Introduction
Fulminant hepatic failure (FHF) is a severe clinical
syndrome that is characteristic of hepatic cell injury resulting
from a variety of hepatic disease processes. FHF leads to hepatic
encephalopathy, severe coagulopathy, jaundice, hydroperitoneum and
high rates of patient mortality. Several mechanisms are responsible
for FHF, including viral infection, drug or toxin intake and
metabolic disorders (1). However,
the pathophysiology of FHF remains poorly understood and further
investigations are required. No therapy is currently available
except liver transplantation, which is limited due to a shortage of
donors, the rapid progression of FHF and the expense of the
surgical procedure (2). Effective
prophylactic or therapeutic interventions are urgently required to
improve the prognosis of patients with FHF.
D-galactosamine (D-GalN) and lipopolysaccharide
(LPS)-induced hepatic failure in mice is a universally used animal
model that resembles acute hepatic failure in the clinic (3). D-GalN has been found to markedly
strengthen the susceptibility of mice to the lethal effects of LPS
(4). Upon stimulation with LPS,
liver macrophages secrete various proinflammatory cytokines,
leading to hepatic necrosis, decreased levels of antioxidant
enzymes and the scavenging of free radicals (5,6).
Therefore, decreasing the generation of proinflammatory mediators
and oxidative substances may be an effective strategy for the
prevention or treatment of FHF.
Produced by interstitial fibroblasts in the kidney
and in the fetal liver, erythropoietin (EPO) is an endogenous
hormone that acts as a potent stimulator of bone marrow activity
and red blood cell production (7).
Although EPO is primarily synthesized in the kidney, it has been
recognized that EPO and EPO receptors (EPORs) are expressed by
other tissues and organs, including the brain, heart, kidney and
liver (8–10). Furthermore, studies have revealed
that EPO exerts antiapoptotic (11,12),
antioxidative (13–15) and anti-inflammatory (16) effects. Activation of the EPOR
activates various intracellular pathways, including the
mitogen-activated protein kinase, c-Jun N-terminal kinase, nuclear
factor κ-light-chain-enhancer of activated B cell and
phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascades
(17,18). In the present study, it was
hypothesized that EPO may protect liver tissues against D-GalN and
LPS-induced liver failure in mice. This study aimed to investigate
this hypothesis.
Materials and methods
Materials
D-GalN and LPS were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Detection kits for alanine aminotransferase
(ALT), aspartate aminotransferase (AST), superoxide dismutase
(SOD), malondialdehyde (MDA) and glutathione peroxidase (GSH-Px)
were purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China).
Experimental animals, groups and
treatment
Six- to eight-week-old BALB/c mice weighing between
18 and 22 g were obtained from the Experimental Animal Center of
Xi’an Jiaotong University College of Medicine (Xi’an, China). All
animals were acclimated to the laboratory environment for one week
prior to the conduction of experimental procedures. The mice were
allowed free access to drinking water and food and were maintained
at 22±2°C in an automatic 12-h light/dark cycle with 50% humidity.
The study was approved by the Ethics Committee of Xi’an Jiaotong
University and performed in accordance with the Practice Guidelines
for Laboratory Animals of China.
The mice were randomly divided into the following
five groups (n=8/group): Normal, D-GalN/LPS, and 1,000, 3,000 and
10,000 U/kg EPO. The dosages were selected based on previous
investigations (9,17) and a preliminary study. The EPO
groups were administered EPO intraperitoneally once per day for 3
days. One hour after the final administration, mice in the normal
group were injected with the same volume of sterile saline. The
mice in the other group were simultaneously intraperitoneally
injected with 700 mg/kg D-GalN and 10 μg/kg LPS. The mice were
fasted for one night (12 h) prior to D-GalN and LPS administration.
Eight hours after D-GalN and LPS injection, all mice were
sacrificed under deep ether anesthesia. Blood samples were taken
from the retro-orbital plexus of the mice. Serum was separated
using centrifugation at 1,368 × g for 10 min, then stored at −80°C
for analysis of the serum levels of ALT and AST. Liver tissue
samples were divided into two sections. One was immersed in 10%
phosphate-buffered formalin for morphological analysis and the
other was stored at −80°C for the analysis of biochemical
indicators of the liver.
