Introduction
The expression of insulin genes in pancreatic islet
cells is affected by plasma glucose or intracellular cAMP (1,2).
cAMP response element binding protein (CREBP) stimulates the
transcription of insulin genes by binding to CREs in the promoter
region (3,4). Among the CREB family members, CRE
modulator (CREM) is a unique gene that encodes either
transcriptional activators or repressors based on alternative
splicing (5). In particular,
inducible cAMP early repressor (ICER) has an important role as a
dominant-negative regulator of CREB and CREM activity, and consists
of DNA binding and leucine zipper domains, while also lacking an
N-terminal transactivation domain. As a strong repressor of
cAMP-induced gene expression, the ICER acts as an endogenous
inhibitor competing with CREB on the CRE sequence, and then ICER Iγ
induces the return of cAMP signaling to the basal state (6). Of note, prolonged expression of ICER
Iγ results in the development of pathological conditions.
Previously, it was reported that the overexpression of ICER Iγ is
associated with an increased incidence of diabetes and decreased
β-cell numbers in transgenic mice (7,8).
When using constructs with a constitutively active
promoter, the unregulated expression of a target gene often
produces unexpected results. To avoid these limitations, a
conditional transgenic technique was adopted, the tetracycline
(tet)-on system. This system enables both temporal and spatial
regulation of transgene expression, and requires a responder
construct and activator construct in a single cell (9). Transcriptional activator protein
(tTA; a transactivator) consists of a modified bacterial tet
repressor (TetR) fused to three minimal activation domains of the
herpes simplex virus transcription activator (VP16). Expression of
this construct is controlled by tetracycline or its analog,
doxycycline (dox) (10). In the
presence of dox, a conformational change in tTA allows the
activator to bind to the tTA-response element (TRE) promoter of a
responder construct, and induces transcription of the target gene
and a green fluorescent protein reporter gene downstream of the TRE
promoter. The TRE promoter consists of seven repeats of a 19-bp tet
operator sequence located upstream of a minimal cytomegalovirus
(CMV) promoter in which the binding sites for endogenous mammalian
transcription factors have been eliminated (11).
In the present study, a pancreas-specific,
dox-inducible ICER Iγ expression vector was constructed, where the
CMV promoter was exchanged for the human insulin promoter in the
activator construct. Additionally, this construct contained a
unitary system combined with an activator cassette and responder
cassette. Although in vivo experiments designed to study
diabetes have been conducted in rodents and other animals (12,13),
the phenotypic hallmarks of diabetes mellitus in rodent models are
not sufficient to fully elucidate the mechanisms underlying the
development of diabetes in humans. Therefore, porcine transgenic
fibroblasts were also established, that were genetically modified
to evoke type 1 diabetes mellitus-like symptoms in a porcine
model.
Materials and methods
Cell culture
The study was approved by the Ethics Committee of
Chungbuk National University (Cheongju, Korea). The porcine
fibroblasts were obtained from miniature pig fetuses (Yucatan pigs;
Optifarm Solution Inc., Gyeonggi-do, Korea) on the thirtieth day of
pregnancy and the cells were routinely maintained in Dulbecco’s
Modified Eagle’s Medium (DMEM) containing 25 mM glucose,
supplemented with 10% fetal bovine serum (FBS; WelGENE, Inc.,
Daejeon, South Korea), 100 U/ml penicillin and 100 μg/ml
streptomycin. The mouse β-cell line, MIN6 (American Type Culture
Collection, Manassas, VA, USA), was cultured in DMEM containing 25
mM glucose, supplemented with 15% FBS, 55 μM 2-mercaptoethanol
(Gibco, Grand island, NY, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin. All cells were grown in a humidified 5%
CO2 atmosphere at 37°C. Unless otherwise indicated, all
cell culture materials were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA).
