Introduction
Combining conventional systemic chemotherapy with
bevacizumab, an angiogenesis inhibitor, is currently recommended as
first-line treatment for patients with metastatic colorectal cancer
(mCRC), as it achieves a median overall survival of 22.7 months
(1).
Bevacizumab was developed by Ferrara et al as
a recombinant humanized antivascular endothelial growth factor
monoclonal immunoglobulin G antibody (2) and it has been approved by the US Food
and Drug Administration as an adjuvant agent in the treatment of
mCRC. However, bevacizumab therapy has been associated with certain
adverse events, such as bleeding (3%), gastrointestinal perforation
(2%), arterial thromboembolism (1%), hypertension (5.3%),
proteinuria (1%) and wound healing complications (1%) (3–6).
Furthermore, hemorrhagic complications, such as pulmonary
hemorrhage and hemoptysis, have been reported, mainly in patients
with lung cancer, whereas gingival and vaginal bleeding have also
been reported, although less commonly (3). In 2015, a retrospective study by
Collins et al (7) reported
that bleeding occurred in 41% of mCRC patients; however, the
incidence of grade 3 bleeding was found to occur in only 1.6% of
the cases, and there were no reported cases of grade 4 or 5
bleeding.
We herein present two cases of pseudoaneurysm with
massive lower gastrointestinal bleeding in patients with mCRC
during treatment with FOLFIRI and bevacizumab.
Case reports
Case 1
A 57-year-old male patient was diagnosed with rectal
carcinoma with liver metastases. The patient had a previous history
of hypertension, which was well-controlled with medication, but no
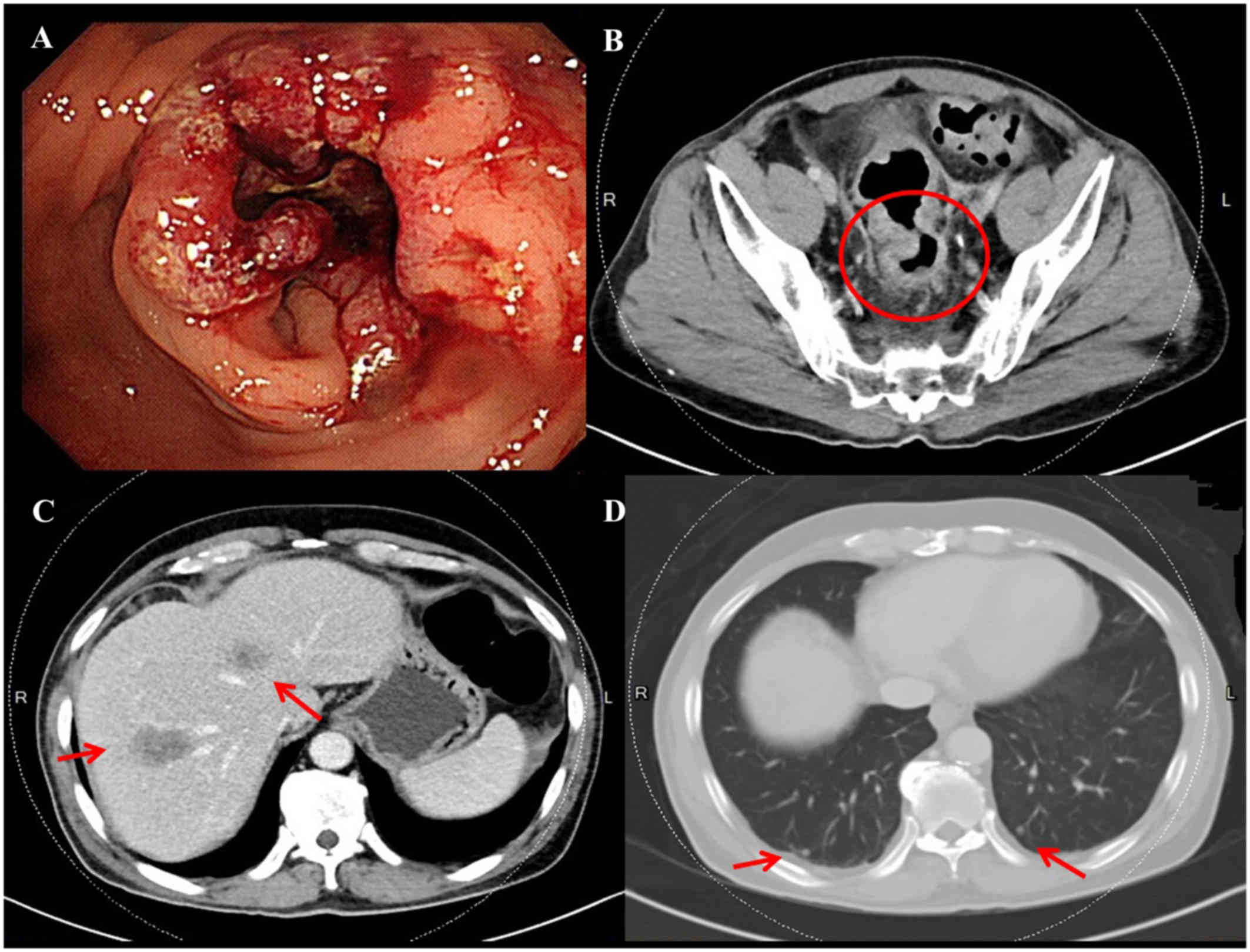
previous history of malignancy. Colonoscopy revealed a polypoid
tumor with superficial ulceration located 8 cm proximal to the anal
verge (Fig. 1A); biopsy of the
rectal tumor was suggestive of moderately differentiated
