Introduction
It has long been known that the brain governs body
functions with feedforward and feedback neuronal signals
transmitted through the peripheral nervous system (PNS), while it
also regulates its own functions through structural connections
among different parts of the central nervous system (CNS) via nerve
fibers. Oligodendrocytes form a laminated, lipid-rich wrapping
known as a myelin sheath around most nerve fibers in the CNS, which
plays an important role as an insulating coating in maintaining the
fast salutatory conduction of action potentials along nerve fibers
(1–3). In the past century, only a few
studies have focused on the structure and function of nerve fibers
compared with nerve cell bodies and synapses. However, over the
past few years, the myelin sheath and nerve fibers have attracted
increasing attention as it has been demonstrated that white matter
exhibits activity-dependent plasticity in a certain period of life
(4,5) and can actively affect how the brain
learns and dysfunctions (2,6).
Several studies (7–11)
have found that the changes in the nerve fibers and myelin sheath,
which have been suggested to be affected by aging, are likely to be
an important factor in the development of age-related cognitive
decline in humans and primates. Myelination is highly specialized
and one of the major events occurring during the development of the
nervous system (1,12). Numerous nerve function deficits
appear with age and have been shown to be the consequence of
peripheral myelin abnormalities (13–16).
Furthermore, age has been indicated to influence the capability of
peripheral nerves to regenerate and reinnervate target organs, but
with different patterns for motor and sensory nerve fibers
(17,18). However, studies on the effect of
aging on myelin sheaths have often been based on comparisons of
only two experimental groups, whereas the lifespan and the duration
of growth periods should be carefully taken into account. The
necessity for assessment at multiple time-points in age studies has
been highlighted (19–21).
Several quantitative studies have been conducted on
the age-dependent morphological changes that occur in the nervous
system; however, these studies have primarily focused on the
effects of age on peripheral nerve trunks (15,22–25).
To date, comprehensive, detailed investigations concerned with
myelin sheaths in the CNS remain limited.
The present study was a controlled morphological
investigation of the sensorimotor white matter in multiple age
groups of male Sprague Dawley rats covering postnatal to aging
periods. The spinal posterior/lateral funiculus and pyramidal tract
were selected to represent the sensory and motor projection fibers,
respectively. Ultrastructural analyses were performed to define the
pattern of changes in the myelinated fibers (MFs) that occur with
age in normal animals.
Materials and methods
Animals and treatment
Experiments were performed on male Sprague Dawley
albino rats [purchased from Laboratory Animal Center of the Fourth
Military Medical University, (FMMU), Xi’an, China]. Time-points of
postnatal day (PND) 14, postnatal month (PNM) 2, PNM 5, PNM 12, PNM
18 and PNM 26 were selected for analysis. The animals were housed
in plastic cages with access to food and water ad libitum
and maintained under a 12-h light/dark cycle at room temperature
(22–26°C). The experimental protocols were approved by the
Institutional Animal Care and Use Committee of the FMMU (Permit no.
SCXK2007-007). The present study was performed in accordance with
the National Institutes of Health (NIH) Guide for the Care and Use
of Laboratory Animals (NIH Publications no. 80-23) revised in
1996.
Electron microscopy examination of
somatic nerve fibers
Five rats per group were infused with 2.5%
glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4) following anesthetization with sodium pentobarbital (80
mg/kg; Sigma-Aldrich, St. Louis, MO, USA). The brain stem and
spinal cord were then collected and fixed with 3% glutaraldehyde in
0.1 M phosphate buffer (pH 7.4) overnight at 4°C. Transverse
sections (1 mm) of the spinal cord were prepared using a vibrating
DTK-1000 microtome (Dosaka, Kyoto, Japan). The posterior and
lateral funiculus, as well as the pyramidal tract, were dissected
and cut into small pieces of similar dimensions, prior to
underdoing osmification in 1% OsO4 in 0.1 M sodium
cacodylate buffer for 2 h at room temperature and dehydration with
an ascending acetone series. The osmicated tissue blocks were
further embedded in Epon-812 (Serva, Heidelberg, Germany) and
trimmed under a light microscope. Ultrathin sections (50–70 nm)
were cut perpendicularly to the axis of the nerve fibers with a
diamond knife on an LKB-11800 ultramicrotome (LKB, Uppsala, Sweden)
and collected by copper grids (300 mesh). The ultrathin sections
stained with uranyl acetate and lead citrate were observed under an
electron microscope (EM; Hitachi, Tokyo, Japan) and
microphotographs were captured at the same time.
Histopathological evaluation
Morphometric evaluation of the MFs was performed by
assessing ≥200 individual MFs from the sets of photographs selected
from five rats at each time-point. Only MFs whose contour was
completely within each photograph were used. Measurements of myelin
sheath thickness, axon and fiber diameters and g-ratio (which was
determined by dividing the axon diameter by the fiber diameter)
were obtained using Image-Pro Plus software (Media Cybernetics,
Silver Spring, MD, USA). The age-related pathological alterations
in the myelin sheath were quantified through pathological grading
and counting of the fibers using a method that was established in a
previous study by our group (26).