Hepatic function tests
Serum AST and ALT levels were determined to assess
liver function using AST and ALT commercial kits (Nanjing Jiancheng
Bioengineering Institute).
MDA, SOD and GSH-Px assays
Liver tissue was washed with cold physiological
saline and the connective tissue was removed. The liver tissue was
then dried using filter paper and weighed. Tissue homogenate was
prepared using cold physiological saline at a ratio of 1/9 (w/v),
followed by centrifugation at 1,596 × g for 10 min. The supernatant
was then collected and the concentration of MDA, and the activities
of SOD and GSH-Px were measured using the corresponding kits
according to the manufacturer’s instructions (Nanjing Jiancheng
Bioengineering Institute).
Histopathological analysis
Liver tissue was fixed in 10% neutral-buffered
formalin and embedded in paraffin. The samples were sliced into
5-μm sections and the slides were stained with hematoxylin and
eosin.
RNA isolation and quantitative polymerase
chain reaction (qPCR) analysis
Total RNA was isolated from mouse liver tissues
using the TriPure RNA isolation reagent (Roche, Basel, Switzerland)
and 2 μg RNA was reverse transcribed using the PrimeScript™ RT
Master Mix (Perfect Real Time) (Takara Bio, Inc., Tokyo, Japan).
qPCR analysis was performed using SYBR® Premix Ex Taq™
II (Perfect Real Time) (Takara Bio, Inc.). PCR reactions were
performed in 96-well plates in an iQ5 Real-Time PCR Detection
system (Bio-Rad Laboratories, Hercules, CA, USA).
All of the primers and probes for qPCR were obtained
from Takara Bio, Inc. Murine EPOR was amplified using the primers
EPOR-F (5′-GCT CCG GGA TGG ACT TCA ACT A-3′) and EPOR-R (5′-CTG GTG
CAG GCT ACA TGA CTT TC-3′); murine PI3K was amplified using the
primers PIK3R1-F (5′-GCT CCT GGA AGC CAT TGA GAA-3′) and PIK3R1-R
(5′-CGT CGA TCA TCT CCA AGT CCA C-3′); and murine GAPDH was
amplified using the primers GAPDH-F (5′-TGT GTC CGT CGT GGA TCT
GA-3′) and GAPDH-R (5′-TTG CTG TTG AAG TCG CAG GAG-3′). GAPDH
served as an endogenous control and the results were normalized to
GAPDH. The efficiency of qPCR for the target genes and the
endogenous control was approximately equal. −ΔCT expresses the
difference between the number of cycles (CT) of the target genes
and the endogenous control. Results are expressed as
2−ΔΔCT and show the fold increase in gene expression
compared with the control group. Bio-Rad iQ5 software (Bio-Rad
Laboratories) was used to perform the data analysis and generate
the standard curve.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. The results were evaluated using one-way analysis of
variance and Tukey’s multiple comparison tests. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of EPO on serum levels of ALT and
AST in a D-GalN/LPS-induced model of FHF
ALT and AST are two important indicators of liver
function. As shown in Fig. 1, ALT
and AST levels were observed to be significantly elevated in the
mice in the D-GalN/LPS group compared with those in the normal
group (P<0.01), indicating severe liver injury in the D-GalN/LPS
mice. Pretreatment with various doses of EPO was found to reduce
the increases in ALT and AST by varying degrees. In the mice in the
10,000 U/kg EPO group, serum AST and ALT activities were
significantly reduced compared with activities in the D-GalN/LPS
group (P<0.01).