adenocarcinoma. Abdominal computed tomography (CT) scans revealed a
rectal tumor (Fig. 1B, circle) with
concomitant multiple liver metastases (Fig. 1C and D, arrows) and regional lymph
nodes metastases; the disease was determined to be clinical stage
cT3N2aM1a (stage IVa). Neoadjuvant concurrent chemoradiotherapy
(CCRT) was administered in accordance with treatment guidelines,
and FOLFIRI [irinotecan 120 mg/m2 as a 120-min IV
infusion, leucovorin (LV) 200 mg/m2 as an IV infusion
over 2 h, and 5-fluorouracil (5-FU) 2,800 mg/m2 as an IV
infusion over a 46-h period biweekly] combined with bevacizumab (5
mg/kg) was administered as a first-line chemotherapeutic regimen.
After a treatment period of 3 months, CT scans were used to
evaluate the response to neoadjuvant therapy; the scans revealed
simultaneous shrinkage of both the liver metastatic lesion and the
primary rectal tumor.
Six days after the 9th cycle of bevacizumab
injections, the patient visited our emergency room after having
passed a massive bloody clot from his anus. The physical findings
on initial presentation included a blood pressure of 124/78 mmHg,
heart rate of 130 beats per min, and hemoglobin levels of 8.6 g/dl.
Contrast-enhanced abdominal CT scans revealed no evidence of
gastrointestinal tract or vascular abnormalities (data not shown).
The patient received a blood transfusion and was admitted to the
hospital with a tentative diagnosis of lower gastrointestinal
bleeding.
On colonoscopic examination performed after
admission, there was blood in the sigmoid colon and rectum;
however, there was no sign of active bleeding in the colon or
rectum. The patient's hemoglobin level decreased to 6.2 g/dl, even
after the blood transfusion, and an angiography was scheduled as a
further diagnostic measure. The angiography revealed a
pseudoaneurysm with contrast extravasation in a branch of the left
internal iliac artery (Fig. 2A).
