Introduction
Primary malignant pericardial mesothelioma (PMPM) is
extremely rare, with an estimated incidence of <0.0017%, as
reported in a large autopsy study of ~50,000 cases (1). PMPM is an aggressive tumor that
originates from the mesothelial cells of the pericardium and is
generally diagnosed following surgical excision. Tumor diagnosis
and staging (including myocardial infiltration) with anatomical
imaging methods, such as computed tomography (CT), echocardiography
and magnetic resonance imaging (MRI), may be particularly
challenging in PMPM due to its diffuse pattern of growth. Early
diagnosis, staging (including assessment of myocardial
infiltration) and response evaluation are crucial for determining
treatment. We herein report our experience with
18F-fluorodeoyglucose (FDG) metabolism imaging in a PMPM
patient for early diagnosis, staging and response evaluation.
Case report
In May 2010, a 28-year-old man was admitted to the
Nanjing First Hospital due to progressive left-sided chest pain and
breathlessness for 4 months, which had worsened over the last 1 h.
The patient had a 10-year history of smoking (1 pack/day). There
was no history of tuberculosis or asbestos exposure. Two months
prior to admission, the patient had been hospitalized with the same
symptoms in another hospital. Physical examination, chest
radiography, computed tomography (CT) and enhanced CT revealed
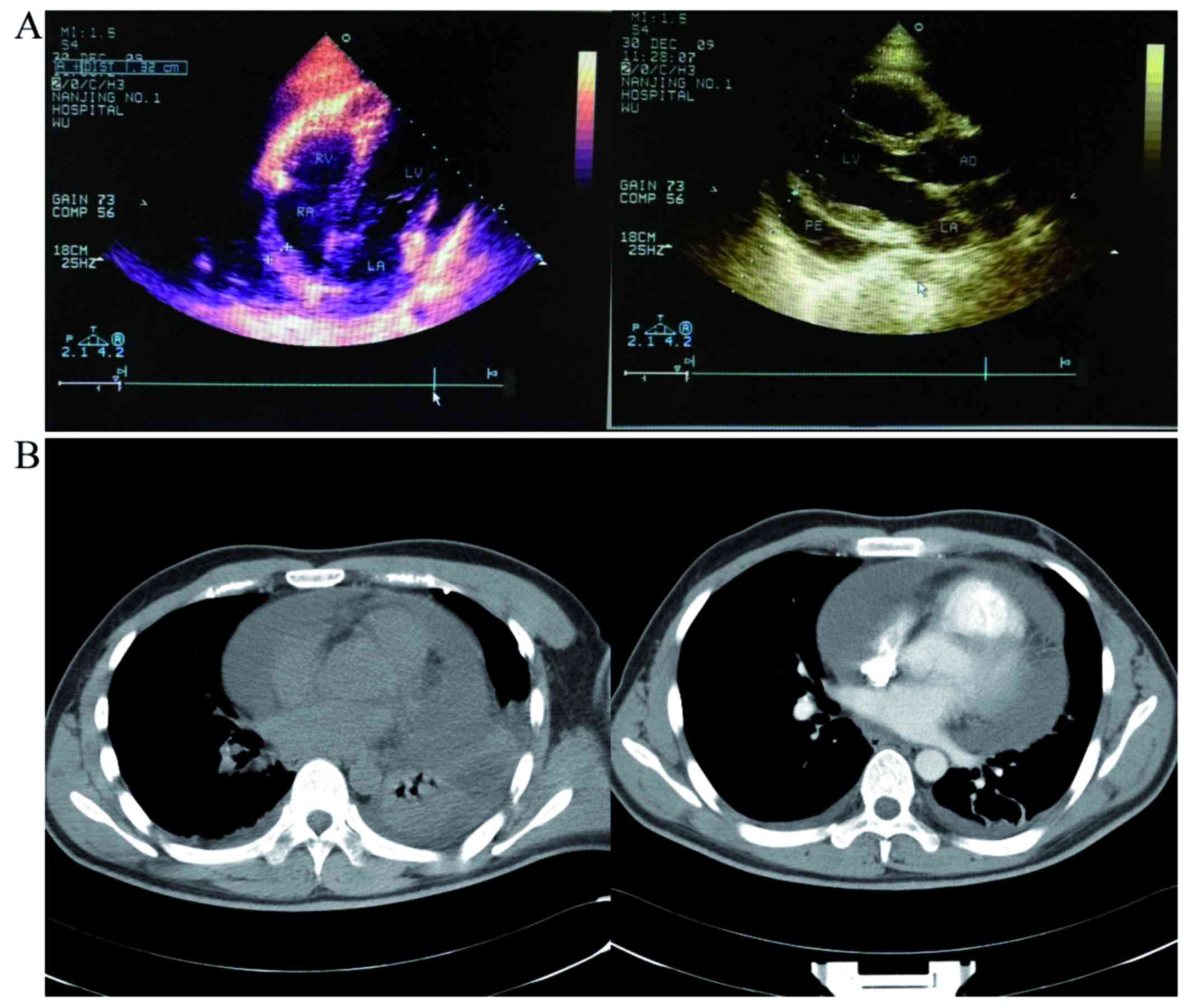
widening of the myocardial boundary, concurrent large pericardial
effusion and little-to-moderate pleural effusion (Fig. 1B). A total of 1,800 ml of pericardial
fluid were evacuated. Cytological examination of the pericardial
fluid only found reactive mesothelial cells, without acid-fast
bacilli or tumor cells. Echocardiography revealed thickening of the
