Introduction
Mucinous carcinomas with signet ring cells in the
ovary are mostly metastatic lesions from a primary tumor.
Particularly when the ovarian carcinoma is predominantly composed
of signet ring cells, referred to as signet ring cell carcinoma
(SRCC), it is usually designated as a Krukenberg tumor, which is
metastatic SRCC that may originate from a number of anatomical
sites, most commonly the stomach. Only rare cases of primary SRCC
of the ovary have been reported in the literature to date (1–3). The
distinction between primary and metastatic SRCC of the ovary has
not been well delineated and may be challenging. We herein report
the case a patient diagnosed with primary SRCC of the ovary.
Case report
A 54-year-old woman was admitted to the Ulsan
University Hospital (Ulsan, South Korea) with a palpable firm
abdominal mass. The patient exhibited no major symptoms and had no
specific past history. The patient underwent an abdominal computed
tomography (CT) scan, which revealed a ~20-cm multiseptated cystic
and solid mass arising from the right ovary. The abdominal CT scan
did not reveal any lesions in the gastrointestinal tract.
The patient underwent total abdominal hysterectomy
with bilateral salpingo-oophorectomy, partial omentectomy and
incidental appendectomy. The removed right ovary exhibited a
sizeable mass, measuring 20.5×16.5×11.5 cm, and had an intact and
smooth external capsular surface. On sectioning, the mass contained
