Introduction
Liposarcoma, a malignant tumor derives from
mesodermal tissues, represents ~20% of all sarcomas. Paratesticular
liposarcoma (PLS) is a rare condition. To the best of our
knowledge, about 200 cases of PLS have been reported to date
(1). Giant PLS is more rare with
only a few cases having been reported (2–6). Due to
the rarity of the disease, there is no standardized guideline as
regards its incidence, diagnosis, recurrence and treatment
(7,8). In this study, we present a case of a
giant dedifferentiated PLS of the right testis with magnetic
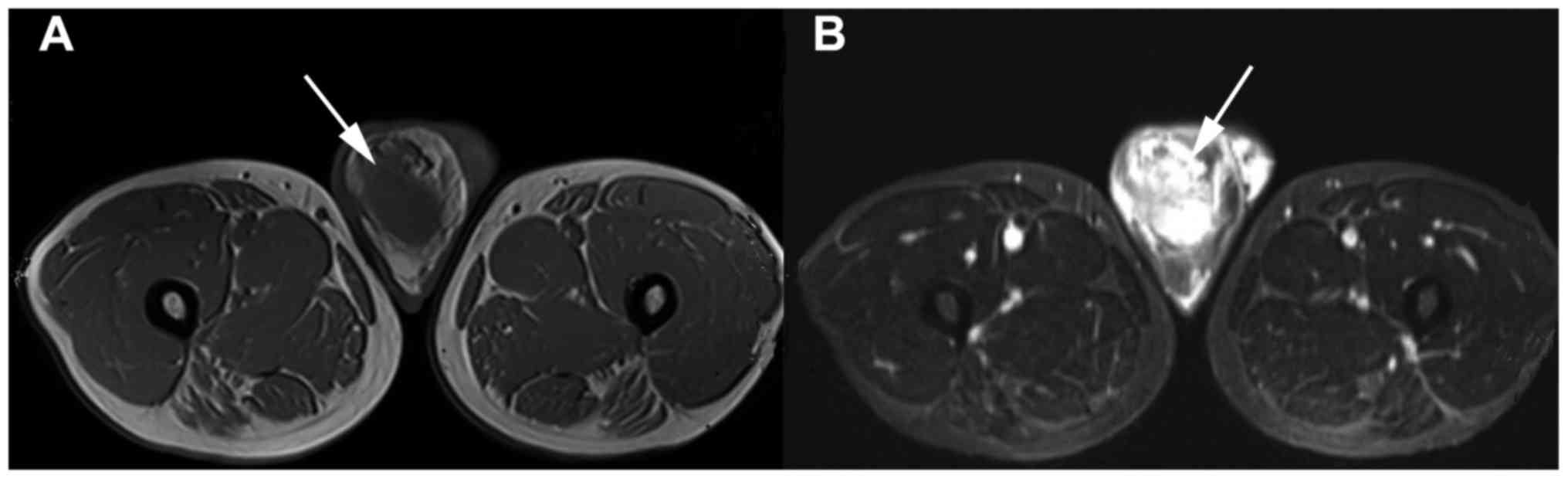
resonance imaging (MRI) measuring 7.8×5.8×10.4 cm and focus on the
discussion about the clinical characteristics, diagnosis and
treatment of this disease. Due to the giant size of this PLS, we
report this case for the characteristics, diagnosis and treatment
of the similar cases. The study was supported by the Ethics
Committee of Peking University Shenzhen Hospital (Shenzhen, China)
and written informed consent was obtained from the patient for the
publication of the case details.
Case report
In July 2017, a 51-year-old man, with a complaint of
swelling of the right scrotum for 2 months, was admitted to the
Department of Urology of our hospital. He presented with a painless
and slow-growing fixed mass in the right scrotum without
conspicuous promoting or alleviating factors. There are no other
signs or symptoms. A rigid mass in the right scrotum, about 8 cm in
maximum diameter, was the only positive finding of physical
examinations. There are no specific abnormalities in the laboratory
and imaging examinations (hemogram, urinalysis, stool routine, ESR,
β-human chorionic gonadotropin, a-fetoprotein, mycobacterium
tuberculosis antibody Ig-G, liver and kidney function tests and
chest X-ray). However, magnetic resonance imaging (MRI)
demonstrated a 7.8×5.8×10.4 cmnonhomogeneous space-occupying lesion
of the right testis (Fig. 1), which
was considered as spermatocytoma at first.
