Introduction
Schwannomas are benign, slowly growing neoplasms
composed of neoplastic Schwann cells (1). Melanocytic schwannoma (MS) is a rare
schwannoma variant composed of melanin-producing cells with the
ultrastructural characteristics of Schwann cells (2), accounting for ~1% of primary peripheral
nerve sheath tumors (1,2). MS is primarily considered to be a
benign tumor, with a relatively rarely reported propensity to
metastasize (2,3); however, recent published literature
suggests that MS must be reconsidered as a malignant neoplasm
(4) with a greater potential to
metastasize. The most common location of the tumor is in the nerve
roots (5–7). MS is also encountered in extramedullary
sites and the peripheral nervous system, but is particularly rare
in intramedullary sites (8,9). There are only 8 reported cases of
intramedullary MS (IMS) (1,8–14); we
herein report the ninth IMS case in a 40-year-old man with a lesion
located in the cervical cord, which was diagnosed based on the
magnetic resonance imaging (MRI) and histopathological findings and
treated with surgery.
Case report
A 40-year-old man presented in the Second Affiliated
Hospital of Zhejiang University School of Medicine in March 2017
with left arm numbness that gradually worsened over a period of 4
months. The patient did not have any other neurological symptoms.
Upon physical examination, no obvious deposition of pigment was