free wall of the right ventricle (RV), with adhesions to the
adjacent pericardium and uneven thickening of the right atrium (RA)
to 0.8–1.4 cm. The thickened pericardium near the output of the RV
has also restricting myocardial motion. A liquid anechoic area was
detected in the pericardial cavity and pericarditis was highly
suspected (Fig. 1A). Experimental
antituberculosis treatment was refused when the Mantoux test was
found to be strongly positive. The patient was referred to our
hospital due to worsening shortness of breath and was tentatively
treated with antituberculotic agents (isoniazid and rifampin), with
a poor therapeutic effect and increasing volume of the pericardial
effusion. 18F-FDG imaging was performed to rule out
malignancy; it revealed highly increased uptake of
18F-FDG in the RA, the pericardium adjacent to the RV
and RA (Fig. 2B and C, arrows), and
mildly increased uptake along the inner thoracic wall (Fig. 2B, arrows). Ring-shaped radioactivity
aggregation and bone destruction in the sacrum were visualized on
18F-FDG imaging (Fig. 2D and
E, arrow) and 99mTc-methyl diphosphonate (MDP)
whole-body scan (Fig. 2A, arrow).
PMPM with RA infiltration and bone metastasis was highly suspected.
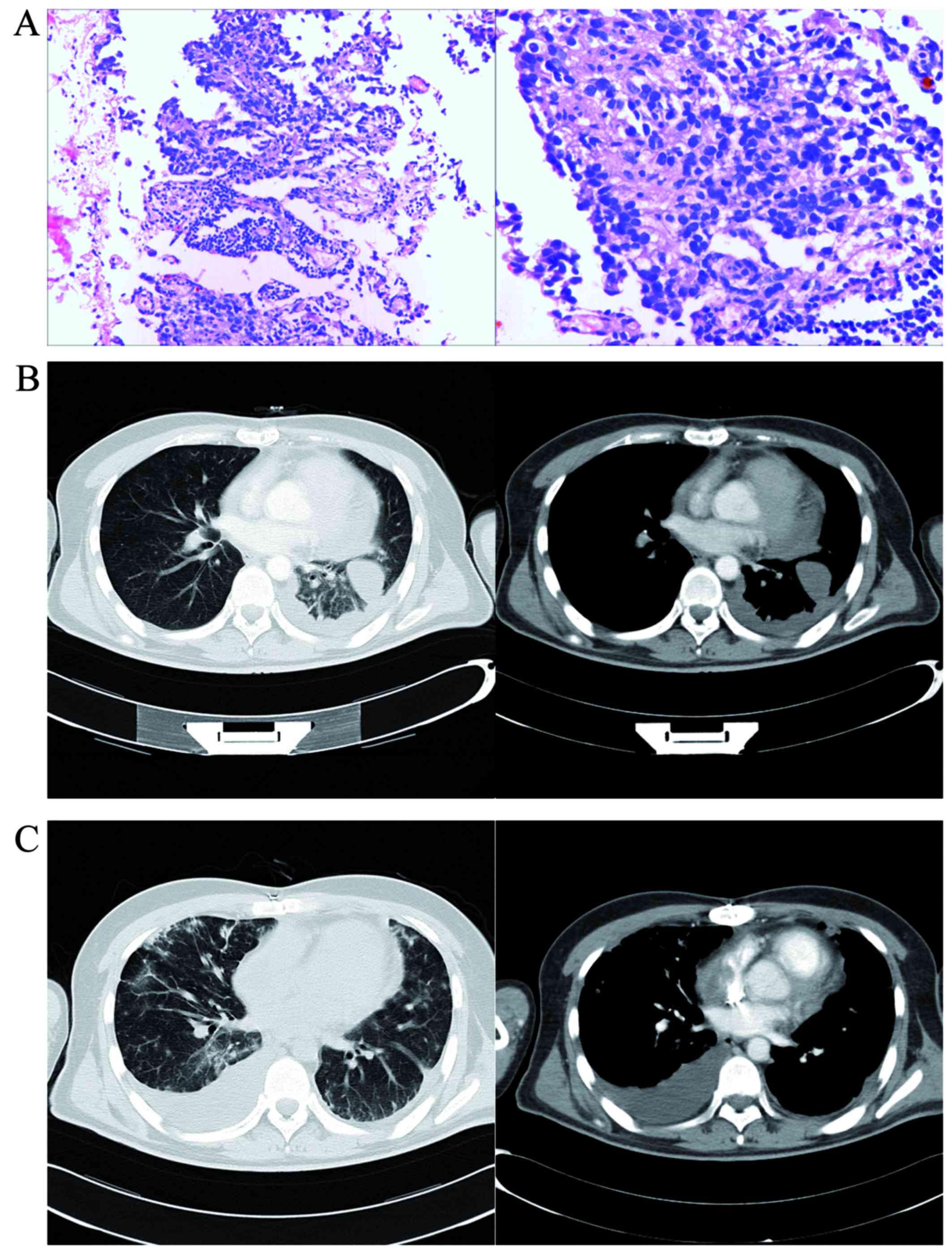
An incisional pericardial biopsy was performed and pathological
examination of the samples obtained by biopsy confirmed the
diagnosis of PMPM with atrial infiltration (Fig. 3A) (cytokeratin5/6+,
D2-40+, calretinin+, carcinoembryonic
antigen−, thyroid transcription factor 1−).
Following surgery, a doublet chemotherapy regimen (pemetrexed 500
mg/m2 + cisplatin 75 mg/m2 were administered
on the first day of each 3-week cycle, for a total of six cycles)
was introduced immediately after definitive diagnosis. During