Damaged MFs were classified into three grades according to the
severity and extent of deterioration, and the percentage of damaged
nerve fibers was assessed. Grading was performed as follows: I,
minor pathological changes, including myelin lamina rarefaction,
focal demyelination or vacuolization, with the axon being less
affected; II, moderate pathological changes, including myelin
lamina reticulation, focal demyelination, vacuolization and axonal
changes, such as increased electron density, lipofuscin deposition
or glycogen granules; III, more severe pathological changes,
including marked myelin damage or disruption accompanied by axonal
degeneration and loss.
Statistical analysis
The Shapiro-Wilk normality test was first used to
determine which data were normally or non-normally distributed.
Normally distributed data are expressed as the mean ± standard
error of the mean, while non-normally distributed data are
expressed as the median with a maximum and minimum. One-way
analysis of variance followed by Fisher’s post hoc least
significant difference analysis was used for comparisons of the
normally distributed data (periodicity of myelin lamellae) between
groups. The Kruskal-Wallis H test and Mann-Whitney U test were used
for the comparison of non-normally distributed data (g-ratios and
the proportions of damaged MFs). P<0.05 was considered to
indicate a significant difference between values.
Results
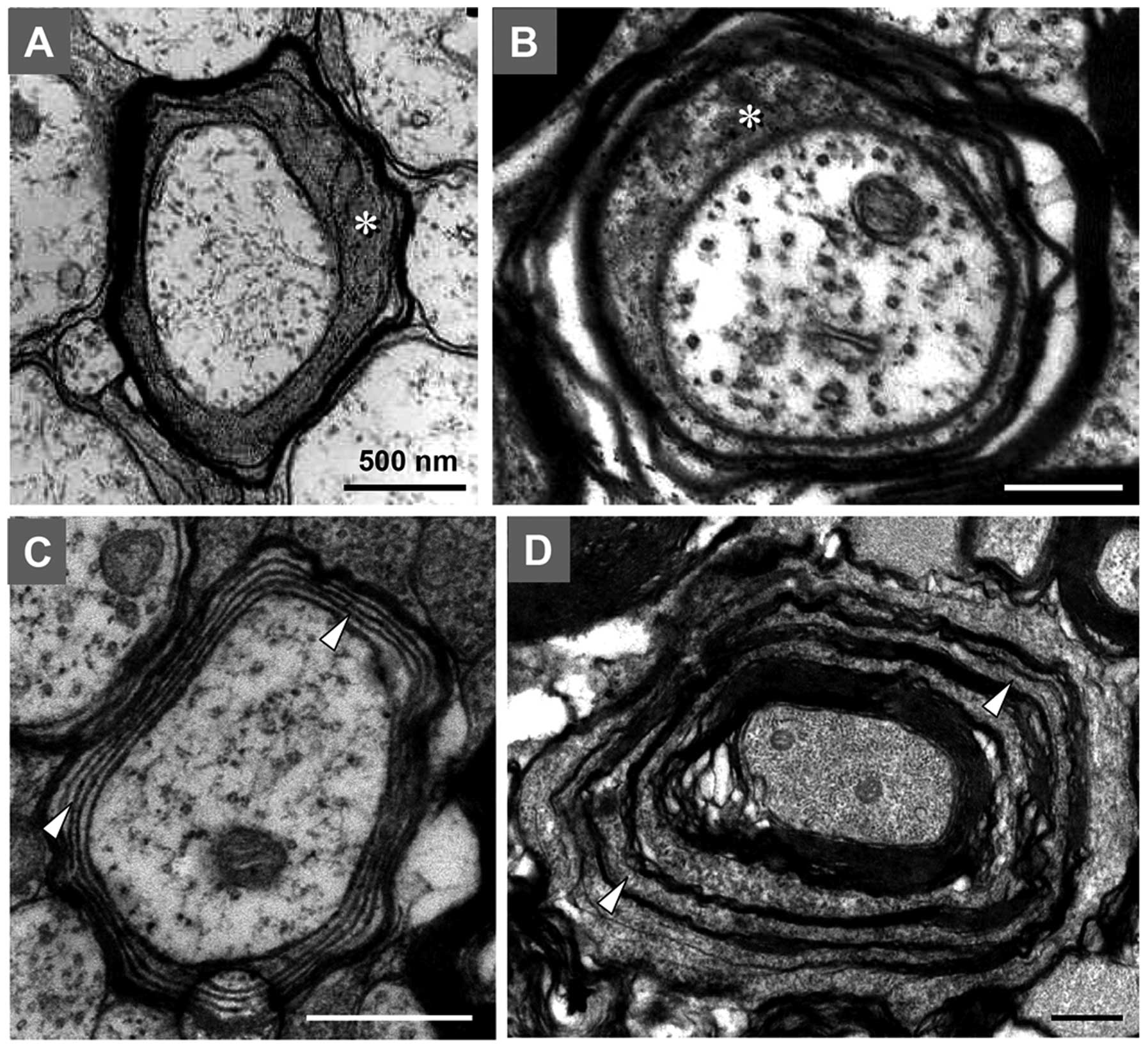
Age-related structural alterations in the
general morphology of the myelin sheath
Based on the analysis of the structure of the myelin
sheath in the posterior funiculus and pyramidal tract, significant
age-related alterations were identified in the sensorimotor
projection MFs of rats. In the spinal posterior funiculus of the
rats, myelination was not fully completed at PND 14, and ~30% of
the fibers appeared as unmyelinated axons. The oligodendrocytes of
this development period were activated and contained little
heterochromatin, which contributed to the comparatively pale
appearance of the nuclei of the oligodendrocytes under the EM
(Fig. 1A). Myelination was not
completed until PNM 2, and nearly all axons were wrapped by an
integrated myelin sheath at this time-point. During this period,
the level of heterochromatin in the nuclei of the oligodendrocytes
increased, which was a typical characteristic of normal
oligodendrocytes (Fig. 1B).