found in the skin and mucosa. There was no previous history of a
surgical procedure for the removal of MS. Upon neurological
examination, the distal pinprick sensation in the left upper limb
was slightly decreased. The muscle strength in all upper and lower
limbs was unaffected (5/5) and the muscle tone was normal. All limb
tendon reflexes were normal. No positive pathological reflexes were
present bilaterally.
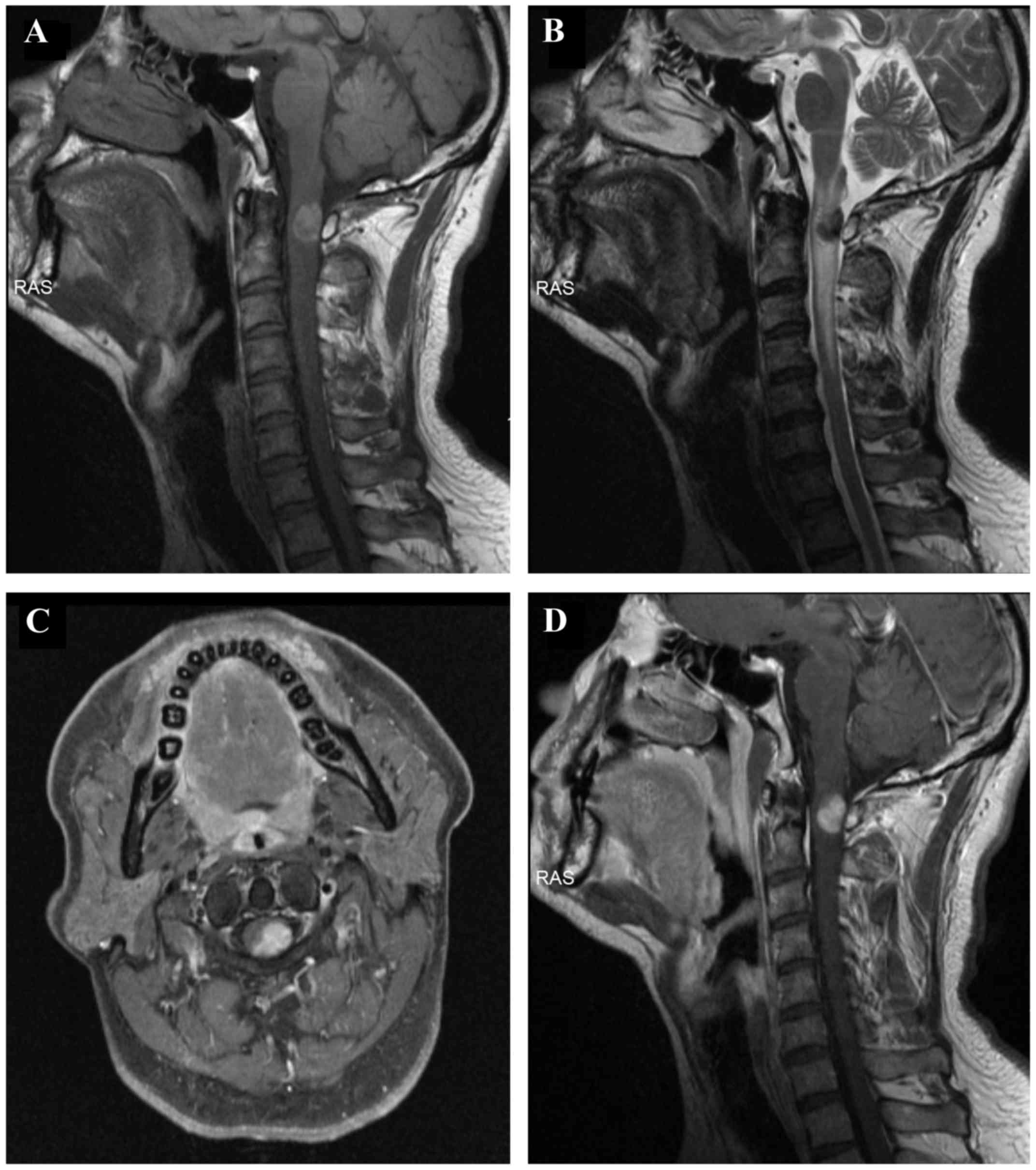
An MRI of the cervical spine revealed an
intramedullary mass, sized 1.5×1.0 cm, within the spinal canal. The
mass occupied 60% of the spinal canal at the level of C1-C2, with
displacement of the spinal cord to the right side (Fig. 1A-D). The mass was T1 hyperintense
(Fig. 1A) and T2 hypointense
(Fig. 1B). Following enhancement,
the mass was homogeneously enhanced (Fig. 1C and D). There was spinal cord
expansion and edema above and below the mass, as evidenced by T2
hyperintensity (Fig. 1B). The
radiological and clinical findings were consistent with melanoma,
including primarily intramedullary melanoma or metastatic malignant
melanoma. As primary tumors were not found anywhere on the skin of
the limbs or the trunk, metastatic malignant melanoma was not
considered in the initial diagnosis. A cavernous malformation with
subacute hematoma was considered in the differential diagnosis;