Genomic DNA extraction and PCR
Genomic DNA from the cells was isolated with a
G-DEX™ IIc Genomic DNA Extraction kit (iNtRON Biotechnology, Inc.,
Seoul, South Korea). Genomic DNA (0.1 μg) was amplified in a 20-μl
PCR reaction containing 1 U i-Start Taq polymerase (iNtRON
Biotechnology, Inc.), 2 mM dNTPs (Takara Bio, Inc., Shiga, Japan)
and 10 pmol of each specific primer. The details of all primers are
described in Table I. The PCR
reactions were denatured at 94°C for 30 sec, annealed at 62°C for
30 sec and extended at 72°C for 1–2 min. The PCR products were
subjected to cloning processes and/or separated on a 1% agarose
gel, stained with ethidium bromide and photographed under UV
illumination. The image was scanned using GelDoc EQ (Bio-Rad,
Hercules, CA, USA).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer name | Restriction
enzyme | Direction | Sequences (5′ to
3′) |
|---|
| Human insulin
promoter (−1,432) | SpeI | Forward | ACT AGT TAC CCC AGG
GGC TCA GCC CAG ATG |
| Human insulin
promoter (+1) | EcoRI | Reverse | GAA TTC GGC CAG CAG
CGC CAG CAG G |
| ICER Iγ cDNA | MluI | Forward | ACG CGT ATG GCT GTA
ACT GGA GAT GA |
| ICER Iγ cDNA | BamHI | Reverse | GGA TCC CTA ATC TGT
TTT AGG AGA GCA AAT G |
| Tet-response
cassette | Bst1107I | Forward | GTA TAC CGA GGC CCT
TTC GTC TTC AAG AAT TC |
| Tet-response
cassette | SpeI | Reverse | ACT AGT GCC GCA GAC
ATG ATA AGA TAC ATT GA |
| Confirming primer
a | | Forward | GTG CTG ACG ACC AAG
GAG AT |
| Confirming primer
a′ | | Reverse | TTT CAG AAG TGG GGG
CAT AG |
| Confirming primer
b | | Forward | GAG GAT GGA GCA GTT
TGC AT |
| Confirming primer
b′ | | Reverse | GCA TTC CAC CAC TGC
TCC CA |
| ICER Iγ | | Forward | ATG GCT GTA ACT GGA
GAT GA |
| ICER Iγ | | Reverse | CTA ATC TGT TTT AGG
AGA GCA AAT G |
| Insulin-1 | | Forward | CCC TGT TGG TGC ACT
TCC TA |
| Insulin-1 | | Reverse | CAC TTG TGG GTC CTC
CAC TT |
| Mouse β-actin | | Forward | ACA GGC ATT GTG ATG
GAC TC |
| Mouse β-actin | | Reverse | ATT TCC CTC TCA GCT
GTG GT |
Vector construction
All restriction enzymes were obtained from Takara
Bio, Inc. The human insulin promoter region (from nucleotides (nt)
−1,431 to +1 nt; +1 = the transcriptional start site) demonstrated
the highest transcriptional activity in a previous study (14) and were prepared by long-range PCR
using human genomic DNA (Clontech Laboratories, Mountain View, CA,
USA) as the template, and specific primers containing restriction
enzyme sites (SpeI at the 5′ end or EcoRI at the 3′ end). Amplified
fragments were digested with SpeI and EcoRI and replaced with the
CMV promoter of the transcriptional activator construct,
pCMV-Tet3G, purchased from Clontech Laboratories. The ICER Iγ cDNA
was prepared by PCR using genomic DNA from pig pancreas as the
template and was inserted into pTRE3G-ZsGreen1 which contains green
fluorescence protein, ZsGreen1 (Clontech Laboratories) through MluI
at the 5′ end or BamHI at the 3′ end. Using the PCR method, the
ICER Iγ-expressing tTA-response region obtained from recombinant
pTRE3G-ZsGreen1-pig ICER Iγ construct was digested with Bst1107I or
SpeI and combined with the recombinant pHINSP-Tet3G vector
controlled by the human insulin promoter.
Establishment of transgenic cell
lines
The fibroblasts were transfected with the linearized
targeting vector using Lipofectamine® 2000 (Invitrogen,
Carlsbad, CA, USA). Following 24 h of transfection, the medium was
replaced with DMEM supplemented with 10% FBS and 250 μg/ml G-418
(Roche Diagnostics, Indianapolis, IN, USA) for four weeks. The
antibiotic-resistant cells were further selected, subjected to
PCR-based genotyping and stored until required for somatic cell
nuclear transfer.
Transient transfection and dox
treatment
Transient transfection was performed using
Lipofectamine™ 2000 according to the manufacturer’s instructions.
Briefly, 3×105 cells were seeded in 6-well tissue
culture plates one day prior to transfection. In total, 4 μg of the
recombinant constructs per well was transfected into the cells
under serum-free DMEM. Following incubation for 4 h, the medium was
replaced with DMEM containing 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin. A total of 20 h later, various concentrations
of dox were treated in transiently transfected cells for an
additional 24 h.
RNA preparation and semi-quantitative
(q)PCR
Total RNA from MIN6 cells was extracted using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. The concentration of the total RNA was
determined by measuring the absorbance at 260 nm. First-strand cDNA
was prepared by subjecting total RNA (1 μg) to reverse
transcription using Moloney murine leukemia virus (MMLV) reverse
transcriptase (Invitrogen Life Technologies) and random primers
(9-mers; Takara Bio, Inc.). To determine the optimal conditions for
logarithmic phase PCR amplification for target cDNA, aliquots of
total cDNA (1 μg) were amplified using different numbers of cycles.
The mouse β-actin gene was amplified as the internal control to
rule out the possibility of RNA degradation and to control for
variations in mRNA concentrations. A linear correlation between PCR
product band visibility and the number of amplification cycles was
observed for the target mRNA. The mouse β-actin and target genes
were quantified using 28 and 30 cycles, respectively. The PCR
reactions were denatured at 94°C for 30 sec, annealed at 58°C for
30 sec and extended at 72°C for 30 sec. The PCR products were on a
2.3% agarose gel, stained with ethidium bromide and photographed
under UV illumination. The image was scanned using GelDoc EQ
(Bio-Rad).