Following the doctors' recommendation, the patient
underwent a radical resection of the tumor combined with a right
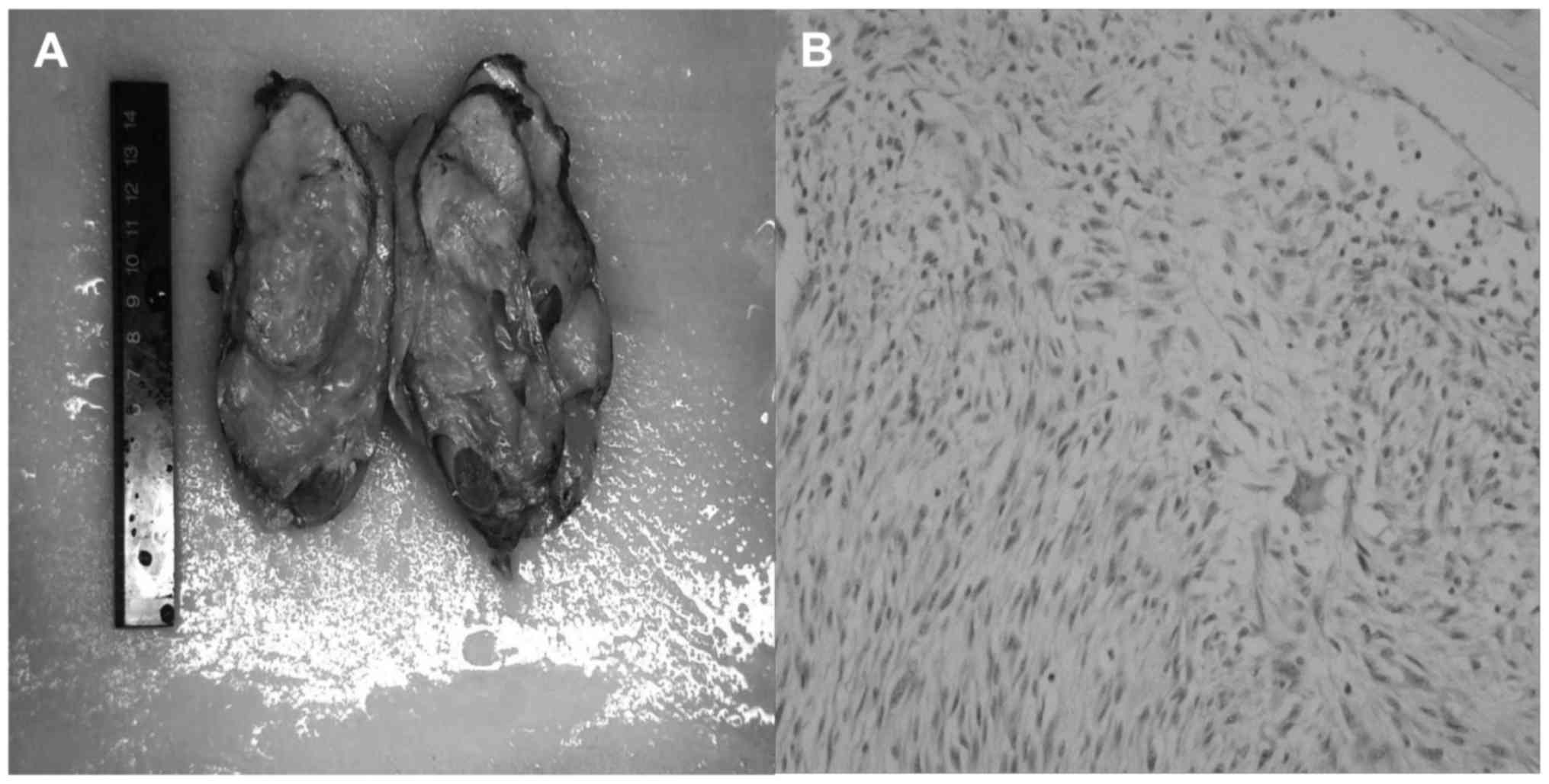
orchiectomy. An enlarged rigid testicle measuring 13×8×6 cm was
removed out of the right scrotum. There is no evident inflammatory
adhesion to the surrounding organs. On gross pathological
examination, the resected specimen was an enlarged mass with a cut
surface having yellowish lipoma-like texture. Final
histopathological examination confirmed that it was a giant
dedifferentiated liposarcoma (Fig.
2). Immunohistochemical analysis showed that CD34(−); SMA(+);
S-100(−)(most of cells); ALK(−) and supported this diagnosis. At
5-month follow-up, there is no evidence of local recurrence or
distant metastasis.
Discussion
Liposarcoma, soft-tissue malignancy derived
embryologically from mesodermal tissue, was first reported by
Lesauvage in 1845 (9). They usually
exist in the lower extremities and retroperitoneum (10,11).