Active bleeding from the pseudoaneurysm was observed; therefore,
transcatheter embolization was performed. A Tornado coil and Nester
coil (both from Cook Inc., Bloomington, IN, USA) were inserted in
this branch of the left internal iliac artery, and ~3 ml of Avitene
mixture (Davol Inc., Woburn, MA, USA) was infused together with a
contrast medium. Post-embolization angiography revealed no signs of
further bleeding (Fig. 2B), and the
patient's hemoglobin level progressively increased to 9.6 g/dl. The
patient was eventually discharged after 2 weeks of uneventful
hospitalization, but he ultimately succumbed to progressive
metastatic multiple liver lesion deterioration 1 year after this
event.
Case 2
A 65-year-old male patient was diagnosed with
rectosigmoid carcinoma and associated liver and lung metastasis.
The patient had a previous history of hypertension and type 2
diabetes mellitus, which was well-controlled with medication, but
no previous history of malignancy. Colonoscopy revealed an
ulcerated tumor located 14–18 cm proximal to the anal verge
(Fig. 3A); biopsy was suggestive of
adenocarcinoma. Abdominal CT scans revealed a rectosigmoid tumor
(Fig. 3B) with concomitant multiple
liver metastases and bilateral lung metastasis; the disease was
determined to be clinical stage T4aN2aM1b (stage IVb). CCRT was
used in accordance with treatment guidelines, and FOLFIRI combined
with bevacizumab was administered as a first-line chemotherapeutic
regimen. After a treatment period of 3 months, CT scans were
performed to evaluate the response to neoadjuvant therapy; the
scans revealed mild progression of the rectosigmoid cancer, with
pericolic fat infiltration.
Eight days after the 5th cycle of bevacizumab
injections, the patient visited our emergency room after having
observed a large amount of blood in the stool. On initial physical
examination, the blood pressure was 97/66 mmHg, the heart rate was
104 beats per min, and the hemoglobin level was 7.8 g/dl. The
patient received a blood transfusion and was admitted to our
hospital with a tentative diagnosis of lower gastrointestinal
bleeding.
Contrast-enhanced abdominal CT scans revealed a
small pseudoaneurysm (0.5 cm), and an angiography was scheduled as
a further diagnostic measure. The angiography revealed a
pseudoaneurysm with contrast extravasation in the superior rectal
artery (Fig. 3C). Active bleeding
from the pseudoaneurysm was observed; therefore, transcatheter
embolization with coils was performed. Straight coils (2×1 and 5×1
mm) were inserted in the pseudoaneurysm feeder, and
post-embolization angiography revealed no further bleeding
(Fig. 3D). The patient was
eventually discharged after 2 weeks of uneventful hospitalization,
but he eventually succumbed to progressive metastatic multiple
liver and lung lesion deterioration 9 months after this event.
Discussion
Radiological imaging plays an important role in
primary diagnostics, staging, evaluation of treatment response,
follow-up, and even for minimally invasive interventions. CT and
MRI are included in national and international guidelines and
structured reporting is strongly recommended (8).
Bleeding complications associated with bevacizumab
therapy may be attributed to the anti-angiogenic effect of this
agent, which inhibits endothelium growth, thus resulting in vessel
wall breach and the formation of pseudoaneurysms. In the present
cases, the exact pathophysiological mechanism involved in
pseudoaneurysm development remains unclear. However, abdominal CT
scans of the patient performed prior to bevacizumab therapy
revealed no radiological signs of aneurysm formation.
Angiogenesis inhibitors have rapidly emerged as
first-line treatment in cancer therapy, usually in combination with
cytotoxic chemotherapy. The most frequent toxicity encountered with
the use of bevacizumab is hypertension, which occurs in up to 32%
of the patients (9). Patients with
hypertension are most commonly treated with oral agents, such as
diuretics or calcium channel blockers; however, a small number of
patients do not respond to this treatment, and bevacizumab must be
discontinued (10,11). Our patient had hypertension prior to
treatment; the condition did not worsen with the concomitant
administration of bevacizumab and was well-controlled with the use
of antihypertensive medication.
