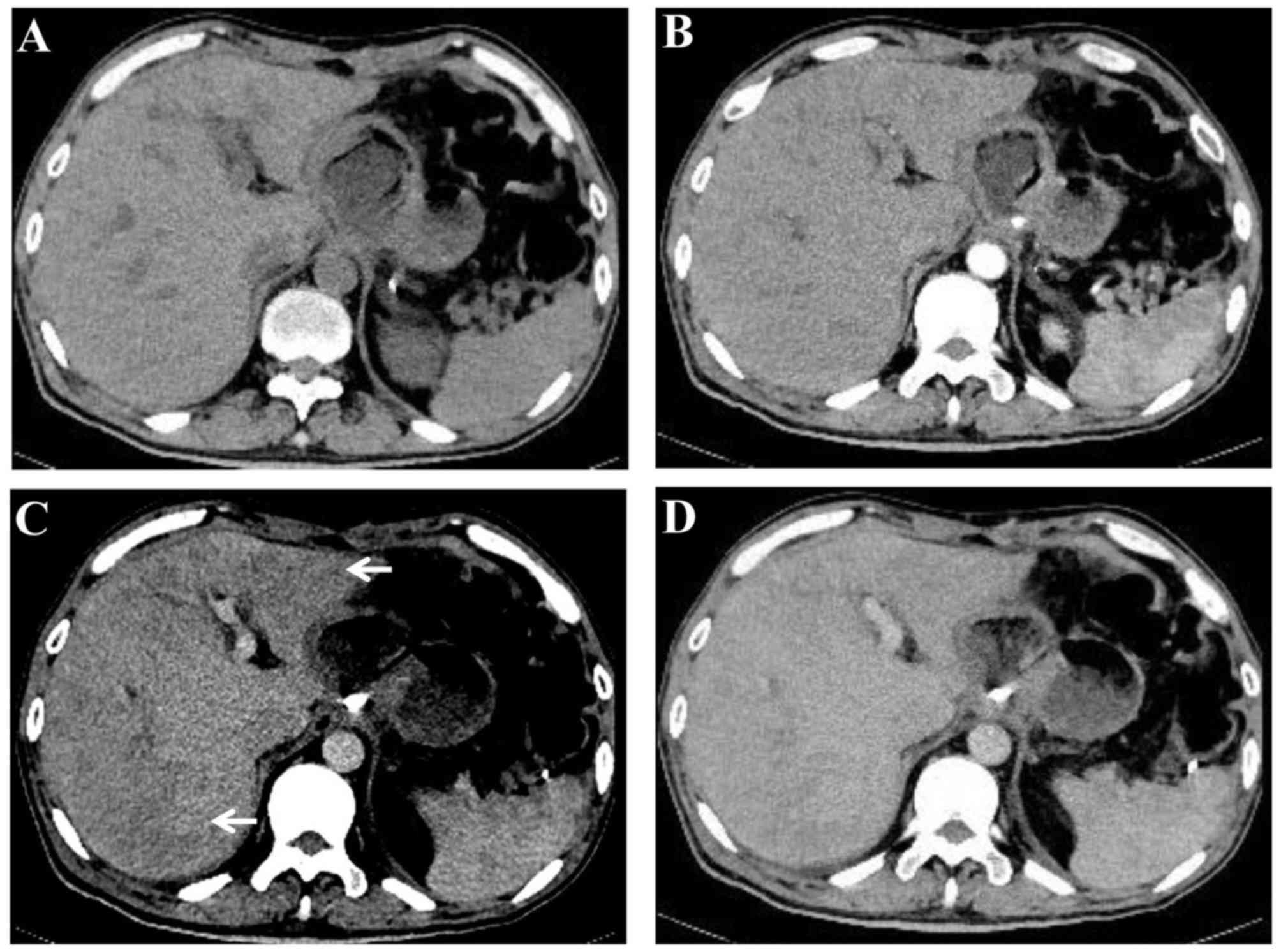
Contrast-enhanced computed tomography (CT) revealed that liver
parenchyma appeared heterogeneous and demonstrated predominantly
moderate hypoattenuation in the left and right lobe of the liver
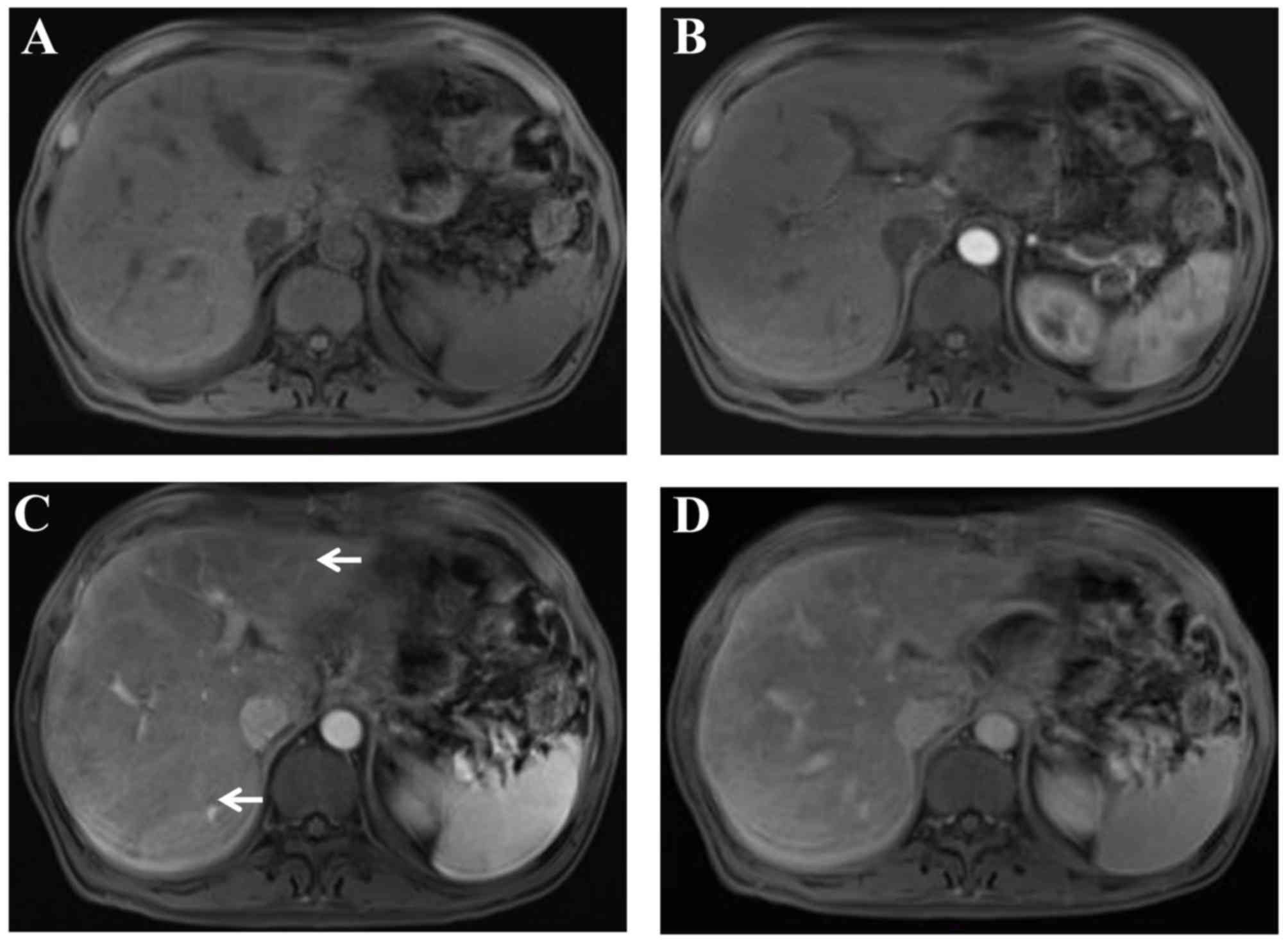
(Fig. 1) (8). On dynamic contrast-enhanced magnetic
resonance imaging (12),