There are four histological subtypes in liposarcoma, which include
well differentiated, dedifferentiated, myxoid and pleomorphic
(12). PLS are rare neoplasm which
compose approximately 12% of all liposarcomas and they originate in
spermatic cord mostly followed by testicular tunics and epididymis
(13). When the diameter of
testicular tumor reaches more than 10 cm, such size will be called
‘giant’ (5). As far as we know, 200
or so cases of PLS have been reported up to date (14), and giant PLS are more rare with only
a few cases having been reported (2–6). The
incidence of PLS has a regional difference with the highest
incidence being in Japan (7). The
tumor attacked adult patients aged 50 to 60 years more frequently
(15), though it occurred in
patients with a range of 16 to 90 years of age on the basis of the
current literature (16,17). PLS mostly present as a painless,
slow-growing inguinal or inguinoscrotal mass and sometimes combine
with a sensation of heaviness (9,14,16) and
the occurrence of wrong diagnosis like scrotal lipoma, inguinal
hernia and epididymitis before surgical intervention attribute to
this clinical presentation (12,15).
Most PLS are primary, but some can be metastasis from liposarcoma
at other sites, such as thigh or the fatty tissue surrounding the
testicle (18,19). Because of the insufficient number of
literature on patients with PLS, no reliable standardized diagnosis
and treatment guidelines have been made (14).
Ultrasonography (US), Computerized Tomography (CT)
and Magnetic Resonance Imaging (MRI) are documented in the
diagnosis of PLS (20,21). On US examination, PLS are identified
as solid, heterogeneous solid, hypoechoic lesions, sometimes
accompanied by colliquation if there is necrosis. However, US
cannot always distinguish PLS from lipomas if the tumor is small or
it is a well-differentiated PLS with homogenous fatty pattern,
which makes PLS similar to lipoma (10,14,22).
Compared to subcutaneous fatty tissue, CT usually demonstrates the
tumor area with lower density. It may be helpful to establish tumor
location, tissue characteristics, staging and follow-up (8,16,23).
MRI, the golden standard in staging soft tissue tumors, not only
provides clear information on the tumor foci but also characterizes
and delineates the degree of local tumor extension (9,14,20).
Diagnosis of PLS mainly depends on histopathology,
immunohistochemistry and cytomorphological features. A Critical
histopathological analysis of dedifferentiated liposarcomas
revealed that CD34 was negative in 9/11 cases; negative rate of
s-100 was 92% (23/25); MDM2 was diffusely positive in
well-differentiated areas and focally in dedifferentiated areas in
the tumor with homologous dedifferentiation; SMA was positive in
2/8 tumors (24). Andrei et
al proved that MDM2 and CDK4 were significative markers for
confirming the diagnosis of well-differentiated liposarcoma
(23). Histologically,
differentiated sarcoma can be subdivided into five main subtype:
Resembling pleomorphic malignant fibrous histiocytoma,
fibrosarcoma, rhabdmyofibrosarcoma, myxofibrosarcoma and
hemangiopericytoma (25,26). A total of 76% of dedifferentiated
liposarcomas was high-grade (24).
Multimodality therapy was suggested by many
researchers (27–29). There is a general consensus that
radical orchiectomy with wide local excision and high ligation of
the spermatic cord are the current standard treatment strategies
due to frequent recurrence that associated with incomplete excision
(7–9,20).
Because the clinical presentation of PLS is similar to scrotal
lipoma or groin hernia, Immediate radical procedure should be
performed to avoid the high risk of local recurrence and
involvement of worsening prognosis, when a suspicious PLS is
diagnosed. It is important to prohibit spillage of malignant cells
and acquire a more safe edge during the operation. A clinical
research showed that the 3-year local-recurrence-free survival was
100% for negative margins compared with 29% for positive margins
(30). Retroperitoneal lymph node
dissection is not recommended except for metastasis (7). It has been reported that occult local
residual lesions were found at least a third of patients after
operation (1), thus not only in
dedifferentiated PLS, considered with a high rate of recurrence and
metastasis, but also in other subtypes of PLS, adjuvant radiation
is quite needed (9,14,31).
Cerda et al (32)reported
that five patients with spermatic cord sarcoma given adjuvant
radiotherapy with a total dose of 54 Gy/27 or 30 fractions were
found no recurrence in median 18 months of follow-up (range 6–28
months). However, whether radiotherapy should be used as
postoperative routine therapy remains to be discussed because
recurrent tumor after radiotherapy may be more aggressive (10). Some suggested that radiotherapy
should be used for local control (8,10,14,30).