Significant levels of myelin breakdown occurred in the spinal
posterior funiculus of rats at PNM 12, including myelin tubercles,
myelin ballooning, the general separation of myelin lamellae and
the atrophy and degeneration of axons (Fig. 1C). A comparable but more extensive
myelin breakdown was observed in the posterior funiculus of rats at
PNM 26. Severe decompaction of lamellae gave the myelin structure a
wave-like appearance. The degeneration of several oligodendrocytes
and axons was identified by an abnormally high electron density
(Fig. 1D).
The age-related changes in the myelin sheath in the
pyramidal tract were similar to those in the posterior funiculus.
However, compared with the posterior funiculus, the development of
the myelin sheath in the pyramidal tract was moderately delayed. A
total of >50% fibers had unmyelinated axons at PND 14 and only a
small number of unmyelinated fibers could be observed in the
pyramidal tract at PNM 2. Myelin breakdown appeared at PNM 12 and
became more aggravated at PNM 26 (Fig.
2A–D).
Quantitative analysis of the age-related
structural alterations in the myelin sheath
Using quantitative image analysis tools, myelin
thickness, axon diameters and g-ratios were measured in the
posterior funiculus and pyramidal tract at PNM 2, 12 and 26. The
rightward shift of the peak in the frequency distribution of axon
diameters indicated that the axons were enlarged in the posterior
funiculus and pyramidal tract at PNM 12 and 26 (Fig. 3A and D). The g-ratio as a function
of axon diameter also changed with increasing age: The slope rate
of the g-ratio fitting line was decreased at PNM 12 and 26, while
the distribution of g-ratios showed no statistical changes in the
posterior funiculus and pyramidal tract among the three age groups
(Fig. 3).
The grading classification of the pathological
changes in the myelin sheath showed the age dependence of myelin
breakdown (Fig. 4A and C). The
percentage of nerve fibers with a pathological alteration in the
myelin structure increased significantly in the posterior funiculus
and pyramidal tract of aging and aged rats; however, the myelin
disruption in the pyramidal tract was less severe than that in the
spinal posterior funiculus (Fig. 4B
and D). The percentage of fibers with myelin disruption reached
37.8 and 28.6% in the posterior funiculus and pyramidal tract at