heterogeneous hypointensity was demonstrated in the portal-venous
phase (Fig. 2). In addition, the
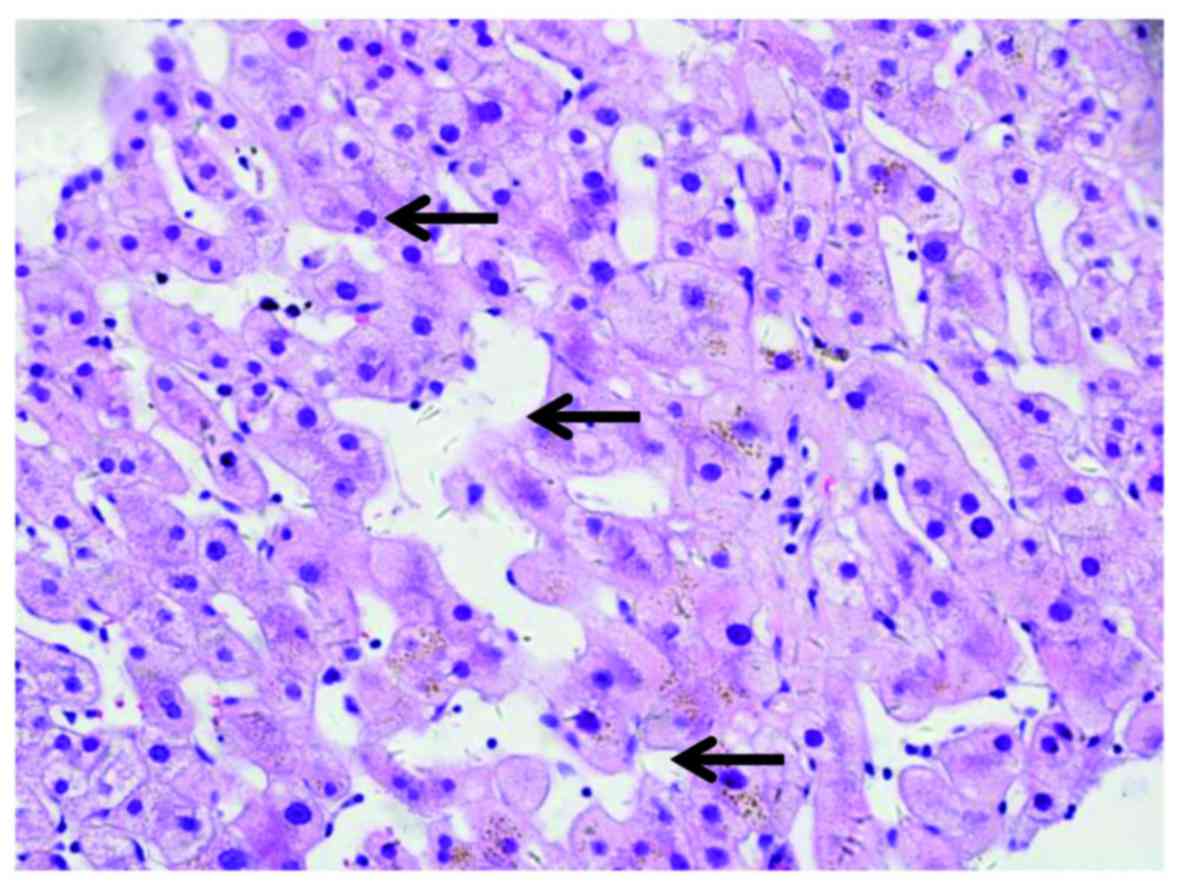
patient received liver biopsy. Tissue sections (5-µm thick) were
fixed in formalin at room temperature, and paraffin-embedded liver
samples were stained at room temperature with hematoxylin and eosin
for standard histological examination using a light microscope.
Pathological examination indicated distinctive multifocal
sinusoidal dilatation without piecemeal necrosis, hepatic rosette
formation and emperipolesis (Fig.
3). Written informed consent was obtained from the patient
prior to publication of the present case report.