The exact pathophysiological mechanism involved in
the development of the pseudoaneurysm in our cases has not been
fully elucidated. However, a possible correlation between the
injections of bevacizumab and the formation of a common iliac or
superior rectal artery aneurysm may be reasonably hypothesized;
thus, it cannot be excluded that bevacizumab may have been a
potential trigger. The aim of the present study was to alert
clinicians to this rare adverse event associated with the use of
bevacizumab.
Based on this observation, it is recommended that
patients selected for anti-angiogenic treatment are closely
monitored through imaging, and whenever a gastrointestinal massive
bleeding from a pseudoaneurysm occurs or a pseudoaneurysm increases
in size, it should be managed with selective embolization.
In conclusion, to the best of our knowledge, cases
of pseudoaneurysm formation in mCRC patients under bevacizumab
therapy have not been previously reported. We herein describe two
such cases, and although there is no direct evidence of an
association between bevacizumab therapy and the formation of
pseudoaneurysms, the need for its consideration during
anti-angiogenic therapy must be emphasized. When a pseudoaneurysm
with bleeding becomes apparent and is life-threatening,
transcatheter embolization is the preferred treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants through
funding from the Ministry of Science and Technology (MOST
107-2321-B-037-003-) and the Kaohsiung Medical University Hospital
(KMUHS10601, KMUHS10608, KMUHA10664). In addition, this study was
supported by the Grant of Biosignature in Colorectal Cancers,
Academia Sinica, Taiwan, R.O.C.; and Grant by the Kaohsiung Medical
University aim for the top University, grant no. KMU-S105011.
Availability of data and materials
Not applicable.
Authors' contributions
CL was responsible for document recording and
manuscript writing. HT, CH and YY were responsible for image
analyses and providing the colonoscopic images. TT was responsible
for angiographic diagnosis and treatment. JW was responsible for
coordinating with investigators. All authors contributed to
reviewing the various drafts of the manuscript and approved the
final version of the article.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Kaohsiung Medical University Hospital
(KMUHIRB-20130020).
Patient consent for publication
The patients have signed an informed consent form
regarding the publication of the case details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Cutsem E, Rivera F, Berry S,
Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL,
Georgoulias V, Peeters M, et al: Safety and efficacy of first-line
bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: The BEAT study. Ann Oncol.
20:1842–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gordon MS and Cunningham D: Managing
patients treated with bevacizumab combination therapy. Oncology. 69
Suppl 3:S25–S33. 2005. View Article : Google Scholar
|
|
4
|
Heinzeling JH and Huerta S: Bowel
perforation from bevacizumab for the treatment of metastatic colon
cancer: Incidence, etiology, and management. Curr Surg. 63:334–337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
You YN, Wolff BG, Boardman LA,
Riegert-Johnson DL and Qin R: Peutz-Jeghers syndrome: A study of
long-term surgical morbidity and causes of mortality. Fam Cancer.
9:609–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kesmodel SB, Ellis LM, Lin E, Chang GJ,
Abdalla EK, Kopetz S, Vauthey JN, Rodriguez-Bigas MA, Curley SA and
Feig BW: Preoperative bevacizumab does not significantly increase
postoperative complication rates in patients undergoing hepatic
surgery for colorectal cancer liver metastases. J Clin Oncol.
26:5254–5260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collins D, Ridgway PF, Winter DC, Fennelly
D and Evoy D: Gastrointestinal perforation in metastatic carcinoma:
A complication of bevacizumab therapy. Eur J Surg Oncol.
35:444–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baeßler B, Maintz D and Persigehl T:
Imaging procedure for colorectal cancer. Visc med. 32:166–171.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao D, Guo CH, Liu JW, Yang X and Li Q:
Bleeding after bevacizumab treatment in patients with metastatic
colorectal cancer. Tumori. 101:46–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kabbinavar FF, Schulz J, McCleod M, Patel
T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B and Novotny WF:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: Results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pañares RL and Garcia AA: Bevacizumab in
the management of solid tumors. Expert Rev Anticancer Ther.
7:433–445. 2007. View Article : Google Scholar : PubMed/NCBI
|