PNM 26, respectively.
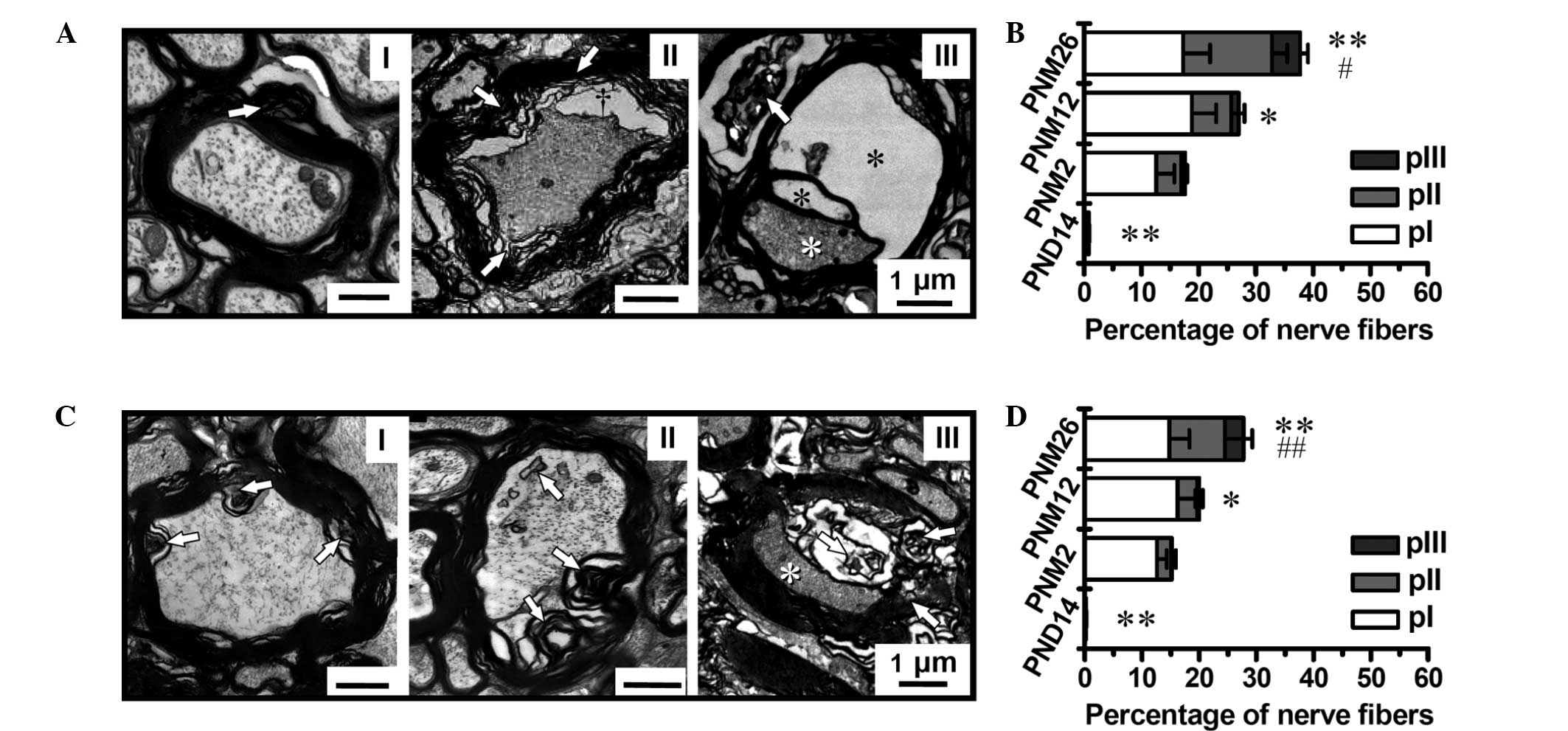 | Figure 4Grading classification of pathological
changes and quantitative analysis of MFs in aged (A and B)
posterior funiculus and (C and D) pyramidal tract (magnification,
×4,000). (A and C) Grading classification of pathological changes
in the posterior funiculus and pyramidal tract of rats between PND
14 and PNM 26: I, Slight pathological changes in the myelin sheath,
shown as focal myelin damage or vacuolization with the axon being
less affected; II, moderate pathological changes in the myelin
sheath, shown as more intensive myelin damage featured by large
lamina decompaction, vacuolization and intra-axonal changes
(increased electron density, lipofuscin deposition and glycogen
granules); III, the most severe pathological changes in the myelin
sheath and axons, shown as dramatic myelin damage or disruption
that were accompanied by axonal degeneration and loss (white
asterisks). Black asterisks indicate ballooning of the myelin
sheath. (B and D) Proportions of MFs with different
pathologically-classified grades (pI–III) in the posterior
funiculus and pyramidal tract, respectively. Scale bars, 1 μm.
*P<0.05 and **P<0.01 versus PNM 2;
#P<0.05 and ##P<0.01 versus PNM 12.
Results in B and D are presented as the mean ± standard error of
the mean. MF, myelinated fiber; PND, postnatal day; PNM, postnatal
month. |
Age-related alterations in the
ultrastructural pattern of myelin lamellae
Using high-resolution electron microscopy, the
ultrastructure of myelin lamellae was observed in the posterior
funiculus of rats at PNM 2, 5, and 18. The ultrastructural pattern
of myelin lamellae also showed significant age-related alterations.
The extracellular surface of the myelin membrane fused completely
at the time of myelination (PNM 2), which made the intraperiod
lines (IPLs) appear as single, thin lines similar to the major
dense lines (MDLs) under the EM. It was difficult to distinguish
between the MDLs and IPLs in the high-magnification images at PNM 2
(Fig. 5a and Ab). The normal,
double-line appearance of the IPLs was observed in myelin lamellae
of rats at PNM 5 and thereafter, which indicated incomplete fusion
of the IPLs at these periods (Fig. 5B
and C). As shown in Fig. 6, at
PNM 26, several IPLs opened to form cavities between the myelin
lamellae in the spinal lateral funiculus (Fig. 6B).