Western blot analysis
Lysates were prepared in RIPA buffer (50 mM Tris, pH
7.4, 150 mM sodium chloride (NaCl), 1% Triton X-100, 0.5% sodium
deoxycholate, 1 mM EDTA and 1 mM PMSF) and protease inhibitor
cocktail (Roche Diagnostics). Protein content was determined using
the Pierce BCA Micro Protein Assay kit (Thermo Fisher Scientific,
Waltham, MA, USA). Total proteins obtained by centrifugation
(13,000 × g for 20 min at 4°C) were denatured at 95°C for 5 min. A
total of 20–50 μg of protein were size-separated by electrophoresis
on 13% SDS-polyacrylamide gels and electrophoretically transferred
to a nitrocellulose membrane. Non-specific binding was blocked with
TBST (20 mM Tris, pH 7.6, 137 mM NaCl and 0.05% Tween-20)
containing 5% non-fat milk for 2 h at room temperature (RT). The
membrane was probed with the following primary antibodies for 2 h
at RT: rabbit polyclonal antibody to Insulin (H-86, sc-9168),
diluted 1:1,000; rabbit polyclonal antibody to CREM (X-12, sc-440),
diluted 1:1,000 and rabbit polyclonal antibody to β-actin (N-21,
sc-130656), diluted 1:1,000. All antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The membrane
was subsequently exposed to a horseradish peroxidase-conjugated
secondary antibody (1:5,000 dilution in TBST) for 1 h at RT.
Results
Establishment of a unitary tet-on ICER Iγ
expression vector
The unitary tet-on system was composed of an
activator cassette and responder cassette (Fig. 1A). The activator cassette had a tTA
under the control of the human insulin promoter (−1,432 to +1 nt)
which is associated with maximum promoter activity in mouse MIN6
β-cells line according to our previous study (14). The responder cassette contained the
porcine ICER Iγ target gene and cDNA encoding ZsGreen1 green
fluorescence protein. The expression of both genes was controlled
by a TRE promoter. tTA was specifically transcribed in pancreas
cells under the control of the human insulin promoter, underwent a
conformational change induced by the administration of dox and then
bound to the TRE promoter of the responder cassette. Finally, the
key gene downstream of the TRE promoter, ICER Iγ, was specifically
transcribed in the pancreas cells. Additionally, expression of the
green fluorescence marker, ZsGreen1, was observed.
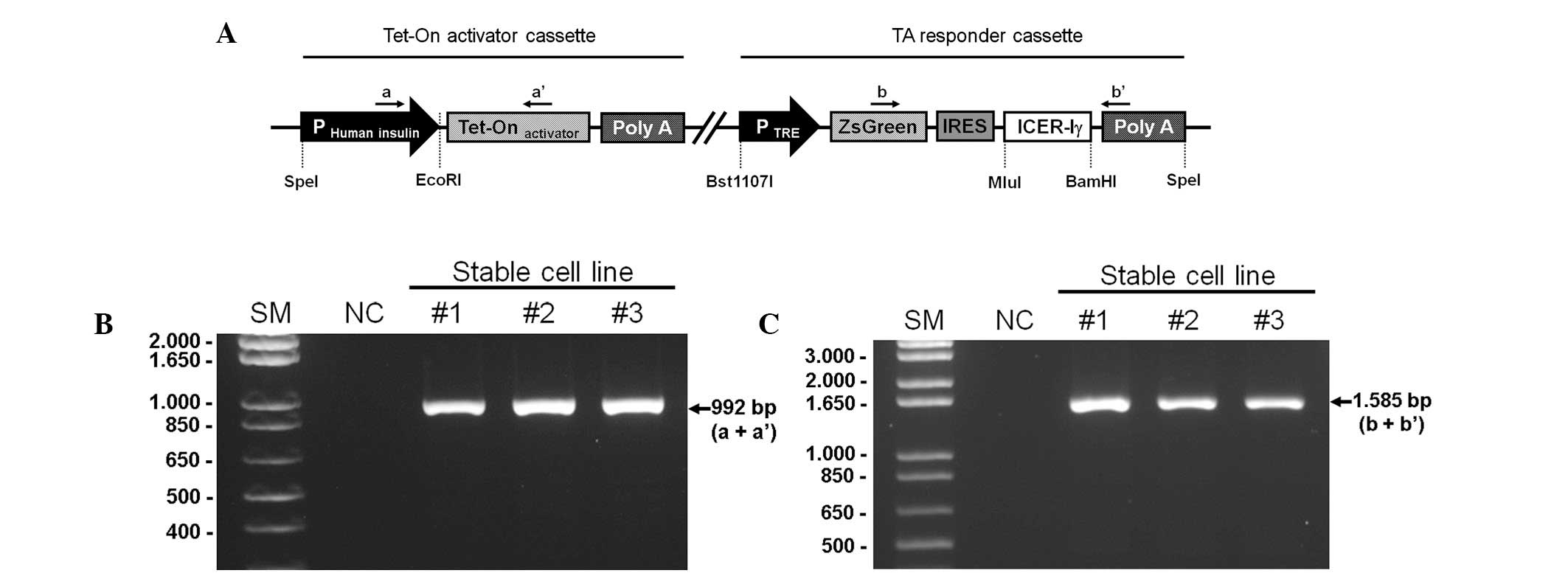 | Figure 1Schematic structure of the targeting
vector and PCR-based confirmation of transgenic fibroblasts. (A)
The unitary tet-on system was composed of two parts in a vector; an
activator cassette and a responder cassette. The activator cassette
had tTA under the control of the human insulin promoter (−1,432 to
+1 nt). The responder cassette contained the target gene, porcine
ICER Iγ and ZsGreen1 cDNA expressing green fluorescence protein,