There are no large studies with respect to the results of
chemotherapy. A meta-analysis of 14 randomized clinical trials
discovered that the improvement of recurrence and recurrence-free
survival were attributed to chemotherapy (14,33).
Some studies reported that we should attach importance to
chemotherapy for high grade LPS (1).
Research report on prognosis of PLS is quite limited
until now. A recent study local-recurrence-free survival was 76% at
3 years and 67% at 5 years (30).
Another study about PLS revealed the 5-year survival rate was 75%
and recurrence rate was 50–70% of all cases (14). Prognosis and overall survival rate
vary in accordance with some risk factors, which include tumor
grade, size, depth of invasion and histopathological classification
(most important). The dedifferentiated types have a worse
prognosis, but local recurrence rate will be smaller (14,34).
In conclusion, PLS represent a rarity of the tumor,
characterized with slow growth, which are often misdiagnosed
preoperatively. US, CT and MRI can redound to diagnose and
differential diagnose, but the final diagnosis of PLS depends on
histopathology and immunohistochemistry. When diagnosed or highly
suspected preoperatively, radical orchiectomy with wide local
excision and high ligation is the best treatment strategy and
multimodality therapy is suggested. Long-term follow-up is
recommended due to the risk of local recurrence and distant
metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 8110122), Science and
Technology Development Fund Project of Shenzhen (nos.
JCYJ20150403091443329 and JCYJ20170307111334308), the fund of
‘San-ming’ Project of Medicine in Shenzhen (no. SZSM201612066) and
the fund of Guangdong Key Medical Subject.
References
|
1
|
Chiodini S, Luciani LG, Cai T, Molinari A,
Morelli L, Cantaloni C, Barbareschi M and Malossini G: Unusual case
of locally advanced and metastatic paratesticular liposarcoma: A
case report and review of the literature. Arch Ital Urol Androl.
87:87–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sopena-Sutil R, Silan F, Butron-Vila MT,
Guerrero-Ramos F, Lagaron-Comba E and Passas-Martinez J:
Multidisciplinary approach to giant paratesticular liposarcoma. Can
Urol Assoc J. 10:E316–E319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandez F and Garcia HA: Giant
dedifferenciated liposarcoma of the spermatic cord. Arch Esp Urol.
62:751–755. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cariati A, Brignole E, Tonelli E and
Filippi M: Giant paratesticular undifferentiated liposarcoma that
developed in a long-standing inguinal hernia. Eur J Surg.
168:511–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kin T, Kitsukawa S, Shishido T, Maeda Y,
Izutani T, Yonese J and Fukui I: Two cases of giant testicular
tumor with widespread extension to the spermatic cord: Usefulness
of upfront chemotherapy. Hinyokika Kiyo. 45:191–194. 1999.(In
Japanese). PubMed/NCBI
|
|
6
|
Martin C, Olivier CM, Rengifo D, Hernandez
Lao A, Ondina LM and Carballido J: Giant liposarcoma of the
spermatic cord. Actas Urol Esp. 17:361–365. 1993.(In Spanish).
PubMed/NCBI
|
|
7
|
Li F, Tian R, Yin C, Dai X, Wang H, Xu N
and Guo K: Liposarcoma of the spermatic cord mimicking a left
inguinal hernia: A case report and literature review. World J Surg
Oncol. 11:182013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alyousef H, Osman EM and Gomha MA:
Paratesticular liposarcoma: A case report and review of the
literature. Case Rep Urol. 2013:8062892013.PubMed/NCBI
|
|
9
|
Vukmirović F, Zejnilović N and Ivović J:
Liposarcoma of the paratesticular tissue and spermatic cord: A case
report. Vojnosanit Pregl. 70:693–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raza M, Vinay HG, Ali M and Siddesh G:
Bilateral paratesticular liposarcoma-a rare case report. J Surg
Tech Case Rep. 6:15–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vinayagam K, Hosamath V, Honnappa S and
Rau AR: Paratesticular liposarcoma-masquerading as a testicular
tumour. J Clin Diagn Res. 8:165–166. 2014.PubMed/NCBI
|
|
12
|
García Morúa A, Lozano Salinas JF, Valdés
Sepúlveda F, Zapata H and Gómez Guerra LS: Liposarcoma of the
espermatic cord: Our experience and review of the literature. Actas
Urol Esp. 33:811–815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noguchi H, Naomoto Y, Haisa M, Yamatsuji
T, Shigemitsu K, Uetsuka H, Hamasaki S and Tanaka N:
Retroperitoneal liposarcoma presenting a indirect inguinal hernia.
Acta Med Okayama. 55:51–54. 2001.PubMed/NCBI
|
|
14
|
Schoonjans C, Servaes D and Bronckaers M:
Liposarcoma scroti: A rare paratesticular tumor. Acta Chir Belg.