 | Figure 5Age-related alterations in the
ultrastructure of myelin lamellae in the spinal posterior
funiculus. Electron microscopic photomicrographs show the
ultrastructure of the posterior funiculus myelin lamellae at (Aa
and Ab) PNM 2, (Ba and Bb) PNM 5 and (Ca and Cb) PNM 18 (Aa-Ca
magnification, ×80,000; Ab-Cb magnification, ×160,000). IPLs (white
arrows) are fused as a less dark line (Aa), and could hardly be
distinguished from MDLs (white triangles) at a high magnification
(Ab) in rats at PNM 2. The ordinary double-line appearance of the
IPLs was observed at PNM 5 (Ba and Bb). Double-line IPLs were more
obvious in the myelin sheath of the posterior funiculus at PNM 18
(Cb) and were clear even at a low magnification (Ca). (D) Bar chart
of the periodicity of myelin lamellae in the posterior funiculus of
young and aged rats. (Aa-Ca) Scale bars, 50 nm; (Ab-Cb) scale bars,
20 nm; *P<0.05 and **P<0.01. Results in
D are presented as the mean ± standard error of the mean. PNM,
postnatal month; IPL, intraperiod line; MDL, major dense line. |
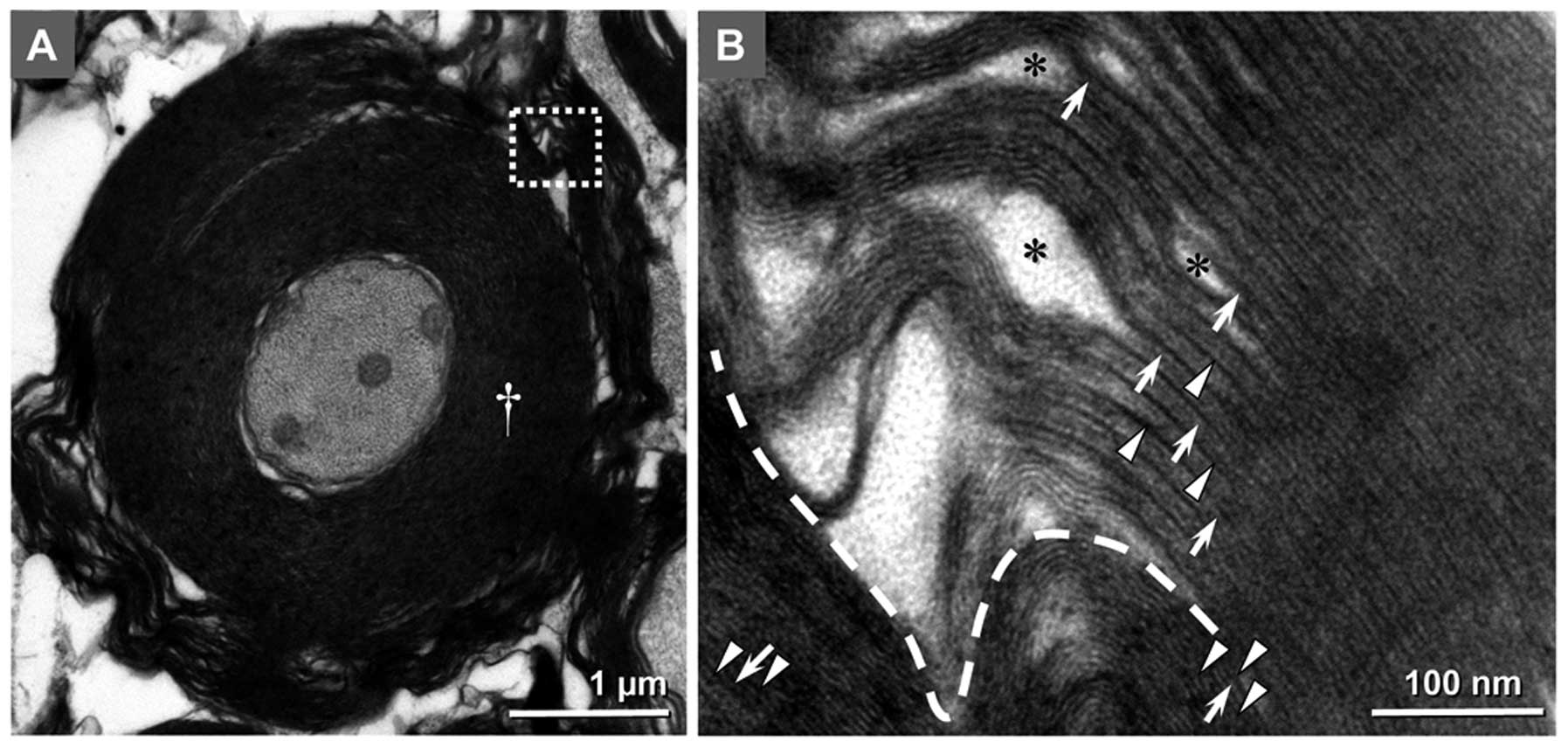 | Figure 6The over-incrassate myelin sheath and
different patterns in the ultrastructure of intra- and
extra-lamellae indicated the continued production of myelin in
aging. (A) Cross-section of a myelinated fiber in the lateral
funiculus of rats at PNM 26, as observed under the electron
microscope (magnification, ×8,000). The over-incrassate myelin
sheath (white cross) decreased the g-ratio to 0.374. Of note, a gap
appeared between the intra- and extra-myelin structures. (B)
High-magnification (magnification, ×80,000) image of the white
square in A. Patterns in the ultrastructure of intra- and
extra-myelin lamellae are significantly different. Gaps between
IPLs are marked as black asterisks. The white triangles and arrows
indicate MDLs and IPLs, respectively. Scale bar in (A), 1 μm and
(B), 100 nm. PNM, postnatal month; IPL, intraperiod line; MDL,
major dense line. |
The assessment of the periodicity of the myelin
lamellae indicated that the distance between adjoining MDLs and
IPLs increased statistically with age. The periodicity of the MDLs
was elevated from 13.09 nm at PNM 2 to 15.23 nm at PNM 18, while
the periodicity of the IPLs increased from 12.88 to 14.98 nm
(Fig. 5D).