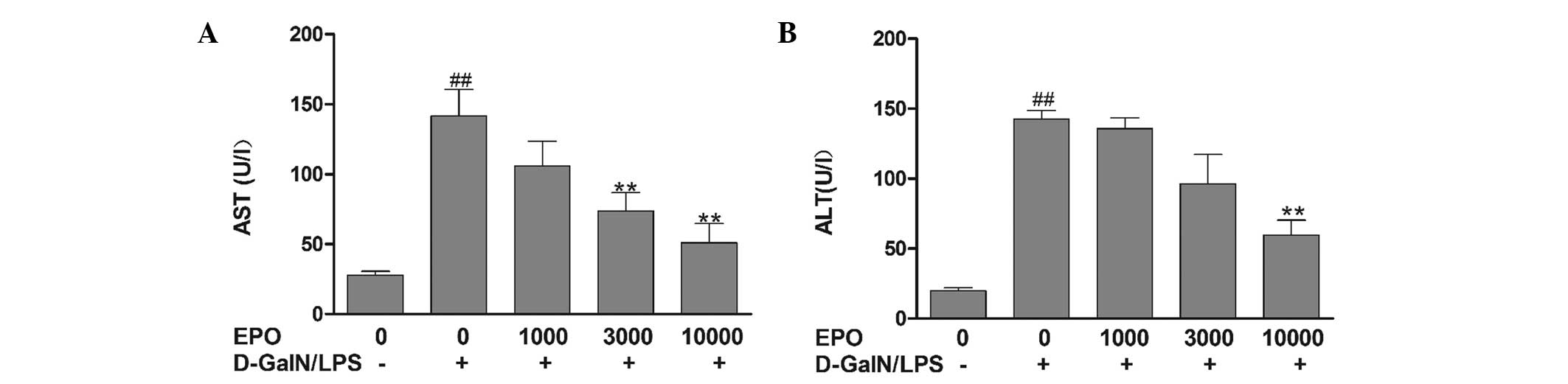 | Figure 1Effect of EPO on serum (A) AST and (B)
ALT levels. Mice were pretreated with 1,000, 3,000 and 10,000 U/kg
EPO per day for 3 days and 1 h prior to D-GalN/LPS injecton. After
8 h, blood samples were collected. Data are presented as the mean ±
standard error of the mean (n=8). ##P<0.01 vs. normal
group; **P<0.01 vs. D-GalN/LPS group. EPO,
erythropoietin; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; D-GalN, D-galactosamine; LPS,
lipopolysaccharide. |
Effect of EPO on MDA, SOD and GSH-Px
levels
As indicated in Fig.
2, 8 h after D-GalN/LPS administration, the concentration of
MDA, a marker of free radical-induced injury, was determined. The
activities of the antioxidant enzymes SOD and GSH-Px were also
analyzed. Liver tissue MDA levels were increased significantly in
the D-GalN/LPS group compared with those in the normal group
(P<0.01). However, the activities of SOD and GSH-Px were
markedly reduced in the mice in the D-GalN/LPS group compared with
those in the normal group (P<0.01). The elevation in MDA was
significantly attenuated with 3,000 and 10,000 U/kg EPO compared
with the D-GalN/LPS group (P<0.01). Furthermore, EPO
pretreatment was found to enhance the activities of SOD and
GSH-Px.
 | Figure 2Effect of EPO on MDA, SOD and GSH-Px
levels. Mice were pretreated with 1,000, 3,000 and 10,000 U/kg EPO
per day for 3 days and 1 h prior to D-GalN/LPS injecton. After 8 h,
liver tissue was collected. (A–C) Effect of EPO on (A) MDA content;
(B) SOD activity; (C) GSH-Px activity. Data are presented as the
mean ± standard error of the mean (n=8). ##P<0.01 vs.
normal group; *P<0.05 and **P<0.01 vs.
D-GalN/LPS group. EPO, erythropoietin; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH-Px, glutathione peroxidase; D-GalN,
D-galactosamine; LPS, lipopolysaccharide. |
EPO alleviates liver injury in
D-GalN/LPS-induced FHF mice
In the normal group, the hematoxylin and
eosin-stained liver sections exhibited an integrated architecture
of hepatic lobules and normal cell structure without necrosis
(Fig. 3). However, injection with
D-GalN/LPS was found to induce destruction of the liver structure,
severe hepatocyte necrosis, hemorrhage and inflammatory cell
infiltration. Liver tissue damage was ameliorated in the mice
pretreated with EPO, compared with those in the D-GalN/LPS
group.
 | Figure 3Morphological analysis of the liver
tissue using hematoxylin and eosin staining. Mice were pretreated
with 1,000, 3,000 and 10,000 U/kg EPO per day for 3 days and 1 h
prior to D-GalN/LPS injecton. After 8 h, liver tissue was collected
and subjected to hematoxylin and eosin staining. (A) Normal group,
(B) D-GalN/LPS group, (C) EPO (1,000 U/kg)+D-GalN/LPS group, (D)
EPO (3,000 U/kg)+D-GalN/LPS group, (E) EPO (10,000 U/kg)+D-GalN/LPS
group (magnification, ×400). EPO, erythropoietin; D-GalN,
D-galactosamine; LPS, lipopolysaccharide. |
Effects of EPO on the expression of EPOR
and PI3K
In order to further investigate the mechanism
underlying the effect of EPO on FHF, the hepatic expression of EPOR
and PI3K was assessed in the different groups. The expression of
EPOR and PI3K was calculated relative to that of the endogenous
control, GAPDH. As shown in Fig.