a multiloculated cystic component filled with mucinous fluid, and
eccentric solid components (Fig. 1).
The right ovary (4 µm) was fixed in 10% neutral buffered formalin
for 12 h at room temperature, and then at 45°C for 44 min in an
automated tissue processor. Subsequently, H& staining was
performed using an automated staining system and was processed for
27 min at room temperature. The tissue was analyzed under a light
microscope. The left ovary, uterus, cervix and appendix appeared to
be normal on macroscopic examination. Histologically, most of the
mass was composed of malignant mucinous epithelial cells arranged
in predominantly solid and slightly glandular patterns.
Characteristically, the tumor predominantly consisted of signet
ring cells, particularly in the area of the solid nests, which were
arranged in small groups or infiltrated as individual cells
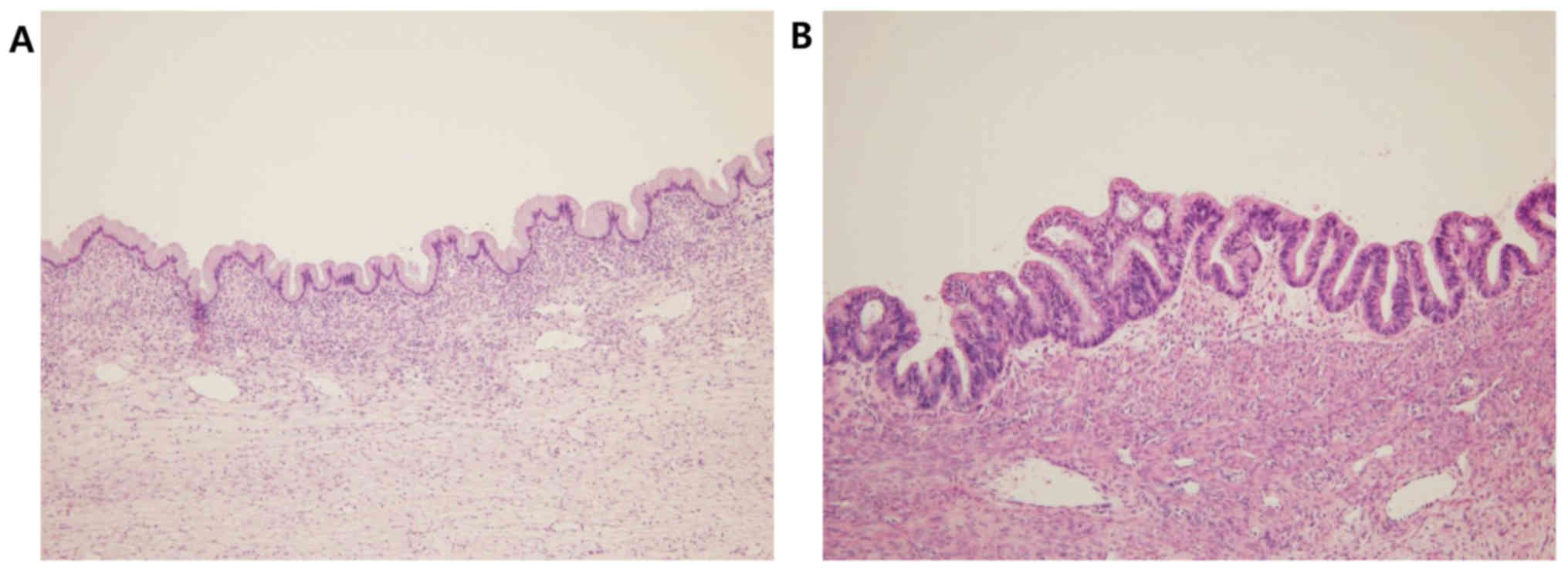
(Fig. 2). In some areas, the tumor
was lined by benign or borderline mucinous epithelium exhibiting
stratification and considerable atypia (Fig. 3). The stroma was generally fibrous
and moderately cellular, and focally loose or edematous. There was
no evidence of lymphovascular invasion, nodular growth pattern,
extracellular mucin or tumor cells on the ovarian surface.
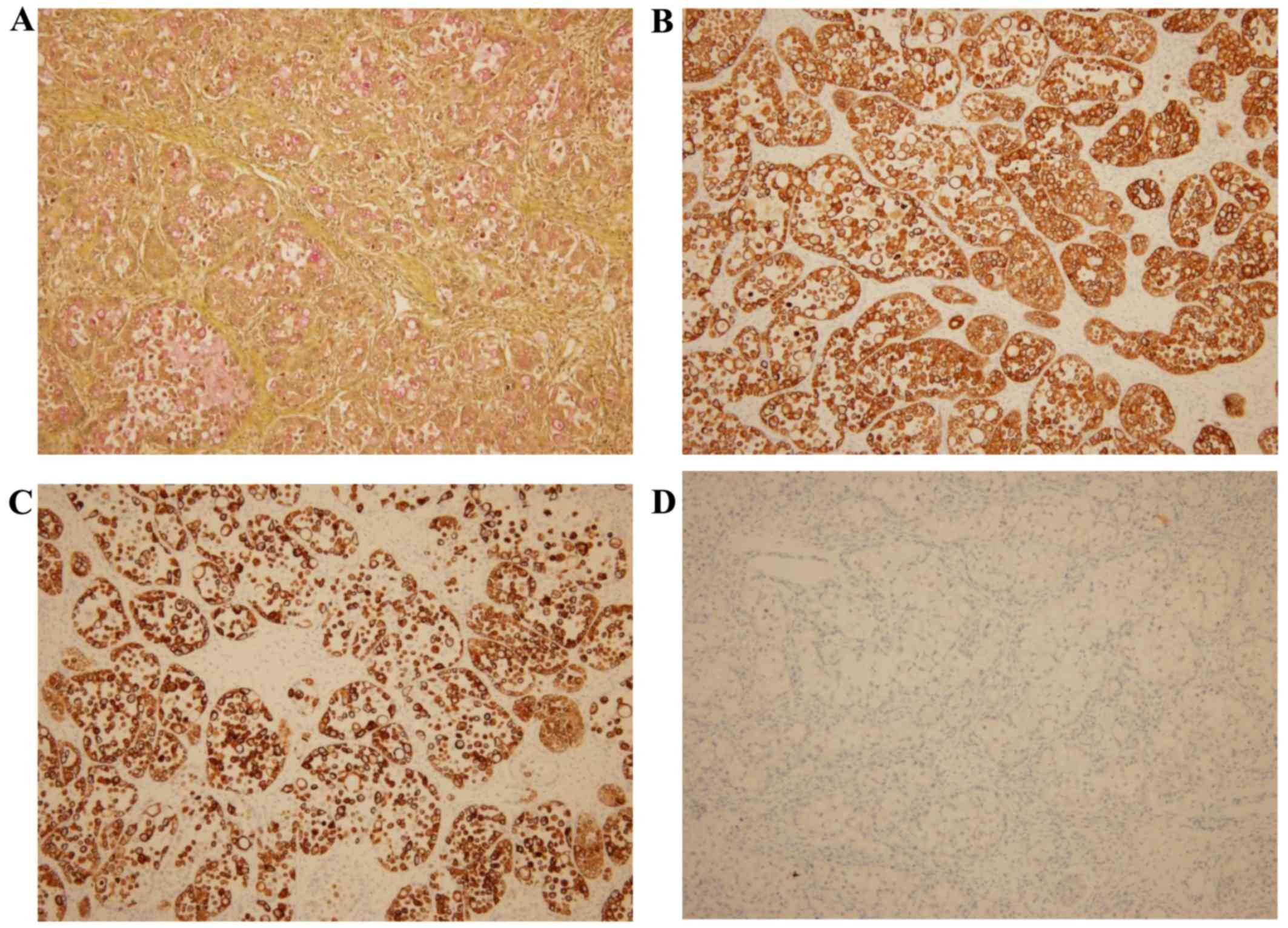
The tumor cells, including the signet ring cells,
exhibited diffuse positivity for cytokeratin (CK)7, CK20, alcian
blue and mucicarmin (Fig. 4).