Evidence of continued myelin production
and remyelination in the CNS of aged rats
In the CNS of rats at PNM 18 and 26, the myelin
sheath of certain fibers was observed to be overly thick. These
fibers had ≥20 myelin lamellae; however, the axon diameters were
not expanded accordingly, which led to g-ratios of <0.4 in
overly thick MFs. In several cases, this overly thick myelin
contained circumferential splits, giving the sheath the appearance
of an inner set of compact lamellae surrounded by an outer
separated loose set (Fig. 6A).
Under the EM at high magnification, the outer set of myelin
lamellae had a similar ultrastructural pattern to that observed in
the CNS of rats at PNM 5 and 18. The IPLs in the outer myelin
sheath fused incompletely, showing the typical double-line pattern
of aging myelin lamellae, while the IPLs in the inner compact
myelin sheath fused completely to form single lines. This pattern
was similar to the ultrastructural pattern of myelin lamellae in
the posterior funiculus at PNM 2 (Fig.
6B).
By comparing the myelin structure in rats during the
aging and developmental periods, it was observed that certain
myelin sheath samples of the posterior and lateral funiculus in the
aging period exhibited similar characteristics to those in the
developmental period (Fig. 7). For
example, certain MFs in aging rats CNS had thin myelin sheaths, a
higher g-ratio, fewer myelin lamellae and a large inner tongue
process of the myelin sheath, which were typical characteristics of
MFs in the developmental process (Fig.
7A and B). Certain other nerve fibers in aged rats had myelin
sheaths that were separated by oligodendrocyte cytoplasmic
components between compacted myelin lamellae, appearing as a
multi-layer concentric structure. This phenomenon was also common
in MFs of rats at the development stage (Fig. 7C and D). These results suggested
the existence of continued myelin production and remyelination in
the spinal posterior and lateral funiculus of aged rats.
Discussion
The assessment of the g-ratio is a conventional
quantitative method used to detect the myelinating capacity of
oligodendrocytes in the CNS and Schwann cells in the PNS.
Furthermore, this technique has been widely used to evaluate the
effects of drugs or gene mutation on myelination (27–29).
In these studies, an increase in the g-ratio was generally
considered to indicate a decline in myelinating capacity, while a
decrease in the g-ratio was suggested to indicate increased
myelination. Variations in the g-ratio of the animals and
age-matched controls were identified as a leftward or rightward
shifting of the g-ratio fitting line to axon diameters. However,
when this method was used to evaluate the effects of aging on rat
myelin integrity, several problems appeared. In contrast to
previous studies, which indicated no change in the visual cortex
axon diameters in monkeys with age (9), the present study suggested that axon
diameters increase in the sensorimotor projection fibers of aging
and aged rats (Fig. 3A and D).
This inconsistency may be due to differences between animal
species. The age-related enlargement of axon diameters disturbed
the g-ratio as a function of axon diameter, which caused a
clockwise rotation of the fitting line rather than a parallel
shift. However, age-related myelin breakdown was more complex than
simple dysmyelination or demyelination. Certain demyelinated fibers
had an increased g-ratio, while fibers with decompacted myelin had
a decreased g-ratio. Therefore, the median g-ratio value in aging
could be maintained at the same level as that in young rats
(Fig. 3C and E). Accordingly, the
conventional method of assessing the g-ratio was not suitable for
evaluating the integrity of the myelin sheath in normal aging.
Using the method for the pathological grading and
counting of fibers established in a previous study conducted by our
group (26), the degree of myelin
disruption in the sensorimotor system of aging rats was
successfully quantified, and it was found that the pathological
alterations in the myelin sheath were significantly age-dependent.
At only PNM 2, the myelin sheath in the sensorimotor projection
fibers of rats began to show minor pathological changes. More
significant pathological alterations were identified in rats at PNM
12, which became more severe in the sensorimotor system of older
rats. A similar age-related myelin breakdown has also been reported
in the PNS of rats (14,15,30,31),
where marked abnormalities in the myelin sheath of peripheral
nerves were observed at 12 and 18 months of age.
Peters et al (11,32,33)
defined two types of the most common age-related morphological
alterations in the myelin sheath in their studies on the CNS of
monkeys: i) Accumulation of pockets of dense cytoplasm between
splits of the lamellae at the MDL; and ii) fluid-filled cavities
that occupy splits in the IPL of the sheath. The cavities formed by
the IPL opening of the myelin sheath were also able to be observed
in the posterior funiculus of aged rats in the present study
(Figs. 4A and 6B); however, the pockets of dense
cytoplasm in the MDL were rare in the CNS of aged rats. The most
common pathological changes in the myelin sheath in the present
study were a wave-like decompaction of myelin lamellae and an
atrophy of axons (Figs. 1,
2 and 4). Other structural alterations included
sclerosis of myelin, splits between myelin lamellae, ballooning of
axons and localized demyelination. Compared with other studies
(15,18,30,34),
the diversity in myelin breakdown in the CNS was not as high as
that in the PNS of rats. These results suggested that there were
differences in age-related myelin breakdown among different species
and locations.