both which are controlled by the TRE promoter. This unitary vector
was linearized and integrated into the genomic DNA of the porcine
fibroblasts. Genomic DNA was isolated from G418-resistant
fibroblast colonies and was identified with specific primer sets
indicated by the arrows (a, a′, b, b′). (B) The PCR products with
primer a and a′ represents the chromosomal insertion of the
activator cassette. (C) Whether the same fibroblast colonies had
responder cassette was confirmed using primer b and b′. IRES,
internal ribosomal entry site; SM, size marker; NC, negative
control; ICER Iγ, inducible cyclic AMP early repressor Iγ; tTa,
tet-controlled transactivator; TRE, tTA-response element. |
Generation and characterization of
fibroblast cell lines containing the dox-inducible porcine ICER Iγ
construct
The dox-inducible porcine ICER Iγ vector was
linearized and used to transfect miniature pig fibroblasts using a
liposomal-mediated gene delivery system. The transfected
fibroblasts were selected and maintained with a medium containing
G418 (250 μg/ml) for four weeks. Chromosomal integration of the
targeting vector was confirmed by a PCR-based method using primer
sets specific for the vector (Table
I). Genomic DNA extracted from G418-resistant colonies was
amplified with primers a and a′ (product size, 992 bp; Fig. 1B) or primers b and b′ (product
size, 1,585 bp; Fig. 1C). In
total, 12 positive colonies were obtained following two rounds of
transfection (Table II).
Fibroblasts from the positive colonies may serve as a cellular
source for somatic cell nuclear transfer to generate a porcine
model that overexpresses ICER Iγ in a dox-inducible,
pancreas-specific manner.
 | Table IITransfection efficiencies of the
porcine fibroblasts. |
Table II
Transfection efficiencies of the
porcine fibroblasts.
| Colony (n) |
|---|
|
|
|---|
| Transfection trials
(n) | G418 resistant | PCR positive |
|---|
| 2 | 12 | 12 |
Observation of dox-inducible fluorescence
in MIN6 cells and porcine fibroblasts
To observe tissue-specific expression, we
transiently transfected mouse pancreatic β-cells (MIN6) and porcine
non-pancreatic fibroblasts. The cells were co-transfected with
pCMV-TA and pTRE-ZsGreen1-ICER Iγ constructs as a positive control
to confirm the activity of the dox-inducible expression system. In
the MIN6 cells expressing pHINSP-TA-TRE-ZsGreen-ICER Iγ, we
observed green fluorescence indicative of dox-inducible and
pancreas-specific ICER Iγ expression (Fig. 2). In this regard, green
fluorescence was also confirmed following dox treatment in a
dose-dependent manner (0, 0.1 and 1 μg/ml). However, no
fluorescence was observed in either the untreated or dox-treated
porcine fibroblasts transiently transfected with
pHINSP-TA-TRE-ZsGreen-ICER Iγ due to the pancreas specific insulin
promoter (Fig. 3).