Discussion
HSOS, also known as veno-occlusive disease, is an
obliterative venulitis of the terminal hepatic venules (6). It occurs as a result of cytoreductive
therapy prior to hematopoietic stem cell transplantation (HSCT),
following oxaliplatin-containing chemotherapy for colorectal
carcinoma metastatic to the liver, in patients taking pyrrolizidine
alkaloid-containing herbal remedies (5,13,14). It
is histologically characterized by sinusoidal dilatation,
hepatocyte necrosis and obliterated hepatic venules due to damage
to the sinusoidal endothelial cells (4). Sinusoidal changes induced by
chemotherapeutic agents or pyrrolizidine alkaloid-containing
herbals are caused by a direct toxicity to sinusoidal endothelial
cells (12). In addition, toxic
agents have an adverse effect on bone marrow progenitors of
endothelial cells that are responsible for the repair of sinusoidal
lesions (15). The clinical
presentations of HSOS include jaundice, right upper-quadrant pain
and tender hepatomegaly, ascites and unexplained weight gain. The
diagnosis of a HSOS result from HSCT or pyrrolizidine alkaloid
(PA)-containing herbals is based on a history of taking
PAs-containing herbs and a classical triad of weight gain, painful
hepatomegaly and jaundice (13,14).
However, clinical manifestations of the patients with
oxaliplatin-induced HSOS appear to be mild or absent (10). Thus, it is challenge to define HSOS
in patients who have received oxaliplatin-contained chemotherapy.
Notably, patients with oxaliplatin-induced HSOS may give rise to
elevated morbidity rates and increased bleeding risk. Thus, it is
important to identify HSOS in patients who receive
oxaliplatin-contained chemotherapy.
The diagnosis of HSOS is established by histological
examination; however, the patchy nature of HSOS incurs sampling
variability that confines the diagnostic yield of the biopsy.
Previous studies have demonstrated that CT and/or MRI scans may
provide excellent strategies for the screening of
oxaliplatin-induced HSOS (8,10,11,16). In
the present study, heterogeneous and predominantly hypoattenuation
of the hepatic parenchyma was a distinctive radiological sign of
oxaliplatin-induced HSOS on the CT scan. In addition, reticular
hypointensity was an important indicator of oxaliplatin-induced
HSOS observed from MRI in the patient. These imaging signs were
also reported in previous studies (8,10,11,16).
Notably, various studies have demonstrated that oxaliplatin-induced
HSOS manifested as focal liver lesions and mimicked metastatic
colon cancer in the liver (17,18).
Thus, HSOS should have been differentiated from metastatic gastric
cancer. Details of history, careful observation of radiological
findings, and histological examination provide assistance in
distinguishing oxaliplatin-induced HSOS from metastatic cancer.
In conclusion, the present case report detailed
oxaliplatin-induced HSOS in a patient with gastric cancer. HSOS
should be considered as one of the potential causes of
newly-developed hepatic lesions in patients with gastric cancer who
receive oxaliplatin. The present case highlights that oxaliplatin
may induce HSOS not only in metastatic colon cancer in the liver,
but also in other types of cancer, such as gastric cancer. Further
studies are required to identify characteristics that predispose
individuals to HSOS secondary to oxaliplatin and the prognosis of
oxaliplatin-induced HSOS in gastric cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570555 and
81270506).
Glossary
Abbreviations
Abbreviations:
|
HSOS
|
hepatic sinusoidal obstruction
syndrome
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
De Manzoni G, Marrelli D, Baiocchi GL,
Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei
MA, Pacelli F, et al: The italian research group for gastric cancer
(GIRCG) guidelines for gastric cancer staging and treatment: 2015.
Gastric Cancer. 20:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabanathan D, Eslick GD and Shannon J: Use
of neoadjuvant chemotherapy plus molecular targeted therapy in
colorectal liver metastases: A Systematic review and meta-analysis.