Another notable finding of the present study was
that the ultrastructural pattern of the myelin lamellae was not
inflexible with age (Fig. 5).
Furthermore, the extracellular surface of the myelin membrane fused
completely at the exact time of myelination, which made the IPL
appear as a single thin line under the EM. The ordinary double-line
appearance of the IPL was not observed until PNM 5. This result
indicated that the stability of the myelin IPL in the sensorimotor
system of rats decreased with age and that the decreased stability
of the IPL in aging may be the structural basis of age-related
myelin breakdown. The mechanism underlying this age-related change
in the IPL remains to be elucidated; however, considering the
important roles of myelin-associated proteins in maintaining the
integrity of myelin lamellae, it is assumed that this is likely to
be linked with the alterations in myelin proteins. Further studies
are required to explore the correlation between age-related changes
in the IPL ultrastructure and age-dependent alterations in myelin
proteins.
Besides the pathological disruption of the myelin
sheath, continued myelin production and remyelination were also
identified in the CNS of aging rats (Figs. 6 and 7). Two types of abnormal myelin sheath
were identified by Peters et al (8,32,33)
and Luebke et al (35) as
evidence of continued myelin production in the CNS of aged monkeys:
i) An overly thick myelin sheath with >20 lamellae; and ii)
redundant myelin which was overly large in relation to the size of
the enclosed axon. In the present study, redundant myelin was only
observed in the PNS of aged rats (data not shown), while an overly
thick myelin sheath was common in the CNS of aged rats (Fig. 6). The same thickened myelin sheath
has also been observed in the paranodal junction of MFs in the
corpus callosum of aged rats (36). Of note, two distinct patterns in
the lamellae existed in one single myelin sheath (Fig. 6B). This difference between the
inner and outer set of lamellae may be a novel piece of evidence of
continued myelin production in the CNS of aging rats, and may imply
that myelin repair during the aging period occurs at the adaxonal
membrane of myelin-forming cells.
The increased frequency of the profiles of paranodes
under the EM has been suggested to evidence remyelination in the
CNS of aged monkeys (37). In the
present study, too few paranodes were found for statistic analysis;
however, by comparing the myelin sheaths in the aging and
developing sensorimotor systems of rats, similar structural
patterns between these two age periods were identified. This
similarity may be a novel piece of evidence for the existence of
remyelination in the CNS of aged rats.
In conclusion, the present study provides a detailed
description of the age-related changes in the myelin sheath in
sensorimotor projection fibers throughout the lifespan of rats.
Marked alterations in the myelin sheath were observed in the
sensorimotor system of aging and aged rats, which became aggravated
with age, resulting in the appearance of a wave-like decompaction
of myelin lamellae, sclerosis of myelin, splits between myelin
lamellae, ballooning of axons and localized demyelination. The
ultrastructural pattern of myelin lamellae also exhibited age
dependence. The transformation of myelin IPL from complete to
incomplete fusion occurred after PNM 5, leading to the expansion of
periodicity in myelin lamellae. These pathological changes in the
myelin structure occurred very early and showed a significant
correlation with age, indicating that myelin was the most
susceptible part of the nervous system to the influence of aging,
and myelin may be the major target of aging effects. Besides myelin
breakdown, continued myelin production and remyelination also
existed in the aging sensorimotor system, which suggested the
presence of endogenous mechanisms of myelin repair. This repair may
provide a basis for future drug research and development on the
modulation of myelin protection.
Acknowledgements
The authors are grateful to X.L. Wang and Y. Yang
for specific pathogen free animal supplies and their collaboration.
The present study was supported by grants from the Major State
Basic Research Development Program of China (973 Program) (nos.
2011CB504100 and 2013BAI04B04) and the National Natural Science
Foundation of China (no. 81171049).
References
|
1
|
Asou H, Hamada K and Sakota T:
Visualization of a single myelination process of an oligodendrocyte
in culture by video microscopy. Cell Struct Funct. 20:59–70. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fields RD: White matter matters. Sci Am.
298:42–49. 2008.
|
|
3
|
Waxman SG: Conduction in myelinated,
unmyelinated, and demyelinated fibers. Arch Neurol. 34:585–589.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bengtsson SL, Nagy Z, Skare S, Forsman L,
Forssberg H and Ullén F: Extensive piano practicing has regionally
specific effects on white matter development. Nat Neurosci.
8:1148–1150. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fields RD: Myelination: an overlooked
mechanism of synaptic plasticity? Neuroscientist. 11:528–531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartzokis G: Age-related myelin breakdown:
a development modal of cognitive decline and Alzheimer’s disease.
Neurobiol Aging. 25:5–18. 2004.
|
|
7
|
Albert M: Neuropsychological and
neurophysiological changes in healthy adult humans across the age
range. Neurobiol Aging. 14:623–625. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters A: The effects of normal aging on
myelin and nerve fibers: a review. J Neurocytol. 31:581–593. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters A, Sethares C and Killiany RJ:
Effects of age on the thickness of myelin sheaths in monkey primary
visual cortex. J Comp Neurol. 435:241–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hinman JD and Abraham CR: What’s behind
the decline? The role of white matter in brain aging. Neurochem
Res. 32:2023–2031. 2007.
|
|
11
|
Peters A and Kemper T: A review of the
structal alterations in the cerebral hemispheres of the aging
rhesus monkey. Neurobiol Aging. 33:2357–2372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Black JA, Foster RE and Waxman SG: Rat
optic nerve: freeze-fracture studies during development of
myelinated axons. Brain Res. 250:1–20. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chase MH, Engelhardt JK, Adinolfi AM and
Chirwa SS: Age-dependent changes in cat masseter nerve: an
electrophysiological and morphological study. Brain Res.