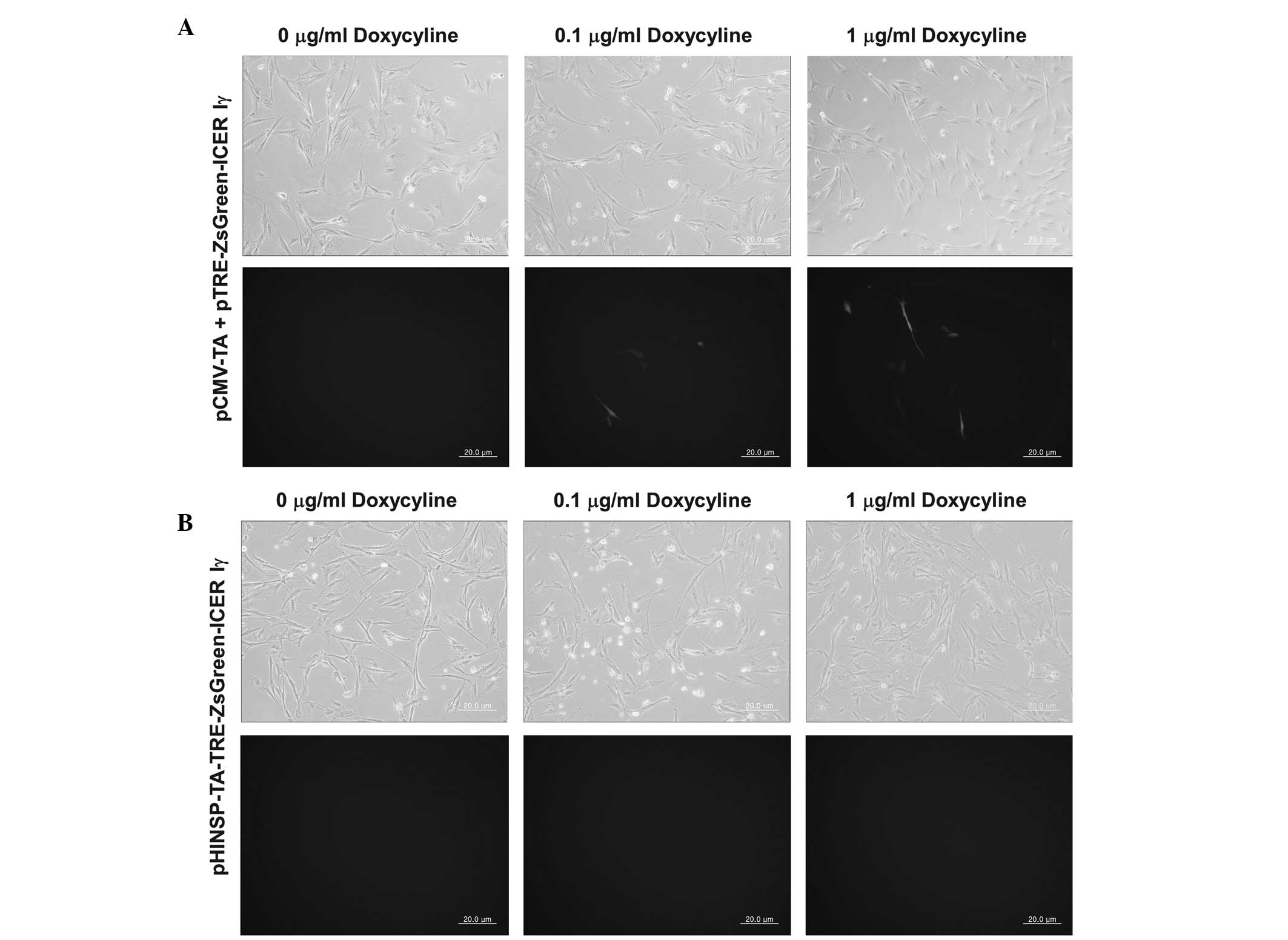 | Figure 2Observation of dox-inducible green
fluorescence in mouse β-cell line, MIN6, by transient transfection
of pCMV-TA with (A) pTRE-ZsGreen-ICER Iγ or (B) unitary
pHINSP-TA-TRE-ZsGreen-ICER Iγ. In the MIN6 cell line, dox-inducible
and pancreas specific expression of green fluorescence by human
insulin promoter was observed. Furthermore, the green fluorescence
demonstrated dox-dose dependent expression, as treated dox
concentration was increased to 0, 0.1 and 1 mg/ml. CMV,
cytomegalovirus promoter; TA, tet-on transcription activator;
HINSP, human insulin promoter; ICER Iγ, inducible cyclic AMP early
repressor Iγ; TRE, tTA-response element. |
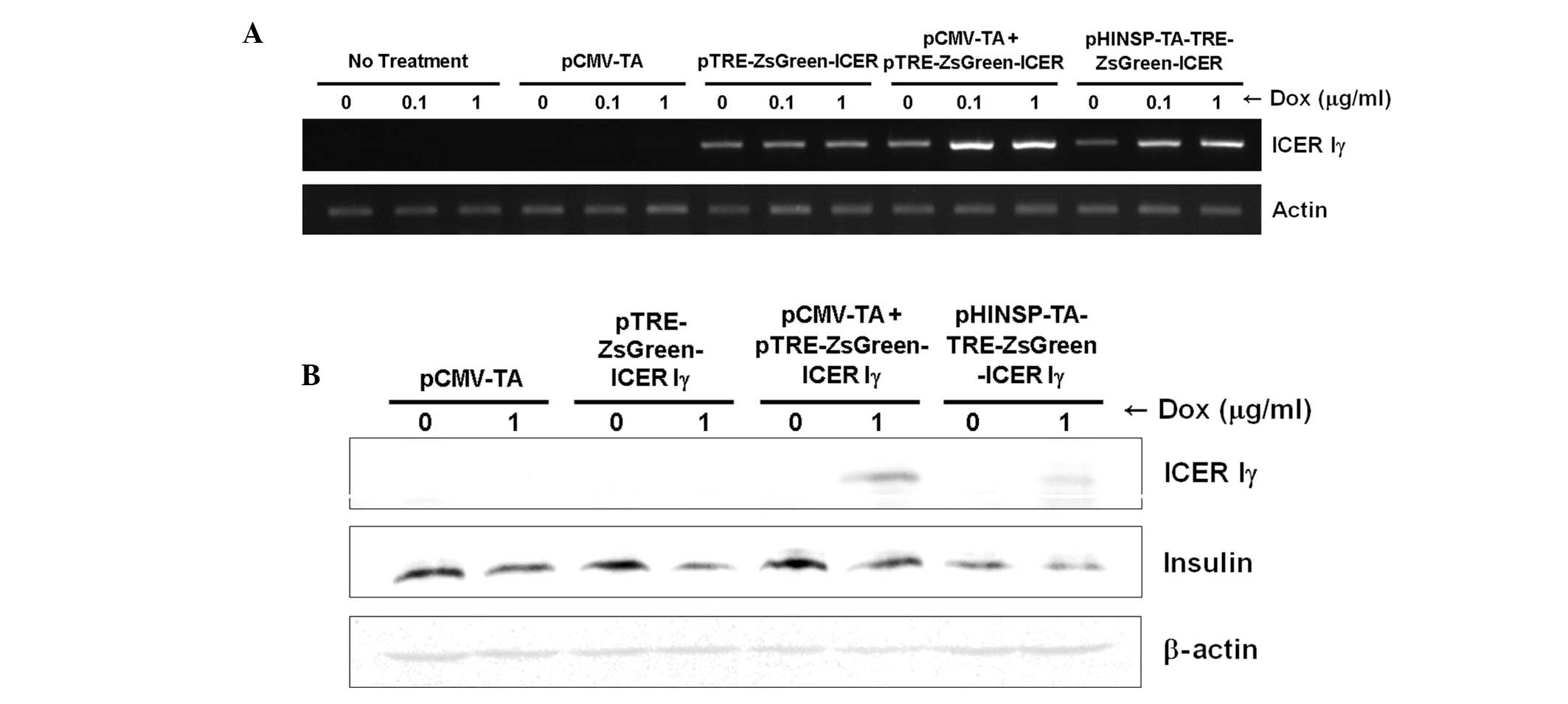
Dox-inducible porcine ICER Iγ mRNA
expression in mouse β-cells
To evaluate dox-inducible ICER Iγ expression under
the control of the human insulin promoter, reverse transcription
(RT)-PCR was performed using mRNA obtained from transiently
transfected MIN6 cells and porcine fibroblasts. Consistent to the
fluorescence microscopy findings, dox-dose dependent and pancreas
specific mRNA expression of ICER Iγ was observed in MIN6 cells
(Fig. 4A) following dox treatment
but not in porcine fibroblasts (data not presented). Additionally,
the expression levels of ICER Iγ in cells transfected with only
pCMV-TA or pTRE-ZsGreen1-ICER Iγ were unchanged regardless of the
absence or presence of dox. Although ICER Iγ expression in MIN6
cells expressing the pTRE-ZsGreen1-ICER Iγ transgene was observed,
the level was consistently low and unaffected regardless of the
presence of dox. It should be considered that unknown factors of
the transcriptional environment may regulate the gene expression of
in vitro systems through introduction of the target
gene.
Insulin production in mouse β-cells
expressing the dox-inducible porcine ICER Iγ gene
Since the ICER Iγ transgene represses insulin
production in vivo (7,8),
western blot analysis was performed to determine whether