Clin Colorectal Cancer. 15:e141–e147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson SM, Mann J, Vasilaki A, Mathers
J, Burt AD, Oakley F, White SA and Mann DA: Pathogenesis of FOLFOX
induced sinusoidal obstruction syndrome in a murine chemotherapy
model. J Hepatol. 59:318–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valla DC and Cazals-Hatem D: Sinusoidal
obstruction syndrome. Clin Res Hepatol Gastroenterol. 40:378–385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan CQ and Crawford JM: Sinusoidal
obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp
Hepatol. 4:332–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Plessier A, Rautou PE and Valla DC:
Management of hepatic vascular diseases. J Hepatol. 56 Suppl
1:S25–S38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubbia-Brandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han NY, Park BJ, Kim MJ, Sung DJ and Cho
SB: Hepatic parenchymal heterogeneity on contrast-enhanced ct scans
following oxaliplatin-based chemotherapy: Natural history and
association with clinical evidence of sinusoidal obstruction
syndrome. Radiology. 276:766–774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han NY, Park BJ, Sung DJ, Kim MJ, Cho SB,
Lee CH, Jang YJ, Kim SY, Kim DS, Um SH, et al: Chemotherapy-induced
focal hepatopathy in patients with gastrointestinal malignancy:
Gadoxetic acid-enhanced and diffusion-weighted MR imaging with
clinical-pathologic correlation. Radiology. 271:416–425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin NY, Kim MJ, Lim JS, Park MS, Chung
YE, Choi JY, Kim KW and Park YN: Accuracy of gadoxetic
acid-enhanced magnetic resonance imaging for the diagnosis of
sinusoidal obstruction syndrome in patients with
chemotherapy-treated colorectal liver metastases. Eur Radiol.
22:864–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ward J, Guthrie JA, Sheridan MB, Boyes S,
Smith JT, Wilson D, Wyatt JI, Treanor D and Robinson PJ: Sinusoidal
obstructive syndrome diagnosed with superparamagnetic iron
oxide-enhanced magnetic resonance imaging in patients with
chemotherapy-treated colorectal liver metastases. J Clin Oncol.
26:4304–4310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anurathapan U, Pakakasama S, Mekjaruskul
P, Sirachainan N, Songdej D, Chuansumrit A, Charoenkwan P,
Jetsrisuparb A, Sanpakit K, Pongtanakul B, et al: Outcomes of
thalassemia patients undergoing hematopoietic stem cell
transplantation by using a standard myeloablative versus a novel
reduced-toxicity conditioning regimen according to a new risk
stratification. Biol Blood Marrow Transplant. 20:2066–2071. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Yang X, Xu D, Li Q, Kong X, Lu Z,
Bai T, Xu K, Ye J and Song Y: Magnetic resonance imaging findings
in patients with pyrrolizidine alkaloid-induced hepatic sinusoidal
obstruction syndrome. Clin Gastroenterol Hepatol. 15:955–957. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kan X, Ye J, Rong X, Lu Z, Li X, Wang Y,
Yang L, Xu K, Song Y and Hou X: Diagnostic performance of
contrast-enhanced CT in pyrrolizidine alkaloids-induced hepatic
sinusoidal obstructive syndrome. Sci Rep. 6:379982016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeLeve LD: Liver sinusoidal endothelial
cells and liver regeneration. J Clin Invest. 123:1861–1866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
ORourke TR, Welsh FK, Tekkis PP, Lyle N,
Mustajab A, John TG, Peppercorn D and Rees M: Accuracy of
liver-specific magnetic resonance imaging as a predictor of
chemotherapy-associated hepatic cellular injury prior to liver
resection. Eur J Surg Oncol. 35:1085–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JH, Won YW, Kim HS, Oh YH, Lim S and
Kim HJ: Oxaliplatin-induced sinusoidal obstruction syndrome
mimicking metastatic colon cancer in the liver. Oncol Lett.
11:2861–2864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arakawa Y, Shimada M, Utsunomya T, Imura
S, Morine Y, Ikemoto T, Hanaoka J, Sugimoto K and Bando Y:
Oxaliplatin-related sinusoidal obstruction syndrome mimicking
metastatic liver tumors. Hepatol Res. 43:685–689. 2013. View Article : Google Scholar : PubMed/NCBI
|