However, there was no expression of chromogranin, synaptophysin,
CD56, caudal type homeobox 2 (CDX2), estrogen receptor,
progesterone receptor, or Wilms' tumor gene 1 (not shown, apart
from CD56).
Postoperative positron emission tomography (PET-CT)
revealed no residual malignancy and no alternative primary site.
The cytology of the peritoneal fluid was negative for malignant
cells. Based on these clinicoradiological and histopathological
findings, this case was diagnosed as primary SRCC of the ovary. One
year after the surgery, a follow-up CT of the abdomen also revealed
no evidence of recurrence or an alternative primary site. The
patient remains alive and well. These investigations helped exclude
other primary foci, and the tumor was definitively diagnosed as
primary ovarian SRCC.
Discussion
Primary ovarian mucinous carcinoma comprises 2–3% of
ovarian epithelial neoplasms (4).
The presence of signet ring cells in an ovarian mucinous carcinoma
is usually highly suspicious for a metastatic neoplasm, the primary
site of which is most likely in the gastrointestinal tract,
referred to as Krukenberg tumor (1,5). In
addition to the signet ring cells, other characteristics suggesting
a secondary mucinous neoplasm include bilaterality, small size, a
nodular element on macroscopic or microscopic examination,
prominent histological variation among different areas, destructive
invasion or individual cell stromal infiltration, microscopic
surface tumor involvement (surface implantations), tumor cells
floating in mucin pools, extraovarian extension and considerable
lymphovascular invasion, particularly at the ovarian hilum
(1,6).
Although the presence of signet ring cells is a key
pathological characteristic highly favoring a metastatic rather
than a primary neoplasm of the ovary, in the present case the
neoplasm was considered to be a primary ovarian tumor due to the
following findings: Unilaterality, large size, malignant glands in
a fibrous stroma, lack of surface implantations, lack of
lymphovascular invasion and no extraovarian spread. Furthermore,
the tumor displayed admixed components of benign mucinous
cystadenoma and borderline mucinous tumor. The absence of several
other characteristics of a metastatic neoplasm and the presence of
admixed benign-appearing areas support that this was a primary
ovarian neoplasm. Based on these findings, the diagnosis was
primary ovarian SRCC.
Immunohistochemistry may be applied as an additional
method to help distinguish between primary and metastatic mucinous
carcinoma of the ovary. In particular, several primary ovarian
mucinous neoplasms display intestinal differentiation and express
enteric markers, such as CK20, carbohydrate antigen 19–9,
carcinoembryonic antigen and CDX2, at least partially, despite
usually maintaining their diffuse CK7 expression (7,8). In the
present case, the tumor was diffusely positive for CK20 and CK7,
similar to primary ovarian mucinous carcinoma of the intestinal
type (8). However, these enteric
markers are also variably positive in the majority of upper and
lower gastrointestinal adenocarcinomas and pancreatobiliary
adenocarcinoma (7). As the
immunophenotypes of a primary ovarian mucinous tumor, particularly
one containing abundant signet ring cells, and a metastatic
mucinous tumor from the stomach, pancreatobiliary tract, appendix,
or colorectum, may overlap, immunohistochemical studies may be of
limited value in confirming the primary or a metastatic nature of
ovarian mucinous tumors. Therefore, the clinical history and
radiological findings also have to be carefully reviewed and
integrated with thorough gross inspection and histopathological
findings to ensure correct diagnosis.