however, the history did not include a sudden onset, and the
patient's symptoms gradually worsened over a relatively long period
of time, which is not consistent with haemorrhagic manifestations.
Finally, the preoperative diagnosis was a primarily intramedullary
melanoma.
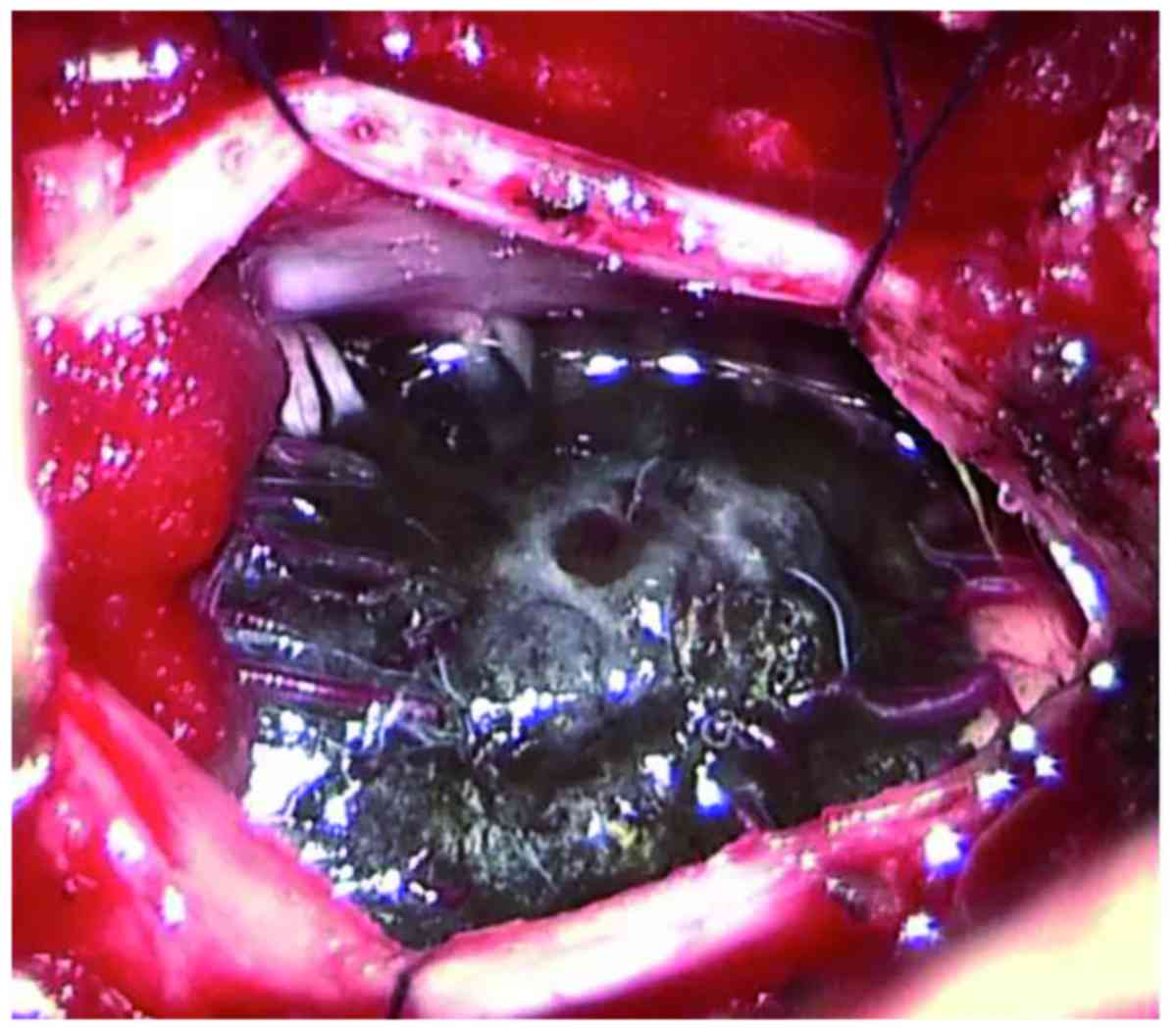
A C1 laminectomy was performed. The dura mater and
arachnoid sheath were opened longitudinally and dark pigmentation
was visible through the dura (Fig.
2). The dura was opened in the midline to expose an
intramedullary tumor, with neurophysiological monitoring during
surgery. The tumor invaded deeply into the spinal cord and had an
unclear boundary. Spinal nerve roots were also invaded by the
tumor. Due to the difficulty of complete removal and an
intraoperative diagnosis of metastatic malignant melanoma by frozen
section biopsy, the lesion was partially resected. The left arm
numbness partially subsided 2 weeks later after the surgery.
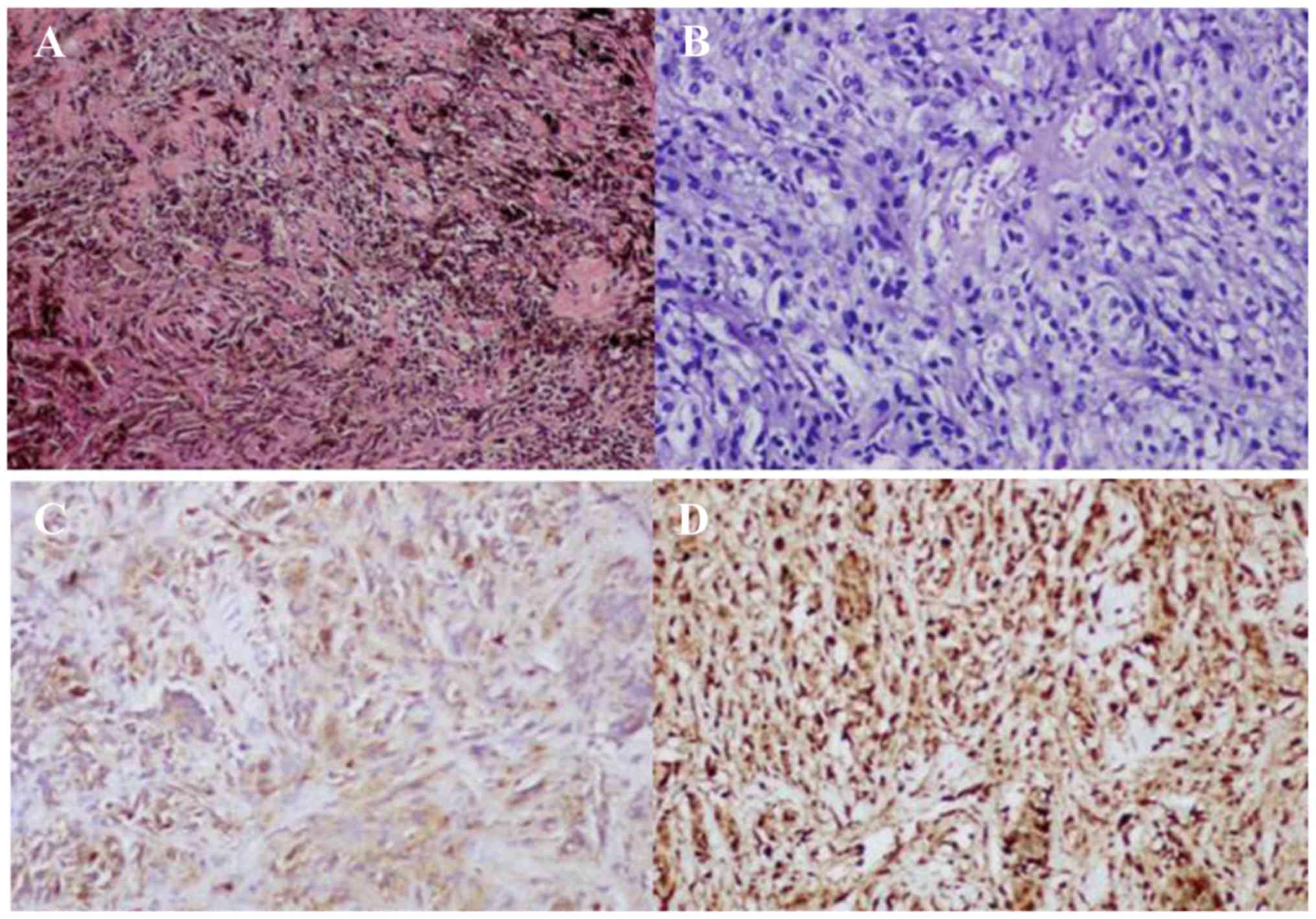
Sections stained with hematoxylin-eosin revealed
that the tumor was composed of polygonal epithelioid and
spindle-shaped cells, with abundant cytoplasm containing melanin
granules (Fig. 3A). Depigmented
sections revealed no obvious atypia of the tumor cell nuclei
(Fig. 3B). Immunocytochemistry for
human melanoma black 45 (Fig. 3C),
p53, vimentin and S-100 (Fig. 3D)