follow-up, CT imaging revealed little pericardial effusion at 4
months postoperatively (Fig. 3B),
but lung metastasis and large pleural effusion were observed 1 year
after the operation (Fig. 3C). The
patient survived for >1.5 years after the diagnosis and
succumbed to severe pericardial effusion and cardiac tamponade in
September 2012.
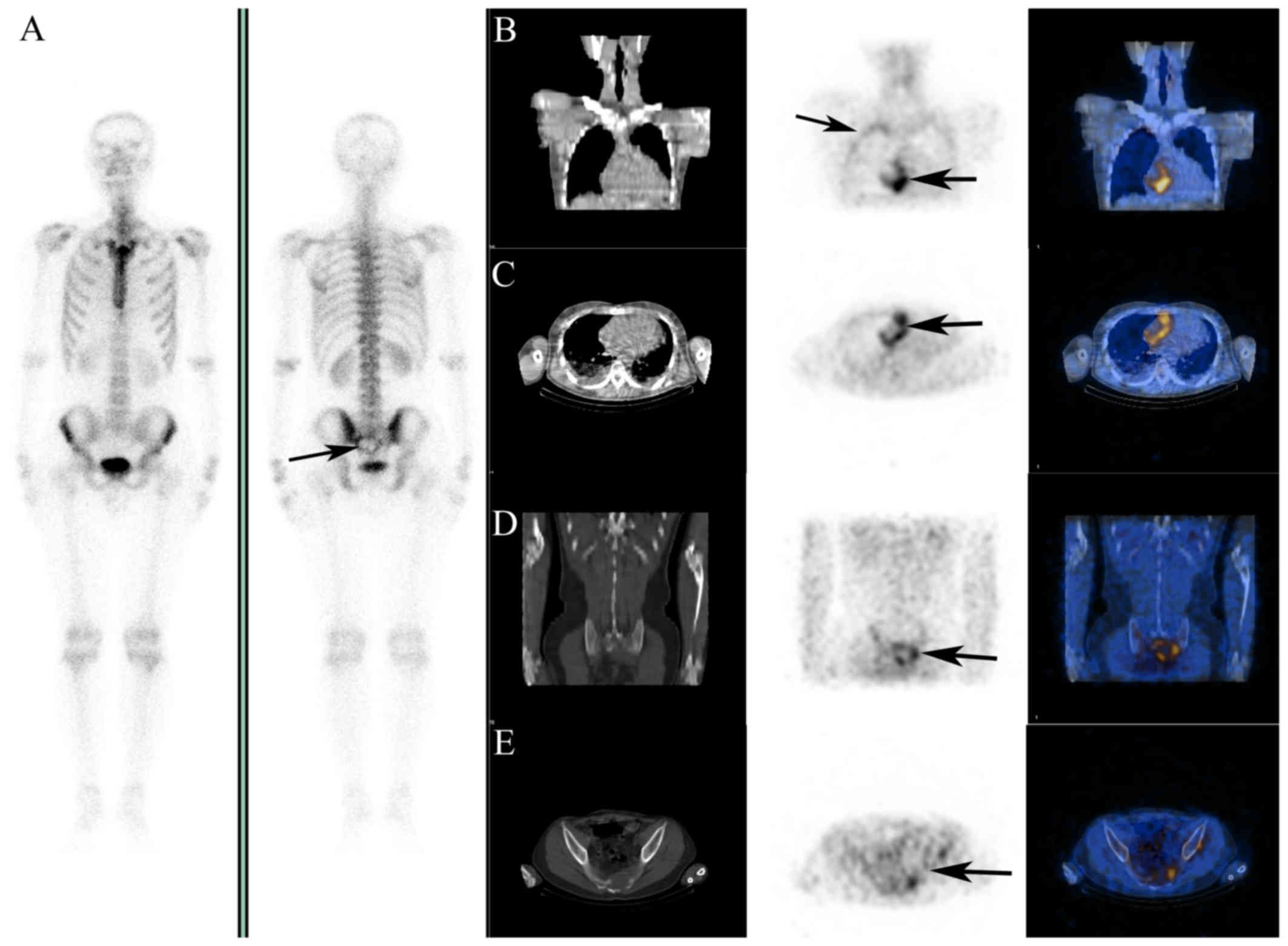 | Figure 2.18F-FDG images and
99mTc-MDP bone scan images. (A) 99mTc-MDP
whole-body scan showing a sacral osteolytic metastasis (arrow)
(left, anterior view; right, posterior view). (B and C) Highly
increased uptake of 18F-FDG was observed in the right
atrium and the pericardium adjacent to the right ventricle and
atrium, with mild increase in the inner wall of the thoracic cavity
(arrows). (D and E) Ring-shaped radioactivity aggregation and
osteolytic bone metastasis in the sacrum were visualized on
18F-FDG coincidence imaging (arrows). B-E: Left panel,
CT; middle panel, 18F-FDG PET; and right panel, PET-CT
fusion images. FDG, fluorodeoxyglucose; MDP, methyl diphosphonate;
CT, computed tomography; PET, positron emission tomography. |
Discussion
Malignant mesothelioma usually occurs in the
peritoneum or pleura, while it rarely occurs in the pericardium.
Primary malignant pericardial mesothelioma (PMPM) is an extremely
rare occurrence with a low incidence (<0.0022%) and a poor
prognosis (2).
Without a definitive etiology or specific clinical
manifestations (3), early diagnosis
and staging of PMPM may be difficult, particularly in the presence
of concurrent pericardial and pleural effusions (4–9).
Asbestos exposure is less frequently associated with pericardial
mesothelioma compared with pleural mesothelioma, and it is not
necessarily considered a risk factor for the development of