dox-inducible expression of porcine ICER Iγ may inhibit insulin
generation. The expression of insulin was evaluated in transiently
transfecting MIN6 cells with four combinations of the constructs
(Fig. 4B). ICER Iγ protein in
cells transfected with only pCMV-TA or pTRE-ZsGreen1-ICER Iγ was
not expressed regardless of the absence or presence of dox.
Dox-inducible expression of ICER Iγ was observed in cells
co-transfected with pCMV-TA and pTRE-ZsGreen1-ICER Iγ, or
transfected with pHINSP-TA-TRE-ZsGreen1-ICER Iγ alone. By contrast,
insulin expression was decreased in the presence of dox. These
findings indicated that dox-inducible ICER Iγ expression decreases
insulin expression in mouse pancreas β-cell. Therefore, the
extensive induction of ICER Iγ expression may interfere with
insulin synthesis and promote the development of type 1 diabetes
mellitus. As a result, our dox-inducible ICER Iγ expression system
may be a useful tool for studying diabetes mellitus and
pre-diabetes in humans by controlling ICER Iγ expression levels
through dox administration.
Discussion
In rodent models, it has been reported that ICERs
have an important role in T-cell mediated downregulated expression
of interleukin (IL)-2, an essential growth factor for
auto-aggressive T-effector cells (15,16).
In pancreatic β-cells, ICER Iγ suppresses not only insulin gene
transcription but also cell replication via the reduction of cyclin
A levels (7,17). Therefore, the prolonged or
constitutive expression of ICER results in the development of
pathological conditions (7)
similar to severe cases of diabetes mellitus, because ICER Iγ
overexpression significantly blocks CRE-mediated transcription by
competing with CRE-binding activators (6,18).
Therefore, in the present study, a tissue-specific ICER Iγ vector
was constructed, whose expression was controlled by dox, and a
porcine fibroblast cell line with this construct was also
established to generate transgenic piglets.