was positive, whereas the tumor cells were negative for melan-A and
epithelial membrane antigen. The Ki-67 proliferative index was
<1%. The pathological diagnosis was IMS. During the follow-up οn
May 2017, the patient status was stable with the left arm
numbness.
Written informed consent was obtained from the
patient regarding the publication of the case details and
associated images.
Discussion
MS is a tumor derived from progenitor neural crest
cells that can differentiate into both Schwann cells and
melanocytes, which is characterized by deposition of melanin in the
Schwann cell cytoplasm (2). Theories
for the production of melanin by these cells include neoplastic
differentiation of neural crest cells into Schwann cells with
melanogenetic properties, and melanocytic transformation of
previously normal Schwann cells (1).
The presence of psammoma bodies is typical of the psammomatous
variant of MS. Approximately 50% of psammomatous MS are part of the
Carney syndrome (along with myxomas, skin pigmentation and
endocrine tumors or overactivity) (15). The most frequent sites of MS are the
dorsal spinal nerve roots, sympathetic chain, acoustic nerve,
cerebellum and orbit (10). IMS is
particularly rare and, to the best of our knowledge, this is the
ninth case reported to date (1,8–14) (Table
I).
 | Table I.Summary of the intramedullary
melanotic schwannoma cases reported in the literature. |
Table I.