4, the expression of EPOR and PI3K in the mice in the
D-GalN/LPS group was significantly decreased compared with that in
the normal group (P<0.05). However, pretreatment with EPO was
found to significantly increase the mRNA expression of EPOR and
PI3K mRNA in comparison with the D-GalN/LPS group (P<0.05).
Discussion
D-GalN/LPS-treated mouse models are frequently used
to study the pathogenesis of liver injury (19–21).
D-GalN is a specific hepatotoxin that selectively depletes uridine
nucleotides, ultimately inhibiting macromolecule synthesis in
hepatocytes, which results in abnormalities in the structure and
function of hepatic cells. In addition, D-GalN may act
synergistically with LPS. Activated neutrophils, which accumulate
around damaged liver cells, undergo bursts of respiration and
degranulation, releasing oxygen free radicals and leading to lipid
peroxidation. Oxygen free radicals and lipid peroxidation target
liver parenchyma and vascular endothelial cells, resulting in cell
damage or death (22,23). In the present study,
intraperitoneal injection of D-GalN/LPS in mice resulted in severe
hepatic injury, which was associated with elevated serum activity
of AST and ALT. Histopathological analysis also showed that
D-GalN/LPS induced severe necrosis in hepatocytes, hemorrhage and
inflammatory cell infiltration. Reactive oxygen species have a
major role in the initiation of D-GalN/LPS-induced liver injury
(24,25), which is consistent with the
findings of the present study. MDA, a marker of lipid peroxidation,
was observed to be present in significantly higher levels in the
liver tissue of mice in the D-GalN/LPS group compared with mice in
the normal group, and the activity of SOD and GSH-Px, antioxidant
markers, was significantly lower.
The present study showed that, in the mice
pretreated with EPO, the serum activity of AST and ALT was
decreased and the degree of liver tissue damage was ameliorated,
compared with mice in the D-GalN/LPS group. This suggests that EPO
has a strong hepatoprotective effect on D-GalN/LPS-induced liver
injury. EPO has been shown to exert antioxidative effects against
hypoxic-ischemic brain injury (26) and chemical toxins (27). In the present study,
intraperitoneal administration of EPO decreased the level of MDA
and increased the activity of antioxidant enzymes, including SOD
and GSH-Px, in the liver tissue compared with the D-GalN/LPS group.
These findings are in accordance with those of other in vivo
studies (28–30) and the histopathological
observations.
EPO has been proposed to be a tissue-protective
hormone with pleiotropic potential. EPO exerts protective effects
against tissue destruction surrounding sites of injury by signaling
through a nonhemopoietic receptor (31). In addition, when exerting its
extra-hematopoietic effects, EPO has been shown to bind to
heterodimeric EPOR, which consists of EPOR and the β common
receptor (32). One of the most
important signaling pathways is the PI3K/Akt pathway. The PI3K/Akt
signaling pathway is an important pathway for survival, and
protects the body against various stresses. The activation of the
PI3K/Akt pathway regulates cell apoptosis, survival and
proliferation through the regulation of its downstream factors.
Studies have shown that this pathway has a positive protective
effect in liver ischemia-reperfusion injury (33–35).
Furthermore, another study found that EPO has a protective effect
against injury in proximal tubule cells and cardiac myocytes by
activating EPOR, which leads to activation of PI3K and ultimately
Akt (36). Thus, it was of
interest to examine the effect of EPO pretreatment on the PI3K/Akt
pathway in D-GalN/LPS-induced acute liver failure in a murine
model. The present study showed that EPOR and PI3K gene expression
was significantly decreased in D-GalN/LPS mice, while pretreatment
with EPO significantly increased EPOR and PI3K mRNA expression in a
dose-dependent manner. Therefore, EPO may exert its
hepatoprotective effects through upregulation of EPOR and PI3K.