586:279–288. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majeed SK: Survey on spontaneous
peripheral neuropathy in aging rats. Arzneimittelforschung.
42:986–990. 1992.PubMed/NCBI
|
|
15
|
Sharma AK, Bajada S and Thomas PK: Age
changes in the tibial and plantar nerves of the rat. J Anat.
130:417–428. 1980.PubMed/NCBI
|
|
16
|
Stanmore A, Bradbury S and Weddell AG: A
quantitative study of peripheral nerve fibres in the mouse
following the administration of drugs. 1 Age changes in untreated
CBA mice from 3 to 21 months of age. J Anat. 127:101–115.
1978.PubMed/NCBI
|
|
17
|
Navarro X and Kennedy WR: The effects of
ageing on regeneration and sprouting of unmyelinated axons.
Peripheral Nerve Changes in the Elderly. New Issues in
Neurosciences. Thomas PK: 1. Wiley & Sons; New York, NY: pp.
125–134. 1989
|
|
18
|
Verdú E, Butí M and Navarro X: Functional
changes of the peripheral nervous system with aging in the mouse.
Neurobiol Aging. 17:73–77. 1996.
|
|
19
|
Coleman P, Finch C and Joseph J: The need
for multiple time points in aging studies. Neurobiol Aging. 25:3–4.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jernigan TL and Fennema-Notestine C: White
matter mapping is needed. Neurobiol Aging. 25:37–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coleman PD: How old is old? Neurobiol
Aging. 25:12004. View Article : Google Scholar
|
|
22
|
Ceballos D, Cuadras J, Verdú E and Navarro
X: Morphometric and ultrastructural changes with ageing in mouse
peripheral nerve. J Anat. 195:563–576. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Behse F: Morphometric studies on the human
sural nerve. Acta Neurol Scand Suppl. 132:1–38. 1990.PubMed/NCBI
|
|
24
|
Arbuthnott ER, Boyd IA and Kalu KU:
Ultrastructural dimensions of myelinated peripheral nerve fibres in
the cat and their relation to conduction velocity. J Physiol.
308:125–157. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friede RL and Samorajski T: Myelin
formation in the sciatic nerve of the rat. A quantitative electron
microscopic, histochemical and radioautographic study. J
Neuropathol Exp Neurol. 27:546–570. 1968. View Article : Google Scholar
|
|
26
|
Xie F, Fu H, Hou JF, Jiao K, Costigan M
and Chen J: High energy diets-induced metabolic and prediabetic
painful polyneuropathy in rats. PLoS One. 8:e574272013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fancy SP, Baranzini SE, Zhao C, et al:
Dysregulation of the Wnt pathway inhibits timely myelination and
remyelination in the mammalian CNS. Genes Dev. 23:1571–1585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
La Marca R, Cerri F, Horiuchi K, et al:
TACE (ADAM17) inhibits Schwann cell myelination. Nat Neurosci.
14:857–865. 2011.PubMed/NCBI
|
|
29
|
Makinodan M, Rosen KM, Ito S and Corfas G:
A critical period for social experience-dependent oligodendrocyte
maturation and myelination. Science. 337:1357–1360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knox CA, Kokmen E and Dyck PJ:
Morphometric alteration of rat myelinated fibers with aging. J
Neuropathol Exp Neurol. 48:119–139. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas PK, King RH and Sharma AK: Changes
with age in the peripheral nerves of the rat. An ultrastructural
study. Acta Neuropathol. 52:1–6. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peters A: The effects of normal aging on
nerve fibers and neuroglia in the central nervous system. Brain
Aging: Models, Methods, and Mechanisms. Riddle DR: CRC Press; Boca
Raton, FL: pp. 97–125. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peters A: The effects of normal aging on
myelinated nerve fibers in monkey central nervous system. Front
Neuroanat. 3:112009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krinke G, Froehlich E, Herrmann M, et al:
Adjustment of the myelin sheath to axonal atrophy in the rat spinal
root by the formation of infolded myelin loops. Acta Anat (Basel).
131:182–187. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luebke J, Barbas H and Peters A: Effects
of normal aging on prefrontal area 46 in the rhesus monkey. Brain
Res Rev. 62:212–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sugiyama I, Tanaka K, Akita M, Yoshida K,
Kawase T and Asou H: Ultrastructural analysis of the paranodal
junction of myelinated fibers in 31-month-old-rats. J Neurosci Res.
70:309–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peters A and Sethares C: Is there
remyelination during aging of the primate central nervous system? J
Comp Neurol. 460:238–254. 2003. View Article : Google Scholar : PubMed/NCBI
|