Summary of the intramedullary
melanotic schwannoma cases reported in the literature.
| Case no. | Authors | Sex | Age, years | Location | Treatment | Outcome | Follow-up | (Refs.) |
|---|
| 1 | Solomon et
al | Male | 69 | Caudal medulla and
C3 | Gross total
removal | – | – | (11) |
| 2 | Marchese et
al | Female | 72 | C4-C6 | Partial removal | Functional
recovery | – | (12) |
| 3 | Sola-Pérez et
al | Female | 63 | C7-T1 | Needle
aspiration | – | – | (13) |
| 4 | Acciarri et
al | Female | 44 | T2-T3 | Gross total
removal | Partial neurological
recovery | – | (14) |
| 5 | Santaguida et
al | Male | 35 | C4-C5 | Gross total
removal | Partial neurological
recovery | Recurrence at 2
years, radiotherapy, and repeatresection at 4 years | (1) |
| 6 | Mouchaty et
al | Female | 56 | Conus | Gross total
removal | Partial neurological
recovery | No recurrence at 12
months | (9) |
| 7 | Hoover et
al | Female | 62 | T11 | Gross total
removal | Good neurological
recovery | No recurrence at 10
months | (8) |
| 8 | Mohamed et
al | Male | 43 | T9-T10 | Gross total
removal | Good neurological
recovery | No recurrence at 12
months | (10) |
| 9 | Present case | Male | 40 | C1-C2 | Partial removal | Partial recovery | – |
|
MRI is currently the optimal diagnostic modality for
evaluating lesions of the spinal cord. Melanin may be associated
with shortened T1 and T2 relaxation times due to its content of
paramagnetic free radicals; thus, melanotic lesions appear
hyperintense on T1-weighted and hypointense on T2-weighted images
(16). By contrast, non-melanotic
tumors are hypointense on T1-weighted and hyperintense on
T2-weighted images, which helps differentiate melanotic from
non-melanotic lesions. However, subacute hematoma is difficult to
differentiate from melanotic lesions, as subacute hematoma may
display similar characteristics on MRI (17). In the present case, a cavernous
malformation with subacute hematoma was also initially considered
on T1- and T2-weighted images. However, considering the patient's
medical history, the onset of the symptoms was not sudden but
rather a chronic process; thus, subacute hematoma was not
considered in the initial preoperative diagnosis. Following
administration of gadolinium, the tumor typically exhibited
homogeneous enhancement; however, enhancement may be heterogeneous
in the presence of haemorrhage within the lesion (10). A diagnosis of intramedullary
schwannoma may be confidently made when there is continuity of the
intramedullary lesion with a contrast-enhanced thickened spinal
root (18); however, no obvious
enhancing thickened spinal root involvement was observed in the
present IMS case.
From the 8 previously reported cases (Table I), the main treatment for IMS is
gross total removal. Approximately 10% of MS cases are reported to
exhibit an aggressive clinical course, with local recurrence and
metastasis (1,16). Due to the rarity of IMS, the role of
radiotherapy has not been established. The fifth documented case
(Table I) of a patient with IMS
recurred 2 years after initial gross total removal. Subsequently,
the patient received radiotherapy, but the tumor progressed 2 years
after radiotherapy and the patient was again treated with surgery.
Considering the recurrence potential of IMS, annual follow-up of
patients with IMS with MRI is required (8). In the present case, the lesion was
partially resected, due to the difficulty of complete removal, and
an intraoperative diagnosis of metastatic malignant melanoma was
made by frozen section biopsy. Due to the rarity of IMS,
intraoperative frozen section diagnosis is challenging without
immunohistochemical examination.
In conclusion, MRI is the preferred method for
evaluating lesions of the spinal cord. A standard IMS would
typically be T1 hyperintense, T2 hypointense and homogeneously
enhanced. However, although IMS has these characteristic MRI
features, preoperative diagnosis as well as intraoperative frozen
section diagnosis are challenging due to the rarity of this tumor.