In conclusion, to the best of our knowledge, the
results of the present study are the first to demonstrate a
protective effect of exogenous EPO against D-GalN/LPS-induced
hepatic injury. In addition, the mechanism underlying the
protective effect of EPO has been shown to be associated with
antioxidant properties and the upregulation of EPOR and PI3K
expression. These findings may contribute to the development of
novel agents for the treatment of FHF. However, further
investigations are required to determine the precise protective
mechanism.
Acknowledgements
This study was supported by grants from the National
Natural Science Youth Foundation of China (no. 30901808), the
Technology and Innovation Project of Shaanxi Province (no.
2011KTCL03-20) and the National College Students Innovation
Training Project (no. 201210698130).
Abbreviations:
|
FHF
|
fulminant hepatic failure
|
|
EPO
|
erythropoietin
|
|
D-GalN
|
D-galactosamine
|
|
LPS
|
lipopolysaccharide
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
GSH-Px
|
glutathione peroxidase
|
|
EPOR
|
erythropoietin receptor
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
References
|
1
|
Schiødt FV and Lee WM: Fulminant liver
disease. Clin Liver Dis. 7:331–349. 2003.
|
|
2
|
Mukherjee S, Mahmoudi TM and Mukherjee U:
Liver transplant for viral hepatitis and fulminant hepatic failure.
Minerva Gastroenterol Dietol. 55:83–100. 2009.PubMed/NCBI
|
|
3
|
Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang
JX and Xu DG: Hepatoprotective effects of IL-22 on fulminant
hepatic failure induced by d-galactosamine and lipopolysaccharide
in mice. Cytokine. 56:174–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin JW, Wang JH, Park HJ, Choi MK, Kim HG
and Son CG: Herbal formula CGX ameliorates
LPS/D-galactosamine-induced hepatitis. Food Chem Toxicol.
49:1329–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong YI, Jung ID, Lee CM, et al: The
novel role of platelet-activating factor in protecting mice against
lipopolysaccharide-induced endotoxic shock. PLoS One. 4:e65032009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thirunavukkarasu C, Uemura T, Wang LF,
Watkins SC and Gandhi CR: Normal rat hepatic stellate cells respond
to endotoxin in LBP-independent manner to produce inhibitor(s) of
DNA synthesis in hepatocytes. J Cell Physiol. 204:654–665. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fisher JW: Erythropoietin: physiology and
pharmacology update. Exp Biol Med (Maywood). 228:1–14.
2003.PubMed/NCBI
|
|
8
|
Coleman T and Brines M: Science review:
recombinant human erythropoietin in critical illness: a role beyond
anemia? Crit Care. 8:337–341. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sepodes B, Maio R, Pinto R, et al:
Recombinant human erythropoietin protects the liver from hepatic
ischemia-reperfusion injury in the rat. Transpl Int. 19:919–926.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewis LD: Preclinical and clinical
studies: a preview of potential future applications of
erythropoietic agents. Semin Hematol. 41(4 Suppl 7): 17–25. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kadota T, Shingo T, Yasuhara T, et al:
Continuous intraventricular infusion of erythropoietin exerts
neuroprotective/rescue effects upon Parkinson’s disease model of
rats with enhanced neurogenesis. Brain Res. 1254:120–127.
2009.PubMed/NCBI
|
|
12
|
Mammis A, McIntosh TK and Maniker AH:
Erythropoietin as a neuroprotective agent in traumatic brain injury
Review. Surg Neurol. 71:527–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lippi G, Franchini M and Banfi G:
Biochemistry and physiology of anabolic androgenic steroids doping.
Mini Rev Med Chem. 11:362–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimitrijevic ZM, Cvetkovic TP, Djordjevic
VM, et al: How the duration period of erythropoietin treatment
influences the oxidative status of hemodialysis patients. Int J Med
Sci. 9:808–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akisu M, Küllahçioğlu Girgin F, Baka M,
Hüsseyinov A and Kültürsay N: The role of recombinant human
erythropoietin in lipid peroxidation and platelet-activating factor
generation in a rat model of necrotizing enterocolitis. Eur J
Pediatr Surg. 11:167–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nairz M, Sonnweber T, Schroll A, Theurl I
and Weiss G: The pleiotropic effects of erythropoietin in infection
and inflammation. Microbes Infect. 14:238–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang CW, Li C, Jung JY, et al:
Preconditioning with erythropoietin protects against subsequent
ischemia-reperfusion injury in rat kidney. FASEB J. 17:1754–1755.