In the present case, diffuse CK7 and CK20 positivity
and CDX2 negativity were helpful in excluding the possibility of
colorectal or appendiceal primaries (1), whereas negativity for chromogranin,
synaptophysin and CD56 help exclude other primary ovarian mucinous
tumors that may comprise signet ring cells, such as goblet cell
carcinoid.
Possible primary lesions in the female genital
tract, such as the cervix, and in the appendix were excluded
following total abdominal hysterectomy, bilateral
salpingo-oophorectomy and incidental appendectomy and examination
of the resected specimens. No other lesions in the gastrointestinal
tract were identified on abdominal CT. Consequently, primary
ovarian SRCC was diagnosed. The remaining point of argument in this
case is that a small occult primary neoplasm in other organs, most
commonly in the stomach or appendix, may have been missed. However,
postoperative PET-CT and follow-up CT 1 year after surgery showed
no residual malignancy or alternative primary site. Taking into
consideration the histopathological findings, these radiological
evaluations support the exclusion of another primary focus, and the
tumor was definitively confirmed as a primary ovarian neoplasm.
In conclusion, we herein described a rare case of
primary ovarian SRCC. The distinction between primary and
metastatic ovarian mucinous carcinomas, particularly those
consisting of predominantly signet ring cells, has not been well
delineated. All aspects of the pathological evaluation and clinical
correlations are crucial for correct diagnosis. The aim of this
case report was to remind pathologists to consider primary ovarian
SRCC as a differential diagnosis when they encounter ovarian tumors
with a major signet ring cell component.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HJ and KR performed the histological examination. JH
and KB wrote the manuscript and KB supervised the study throughout.
All the authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient prior to surgery.
Patient consent for publication
Written informed consent was obtained from the
patient regarding the publication of the case. details and
associated images.
Competing interests
The authors have no competing interests to
disclose.
References
|
1
|
McCluggage WG and Young RH: Primary
ovarian mucinous tumors with signet ring cells: Report of 3 cases
with discussion of so-called primary Krukenberg tumor. Am J Surg
Pathol. 32:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Safadi S, Stahl U, Tinneberg HR,
Hackethal A and Muenstedt K: Primary signet ring cell mucinous
ovarian carcinoma: A case report and literature review. Case Rep
Oncol. 3:451–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
P JG, . R VC, P KM and Narasimhan L:
Primary ovarian mucinous carcinoma with signet ring cells - report
of a rare case. J Clin Diagn Res. 8:FD12–FD13. 2014.
|
|
4
|
Seidman JD, Cho KR, Ronnett BM and Kurman
RJ: Surface epithelial tumours of the ovaryBlausteins Pathology of
Female Genital Tract. 6th edition. Springer; New York: pp. 745–749.
2010
|
|
5
|
Kiyokawa T, Young RH and Scully RE:
Krukenberg tumors of the ovary: A clinicopathologic analysis of 120
cases with emphasis on their variable pathologic manifestations. Am
J Surg Pathol. 30:277–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidman JD, Kurman RJ and Ronnett BM:
Primary and metastatic mucinous adenocarcinomas in the ovaries:
Incidence in routine practice with a new approach to improve
intraoperative diagnosis. Am J Surg Pathol. 27:985–993. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCluggage WG and Young RH:
Immunohistochemistry as a diagnostic aid in the evaluation of
ovarian tumors. Semin Diagn Pathol. 22:3–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SY, Kim HS, Hong EK and Kim WH:
Expression of cytokeratins 7 and 20 in primary carcinomas of the
stomach and colorectum and their value in the differential
diagnosis of metastatic carcinomas to the ovary. Hum Pathol.
33:1078–1085. 2002. View Article : Google Scholar : PubMed/NCBI
|