Correct diagnosis is crucial for management planning; therefore,
immunohistochemical examination is warranted. In addition, careful
follow-up is required for all IMS patients, particularly when the
mass cannot be completely resected.
References
|
1
|
Santaguida C, Sabbagh AJ, Guiot MC and Del
Maestro RF: Aggressive intramedullary melanotic schwannoma: Case
report. Neurosurgery. 55:14302004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang HY, Yang GH, Chen HJ, Wei B, Ke Q,
Guo H, Ye L, Bu H, Yang K and Zhang YH: Clinicopathological,
immunohistochemical and ultrastructural study of 13 cases of
melanotic schwannoma. Chin Med J (Engl). 118:1451–1461.
2005.PubMed/NCBI
|
|
3
|
Vallat-Decouvelaere AV, Wassef M, Lot G,
Catala M, Moussalam M, Caruel N and Mikol J: Spinal melanotic
schwannoma: A tumour with poor prognosis. Histopathology.
35:558–566. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torres-Mora J, Dry S, Li X, Binder S, Amin
M and Folpe AL: Malignant melanotic schwannian tumor: A
clinicopathologic, immunohistochemical and gene expression
profiling study of 40 cases, with a proposal for the
reclassification of ‘melanotic schwannoma’. Am J Surg Pathol.
38:94–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Cerchio L, Contratti F and Fraioli MF:
Dorsal dumb-bell melanotic schwannoma operated on by posterior and
anterior approach: Case report and a review of the literature. Eur
Spine J. 15 Suppl 5:S664–S669. 2006. View Article : Google Scholar
|
|
6
|
Er U, Kazanci A, Eyriparmak T, Yigitkanli
K and Senveli E: Melanotic schwannoma. J Clin Neurosci. 14:676–678.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Reay DG, Shattuck MC and Guthrie FW
Jr: Psammomatous melanotic schwannoma: An additional component of
Carney's complex. Report of a case. Am J Clin Pathol. 95:484–489.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoover JM, Bledsoe JM, Giannini C and
Krauss WE: Intramedullary melanotic schwannoma. Rare Tumors.
4:e32012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mouchaty H, Conti R, Buccoliero AM and
Conti P: Intramedullary melanotic schwannoma of the conus
medullaris: A case report. Spinal Cord. 46:703–706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohamed M, Panos S, Baborie A, Das K and
Pillay R: Atypical benign melanotic thoracic intradural schwannoma.
Br J Neurosurg. 28:411–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solomon RA, Handler MS, Sedelli RV and
Stein BM: Intramedullary melanotic schwannoma of the
cervicomedullary junction. Neurosurgery. 20:36–38. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marchese MJ and McDonald JV:
Intramedullary melanotic schwannoma of the cervical spinal cord:
Report of a case. Surg Neurol. 33:353–355. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sola-Pérez J, Pérez-Guillermo M,
Bas-Bernal A, Giménez-Bascuñana A and Montes-Clavero C: Melanocytic
schwannoma: The cytologic aspect in fine-needle aspiration cytology
(FNAC): Report of a case located in the spinal cord. Diagn
Cytopathol. 11:291–296. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Acciarri N, Padovani R and Riccioni L:
Intramedullary melanotic schwannoma. Report of a case and review of
the literature. Br J Neurosurg. 13:322–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shields LB, Glassman SD, Raque GH and
Shields CB: Malignant psammomatous melanotic schwannoma of the
spine: A component of carney complex. Surg Neurol Int. 2:1362011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tawk RG, Tan D, Mechtler L and
Fenstermaker RA: Melanotic schwannoma with drop metastases to the
caudal spine and high expression of CD117 (c-kit). J Neurooncol.
71:151–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Höllinger P, Godoy N and Sturzenegger M:
Magnetic resonance imaging findings in isolated spinal psammomatous
melanotic schwannoma. J Neurol. 246:1100–1102. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colosimo C, Cerase A, Denaro L, Maira G
and Greco R: Magnetic resonance imaging of intramedullary spinal
cord schwannomas. Report of two cases and review of the literature.
J Neurosurg. 99 Suppl 1:S114–S117. 2003.
|