The insulin promoter directly regulates insulin gene
transcription according to plasma glucose levels and enables
β-cells to produce insulin (19,20).
On this basis, the insulin promoter was utilized in the present
study as a β-cell-specific regulator of gene transcription. The
human insulin promoter region spanning −1,431 to +1 nt
significantly increased ICER Iγ gene expression in MIN6 cells in
the presence of high glucose levels and dox (Fig. 4A). However, induction of ICER Iγ
expression in MIN6 cells slightly downregulated insulin production
(Fig. 4B). It was hypothesized
that the activity of the porcine ICER Iγ protein would not be
maximized in mouse β-cells because the porcine ICER Iγ protein is
documented as having 92.6–94.4% identity to mouse ICERs, using a
BLAST tool. By contrast, it may be possible to reduce the
interaction between porcine ICER Iγ and mouse insulin promoter
regulatory elements.
It was reported that the relative number and
composition of principal insulin promoter regulatory elements in
different species was determined through transcription factor
binding site turnover and accretion (21–23).
In particular, CRE is a key determinant of gene expression
(24,25) and binds to the widest array of
transcription factors in the insulin promoter (26). Unlike the human insulin promoter
that contains four copies of CREs, the mouse insulin promoter only
has a single copy (23). For this
reason, the binding rate of overexpressed ICER Iγ within the mouse
insulin promoter may decrease and the repression of insulin
expression by ICER Iγ may be weaker in spite of ICER Iγ
overexpression via the human insulin promoter in mouse β-cells.
MIN6 cells were derived from a transgenic C57BL/6
mouse insulinoma expressing an insulin-promoter/T-antigen construct
and possess characteristics of pancreatic β-cells, including the
ability to secrete insulin in response to glucose (27,28).
Although the amount of insulin secreted from MIN6 cells in the
presence of a high (25 mM) glucose concentration is six-to
seven-fold greater than that observed with a low (5 mM) glucose
level, it has been noted that glucose-induced insulin secretion
from the MIN6 cells may suddenly be lost during the course of
passaging (28). This may result
in a poor response to the downregulation of glucose-mediated
insulin production via dox-inducible ICER Iγ expression.
To generate a porcine model for diabetes or
metabolic syndromes, pig fibroblast cell lines expressing
dox-inducible ICER Iγ were established. These cells should be a
useful source for somatic cell nuclear transfer procedures. Porcine
models provide various advantages for studying human metabolic
syndromes because pigs are mono-gastric omnivores and have
anatomical/physiological characteristics similar to that of humans
(29). The porcine model
established using the created cells may offer information crucial
for understanding the mechanisms underlying human diabetes
mellitus.
In the present study, the dox-inducible,
tissue-specific expression of the transcriptional repressor ICER Iγ
was observed in a mouse pancreatic β-cell line. This result was
compared with the expression of ICER Iγ observed in transiently
transfected primary porcine fibroblasts. The unitary tet-on ICER Iγ
induction system was pancreas-specific and dox-inducible. When
generating transgenic animals, these cellular traits may reduce
stillbirth rates during pregnancy and unwanted outcomes caused by
uncontrollable gene expression.
Acknowledgments
This study was supported by a grant from the
Next-Generation BioGreen 21 Program (no. PJ00956301), Rural
Development Administration, Republic of Korea.
References
|
1
|
Leonard J, Serup P, Gonzalez G, Edlund T
and Montminy M: The LIM family transcription factor Isl-1 requires
cAMP response element binding protein to promote somatostatin
expression in pancreatic islet cells. Proc Natl Acad Sci USA.
89:6247–6251. 1992. View Article : Google Scholar
|
|
2
|
Barnett DW, Pressel DM, Chern HT, Scharp
DW and Misler S: cAMP-enhancing agents ‘permit’ stimulus-secretion
coupling in canine pancreatic islet beta-cells. J Membr Biol.
138:113–120. 1994.
|
|
3
|
Ortmeyer HK: Insulin decreases skeletal
muscle cAMP-dependent protein kinase (PKA) activity in normal
monkeys and increases PKA activity in insulin-resistant rhesus
monkeys. J Basic Clin Physiol Pharmacol. 8:223–235. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seino S, Takahashi H, Fujimoto W and
Shibasaki T: Roles of cAMP signalling in insulin granule
exocytosis. Diabetes Obes Metab. 11(Suppl 4): 180–188. 2009.
View Article : Google Scholar
|
|
5
|
Foulkes NS, Borrelli E and Sassone-Corsi
P: CREM gene: use of alternative DNA-binding domains generates
multiple antagonists of cAMP-induced transcription. Cell.