pericardial mesothelioma. Pericardial effusion/tamponade or
constrictive pericarditis is common, and its causes may be
infectious (tuberculosis, viral or bacterial infection) or
non-infectious diseases (tumor, rheumatism, endocrine and metabolic
diseases).
The clinical misdiagnosis rate of PMPM is extremely
high due to its non-specific symptoms, ranging from cough, dyspnea
and dysphagia to chest pain, as in the present case. The clinical
signs are often misdiagnosed as other conditions, such as coronary
heart disease, tuberculous pericarditis, atrial myxoma and
cardiomyopathy. In the present case, tentative antituberculosis
treatment was introduced due to a misdiagnosis based on a positive
PPD test. Aspiration and evaluation of pericardial fluid was
inconclusive, as it is difficult to differentiate malignant
mesothelioma cells from reactive cells.
The characteristic feature of PMPM is focal or
diffuse uneven solid growth of the mesothelium, with atypical
cavities surrounded by fibrous stroma (10). Among anatomical imaging tools,
echocardiography is the one most commonly used. CT and MRI may not
clearly delineate the mass and the boundary of PMPM, particularly
when adjacent myocardium is infiltrated. 18F-FDG
metabolism imaging is an alternative tool for the diagnosis and
accurate staging of most malignant tumors, although it is rarely
reported for pericardial mesothelioma (11). Increased tumor 18F-FDG
metabolism may be evident prior to the appearance of anatomical
changes. When increased FDG aggregation is demonstrated in the
pericardium of patients presenting with recurrent or unexplained
pericardial effusion, PMPM should be suspected (12). Multimodal imaging and clinical data
are important, while pathology remains the gold standard for the
definitive diagnosis of PMPM (13).