116:122–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fitzgerald S and Maclennan GT:
Paratesticular liposarcoma. J Urol. 181:331–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabriele R, Ferrara G, Tarallo MR,
Giordano A, De Gori A, Izzo L and Conte M: Recurrence of
paratesticular liposarcoma: A case report and review of the
literature. World J Surg Oncol. 12:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bostwick DG: Spermatic cord and testicular
adnexa. 1997.
|
|
18
|
Fahsi O, Kallat A, Ouazize H, Dergamoun H,
Sayegh HE, Iken A, Benslimane L and Nouini Y: Metastatic
paratesticular liposarcoma. Pan Afr Med J. 27:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thinyu S and Muttarak M: Role of
ultrasonography in diagnosis of scrotal disorders: A review of 110
cases. Biomed Imaging Interv J. 5:e22009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pergel A, Yucel AF, Aydin I, Sahin DA,
Gucer H and Kocakusak A: Paratesticular liposarcoma: A radiologic
pathologic correlation. J Clin Imaging Sci. 1:572011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshino T, Yoneda K and Shirane T: First
report of liposarcoma of the spermatic cord after radical
prostatectomy for prostate cancer. Anticancer Res. 29:677–680.
2009.PubMed/NCBI
|
|
22
|
Montgomery E and Fisher C: Paratesticular
liposarcoma: A clinicopathologic study. Am J Surg Pathol. 27:40–47.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pănuş A, Meşină C, Pleşea IE, Drăgoescu
PO, Turcitu N, Maria C and Tomescu PI: Paratesticular liposarcoma
of the spermatic cord: A case report and review of the literature.
Rom J Morphol Embryol. 56:1153–1157. 2015.PubMed/NCBI
|
|
24
|
Rekhi B, Navale P and Jambhekar NA:
Critical histopathological analysis of 25 dedifferentiated
liposarcomas, including uncommon variants, reviewed at a Tertiary
Cancer Referral Center. Indian J Pathol Microbiol. 55:294–302.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCormick D, Mentzel T, Beham A and
Fletcher CD: Dedifferentiated liposarcoma. Clinicopathologic
analysis of 32 cases suggesting a better prognostic subgroup among
pleomorphic sarcomas. Am J Sur Pathol. 18:1213–1223. 1994.
View Article : Google Scholar
|
|
26
|
Coindre JM, Pedeutour F and Aurias A:
Well-differentiated and dedifferentiated liposarcomas. Virchows
Arch. 456:167–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brennan MF, Casper ES, Harrison LB, Shiu
MH, Gaynor J and Hajdu SI: The role of multimodality therapy in
soft-tissue sarcoma. Ann Surg. 214:328–338. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanso C, Roussel H, Zerbib M, Flam T,
Debré B and Vieillefond A: Spermatic cord sarcoma in adults:
Diagnosis and management. Prog Urol. 21:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sherman KL, Wayne JD, Chung J, Agulnik M,
Attar S, Hayes JP, Laskin WB, Peabody TD, Bentrem DJ, Pollock RE
and Bilimoria KY: Assessment of multimodality therapy use for
extremity sarcoma in the United States. J Surg Oncol. 109:395–404.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khandekar MJ, Raut CP, Hornick JL, Wang Q,
Alexander BM and Baldini EH: Paratesticular liposarcoma: Unusual
patterns of recurrence and importance of margins. Ann Surg Oncol.
20:2148–2155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song CH, Chai FY, Saukani MF, Singh H and
Jiffre D: Management and prevention of recurrent paratesticular
liposarcoma. Malays J Med Sci. 20:95–97. 2013.PubMed/NCBI
|
|
32
|
Cerda T, Martin É, Truc G, Créhange G and
Maingon P: Safety and efficacy of intensity-modulated radiotherapy
in the management of spermatic cord sarcoma. Cancer Radiother.
21:16–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gago Juan A, Luján Galán M, Bustamante
Alarma S, Fernández Lobato R, Zárate Rodríguez E, Martín Osés E and
Berenguer Sánchez A: A paratesticular myxoid liposarcoma as a
simulator of a hernial process. A case report. Arch Esp Urol.
50:921–923. 1997.PubMed/NCBI
|
|
34
|
Stranne J, Hugosson J and Lodding P:
Post-radical retropubic prostatectomy inguinal hernia: An analysis
of risk factors with special reference to preoperative inguinal
hernia morbidity and pelvic lymph node dissection. J Urol.
176:2072–2076. 2006. View Article : Google Scholar : PubMed/NCBI
|