2003.PubMed/NCBI
|
|
18
|
Chong ZZ, Kang JQ and Maiese K:
Hematopoietic factor erythropoietin fosters neuroprotection through
novel signal transduction cascades. J Cereb Blood Flow Metab.
22:503–514. 2002. View Article : Google Scholar
|
|
19
|
Kudo H, Takahara T, Yata Y, Kawai K, Zhang
W and Sugiyama T: Lipopolysaccharide triggered TNF-alpha-induced
hepatocyte apoptosis in a murine non-alcoholic steatohepatitis
model. J Hepatol. 51:168–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuhla A, Eipel C, Abshagen K, Siebert N,
Menger MD and Vollmar B: Role of the perforin/granzyme cell death
pathway in D-Gal/LPS-induced inflammatory liver injury. Am J
Physiol Gastrointest Liver Physiol. 296:G1069–G1076. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikeda T, Abe K, Kuroda N, et al: The
inhibition of apoptosis by glycyrrhizin in hepatic injury induced
by injection of lipopolysaccharide/D-galactosamine in mice. Arch
Histol Cytol. 71:163–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JC, Ng CJ, Chiu TF and Chen HM:
Altered neutrophil apoptosis activity is reversed by melatonin in
liver ischemia-reperfusion. J Pineal Res. 34:260–264. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury: Present concepts. J Gastroenterol
Hepatol. 26(Suppl 1): 173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han D, Hanawa N, Saberi B and Kaplowitz N:
Hydrogen peroxide and redox modulation sensitize primary mouse
hepatocytes to TNF-induced apoptosis. Free Radic Biol Med.
41:627–639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou W, Roth RA, Younis HS, Burgoon LD and
Ganey PE: Oxidative stress is important in the pathogenesis of
liver injury induced by sulindac and lipopolysaccharide
cotreatment. Toxicology. 272:32–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan X and van Bel F: Pharmacological
neuroprotection after perinatal asphyxia. J Matern Fetal Neonatal
Med. 23(Suppl 3): 17–19. 2010. View Article : Google Scholar
|
|
27
|
Shang Y, Li X, Prasad PV, et al:
Erythropoietin attenuates lung injury in lipopolysaccharide treated
rats. J Surg Res. 155:104–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cetin H, Olgar S, Oktem F, Ciris M, Uz E,
Aslan C and Ozguner F: Novel evidence suggesting an anti-oxidant
property for erythropoietin on vancomycin-induced nephrotoxicity in
a rat model. Clin Exp Pharmacol Physiol. 34:1181–1185.
2007.PubMed/NCBI
|
|
29
|
Guneli E, Cavdar Z, Islekel H, et al:
Erythropoietin protects the intestine against ischemia/reperfusion
injury in rats. Mol Med. 13:509–517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oztürk E, Demirbilek S, Köroğlu A, et al:
Propofol and erythropoietin antioxidant properties in rat brain
injured tissue. Prog in Neuropsychopharmacol Biol Psychiatry.
32:81–86. 2008.PubMed/NCBI
|
|
31
|
Brines M and Cerami A: Emerging biological
roles for erythropoietin in the nervous system. Nat Rev Neurosci.
6:484–494. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brines M, Grasso G, Fiordaliso F, et al:
Erythropoietin mediates tissue protection through an erythropoietin
and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA.
101:14907–14912. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meier R, Alessi DR, Cron P, Andjelković M
and Hemmings BA: Mitogenic activation, phosphorylation, and nuclear
translocation of protein kinase Bbeta. J Biol Chem.
272:30491–30497. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abdelrahman M, Sharples EJ, McDonald MC,
Collin M, Patel NS, Yaqoob MM and Thiemermann C: Erythropoietin
attenuates the tissue injury associated with hemorrhagic shock and
myocardial ischemia. Shock. 22:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|


