The prognosis of PMPM is extremely poor, with a
median survival of ~6 months (13,14).
Early and systemic therapy, such as surgical resection,
radiotherapy and chemotherapy, are required to prolong patient
survival. A doublet chemotherapy regimen (pemetrexed + cisplatin)
with pemetrexed maintenance was administered to our patient
immediately after surgical resection (14,15).
Multiple metastases in the lungs were diagnosed 1 year after the
operation and the patient survived for >1.5 years after the
diagnosis. 18F-FDG imaging may be a particularly useful
tool for early diagnosis, staging and response evaluation in
patients with PMPM (7,11,15).
References
|
1
|
Fine G: Primary tumors of the pericardium
and the heart. Cardiovasc Clin. 5:207–238. 1973.PubMed/NCBI
|
|
2
|
De Rosa AF, Cecchin GV, Kujaruk MR, Gayet
EG, Grasso LE and Rigou DG: Malignant mesothelioma of the
pericardium. Medicina (B Aires). 54:49–52. 1994.(In Spanish).
PubMed/NCBI
|
|
3
|
Rizzardi C, Barresi E, Brollo A, Cassetti
P, Schneider M and Melato M: Primary pericardial mesothelioma in an
asbestos-exposed patient with previous heart surgery. Anticancer
Res. 30:1323–1325. 2012.
|
|
4
|
Furst B, Liu CJ, Hansen P and Musuku SR:
Concurrent pericardial and pleural effusions: A double jeopardy. J
Clin Anesth. 33:341–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel J and Sheppard MN: Primary malignant
mesothelioma of the pericardium. Cardiovasc Pathol. 20:107–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mensi C, Giacomini S, Sieno C, Consonni D
and Riboldi L: Pericardial mesothelioma and asbestos exposure. Int
J Hyg Environ Health. 214:276–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Small GR, Nicolson M, Buchan K and
Broadhurst P: Pericardial malignant mesothelioma: A latent
complication of radiotherapy? Eur J Cardiothorac Surg. 33:745–747.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peregud-Pogorzelska M, Kaźmierczak J and
Wojtarowicz A: Intracavitary mass as the initial manifestation of
primary pericardial mesothelioma: A case report. Angiology.
58:255–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yakirevich E, Sova Y, Drumea K, Bergman I,
Quitt M and Resnick MB: Peripheral lymphadenopathy as the initial
manifestation of pericardial mesothelioma: A case report. Int J
Surg Pathol. 12:403–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Butz T, Faber L, Langer C, Körfer J,
Lindner O, Tannapfel A, Müller KM, Meissner A, Plehn G, Trappe HJ,
et al: Primary malignant pericardial mesothelioma-a rare cause of
pericardial effusion and consecutive constrictive pericarditis: A
case report. J Med Case Rep. 3:92562009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ost P, Rottey S, Smeets P, Boterberg T,
Stragier B and Goethals I: F-18 fluorodeoxyglucose PET/CT scanning
in the diagnostic work-up of a primary pericardial mesothelioma: A
case report. J Thorac Imaging. 23:35–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
AkSivrikoz İ, Önner H, Dündar Kasapoğlu E,
Çavuşoğlu Y and Dernek S: F-18 FDG PET/CT images of a rare primer
cardiac tumour: Primary pericardial mesothelioma. Anatol J Cardiol.
16:635–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papi M, Genestreti G, Tassinari D,
Lorenzini P, Serra S, Ricci M, Pasquini E, Nicolini M, Pasini G,
Tamburini E, et al: Malignant pericardial mesothelioma. Report of
two cases, review of the literature and differential diagnosis.
Tumori. 91:276–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama R, Sakai M, Nakamura T, Suemitsu
R, Okamoto T, Wataya H, Nishiyama K, Kamei T and Ichinose Y:
Triplet chemotherapy for malignant pericardial mesothelioma: A case
report. Jpn J Clin Oncol. 36:245–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung SM, Choi SJ, Kim MJ, Choi JY, Kim
HJ, Lee SY and Kang EJ: Positive response of a primary malignant
pericardial mesothelioma to pemetrexed plus cisplatin followed by
pemetrexed maintenance chemotherapy: A case report. Oncol Lett.
12:213–216. 2016. View Article : Google Scholar : PubMed/NCBI
|