64:739–749. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mioduszewska B, Jaworski J and Kaczmarek
L: Inducible cAMP early repressor (ICER) in the nervous system - a
transcriptional regulator of neuronal plasticity and programmed
cell death. J Neurochem. 87:1313–1320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inada A, Hamamoto Y, Tsuura Y, Miyazaki J,
Toyokuni S, Ihara Y, Nagai K, Yamada Y, Bonner-Weir S and Seino Y:
Overexpression of inducible cyclic AMP early repressor inhibits
transactivation of genes and cell proliferation in pancreatic beta
cells. Mol Cell Biol. 24:2831–2841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inada A, Someya Y, Yamada Y, Ihara Y,
Kubota A, Ban N, Watanabe R, Tsuda K and Seino Y: The cyclic AMP
response element modulator family regulates the insulin gene
transcription by interacting with transcription factor IID. J Biol
Chem. 274:21095–21103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gossen M and Bujard H: Tight control of
gene expression in mammalian cells by tetracycline-responsive
promoters. Proc Natl Acad Sci USA. 89:5547–5551. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Vink M, Klaver B, Berkhout B and
Das AT: Optimization of the Tet-On system for regulated gene
expression through viral evolution. Gene Ther. 13:1382–1390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gossen M, Freundlieb S, Bender G, Müller
G, Hillen W and Bujard H: Transcriptional activation by
tetracyclines in mammalian cells. Science. 268:1766–1769. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saudek F: Gene therapy in the treatment of
diabetes mellitus. Cas Lek Cesk. 142:523–527. 2003.(In Czech).
|
|
13
|
Driver JP, Serreze DV and Chen YG: Mouse
models for the study of autoimmune type 1 diabetes: a NOD to
similarities and differences to human disease. Semin Immunopathol.
33:67–87
|
|
14
|
Jung EM, Kim YK, Lee GS, Hyun SH, Hwang WS
and Jeung EB: Establishment of inducible cAMP early repressor
transgenic fibroblasts in a porcine model of human type 1 diabetes
mellitus. Mol Med Rep. 6:239–245
|
|
15
|
Bodor J, Fehervari Z, Diamond B and
Sakaguchi S: ICER/CREM-mediated transcriptional attenuation of IL-2
and its role in suppression by regulatory T cells. Eur J Immunol.
37:884–895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodor J, Spetz AL, Strominger JL and
Habener JF: cAMP inducibility of transcriptional repressor ICER in
developing and mature human T lymphocytes. Proc Natl Acad Sci USA.
93:3536–3541. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inada A, Weir GC and Bonner-Weir S:
Induced ICER Igamma down-regulates cyclin A expression and cell
proliferation in insulin-producing beta cells. Biochem Biophys Res
Commun. 329:925–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Molina CA, Foulkes NS, Lalli E and
Sassone-Corsi P: Inducibility and negative autoregulation of CREM:
an alternative promoter directs the expression of ICER, an early
response repressor. Cell. 75:875–886. 1993. View Article : Google Scholar
|
|
19
|
Odagiri H, Wang J and German MS: Function
of the human insulin promoter in primary cultured islet cells. J
Biol Chem. 271:1909–1915. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burkhardt BR, Loiler SA, Anderson JA,
Kilberg MS, Crawford JM, Flotte TR, Goudy KS, Ellis TM and Atkinson
M: Glucose-responsive expression of the human insulin promoter in
HepG2 human hepatoma cells. Ann NY Acad Sci. 1005:237–241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rockman MV and Wray GA: Abundant raw
material for cis-regulatory evolution in humans. Mol Biol Evol.
19:1991–2004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ludwig MZ and Kreitman M: Evolutionary
dynamics of the enhancer region of even-skipped in Drosophila. Mol
Biol Evol. 12:1002–1011. 1995.PubMed/NCBI
|
|
23
|
Hay CW and Docherty K: Comparative
analysis of insulin gene promoters: implications for diabetes
research. Diabetes. 55:3201–3213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daniel PB, Walker WH and Habener JF:
Cyclic AMP signaling and gene regulation. Annu Rev Nutr.
18:353–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inagaki N, Maekawa T, Sudo T, Ishii S,
Seino Y and Imura H: c-Jun represses the human insulin promoter
activity that depends on multiple cAMP response elements. Proc Natl
Acad Sci USA. 89:1045–1049. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foulkes NS and Sassone-Corsi P:
Transcription factors coupled to the cAMP-signalling pathway.
Biochim Biophys Acta. 1288:F101–F121. 1996.PubMed/NCBI
|
|
27
|
Ishihara H, Asano T, Tsukuda K, Katagiri
H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI and Oka Y:
Pancreatic beta cell line MIN6 exhibits characteristics of glucose
metabolism and glucose-stimulated insulin secretion similar to
those of normal islets. Diabetologia. 36:1139–1145. 1993.
View Article : Google Scholar
|
|
28
|
Miyazaki J, Araki K, Yamato E, Ikegami H,
Asano T, Shibasaki Y, Oka Y and Yamamura K: Establishment of a
pancreatic beta cell line that retains glucose-inducible insulin
secretion: special reference to expression of glucose transporter
isoforms. Endocrinology. 127:126–132. 1990. View Article : Google Scholar
|
|
29
|
Petersen B, Carnwath JW and Niemann H: The
perspectives for porcine-to-human xenografts. Comp Immunol
Microbiol Infect Dis. 32:91–105. 2009. View Article : Google Scholar : PubMed/NCBI
|