Introduction
A study from 2008 suggested that the incidence of
malignant tumors in individuals with diabetes mellitus (DM) is
higher than that in those who do not suffer from DM (1). There is much evidence suggesting that
CCL5 is one of the chemoattractant cytokines involved in DM with
diffuse large B-cell lymphoma (DLBCL). However, the pathological
impact is unclear (2).
CCL5 is a chemoattractant cytokine, and a number of
studies have noted high levels of CCL5 expression in DM or
indicated that DM is associated with CCL5 gene polymorphism
(3,4). Previous studies have demonstrated
that the expression of CCL5 in DLBCL cells is upregulated by
monocytes, and lymphoma may result from CCL5 reducing the ability
of immunologic surveillance (5).
Human DLBCL cell lines express CCL5 at high levels (6), and bioinformatic analyses suggest
that CCL5 is an important cytokine involved in DM with DLBCL. Thus,
it is essential to establish whether CCL5 accelerates DLBCL
formation in DM.
The aim of the present study was to examine the
expression of CCL5 mRNA in the A20 mouse DLBCL cell line for the
first time to the best of our knowledge, by subcutaneously
injecting A20-CCL5+ and A20-CCL5− subclones
into BALB/c DM mice and normal mice. A comparison of the tumor
growth in these mice may improve the understanding of the role of
CCL5 in DM with DLBCL.
Materials and methods
Materials
BALB/c mice (n=90, 6–8 week-old males, weighing 25±3
g) were purchased from the Central Laboratory of Animal Science of
the Nanchang University Medical College (Nanchang, China). All
animals received appropriate care according to the criteria
outlined in the ‘Guide for Care and Use of Laboratory Animals’
[SYXK (gan) 2010-0002]. Normal B cells, human-derived DLBCL cell
lines (Ly1, Ly8 and Ly10), and the BALB/c mouse-derived A20 DLBCL
cell line were kindly provided by Professor Tong Zhao (Nanfang
Hospital, Southern Medical University, Guangzhou, China).
Quantitative polymerase chain reaction
(qPCR)
All cell lines were cultured in RPMI-1640 medium
with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan,
UT, USA), and were maintained in 37°C humidified cell culture
incubators with 5% CO2. The Ly1, Ly8, Ly10 and A20 cell
lines were cultured in RPMI-1640 with 5 or 30 mmol/l glucose (Glu).
Total RNA was extracted from cells using TRIzol reagent (Omega
Bio-Tek Inc, Norcross, GA, USA) and the cDNA synthesis was
performed using an RT-PCR kit (All-in-One™ qPCR mix; GeneCopoeia,
Rockville, MD, USA) according to the manufacturer’s instructions.
PCR primers were purchased from Invitrogen (Carlsbad, CA, USA) with
the following sequences: hCCL5: F, 5′-CGCTGTCATCCTCATTGCTA-3′; and
R, 5′-GCACTTGCCACTGGTGTAGA-3′. GAPDH: F,
5′-ACAGTCAGCCGCATCTTCTT-3′; and R, 5′-GACAAG CTTCCCGTTCTCAG-3′.
mCCL5: F, 5′-CCCTCACCATCA TCCTCACT-3′; and R,
5′-CTTCTTCTCTGGGTTGGCAC-3′. SDHA: F, 5′-AGGTATCAATGCTGCTCTGG-3′;
and R, 5′-AAGTAGGTTCGCCCGTAG-3′. All reactions were performed in
triplicate. The results were analyzed using Image-Pro Plus
software, version 6.0 (IPP; Media Cybernetics, Rockville, MD,
USA).
Cell transduction
293T human embryonic kidney (HEK293T) cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Solarbio,
Beijing, China) culture solution with 10% FBS. The murine leukemia
cell line A20 (B lymphocytic) was cultured in RPMI-1640 medium
supplemented with 10% FBS and 0.05 mM 2-mercaptoethanol. pLKO-TRC
short hairpin RNA (shRNA) clones (TRCN0000068102, TRCN0000068101,
TRCN0000068100, TRCN0000068099 and TRCN0000068098) targeting mouse
CCL5 were purchased from Open Biosystems (Huntsville, AL, USA).
cDNA encoding human CCL5 was synthesized and cloned into a pBABE
retroviral vector (Cell Biolabs, Inc., San Diego, CA, USA). For
cell transduction, retroviruses were prepared by transient
co-transfection with helper viral vector into HEK293T cells using
calcium phosphate precipitation (Macgene Biotechnology Ltd.,
Beijing, China). HEK293T cells were transfected with plasmid DNA
and cultured at 37°C for 6 h, then the medium was replaced with
RPMI-1640 medium supplemented with 10% FBS. At 36–48 h
post-transfection, the supernatant was collected and filtered
through a 0.45-μm filter. Cells were infected at a density of
~2×106 per 10-cm cell culture dish in DMEM and RPMI-1640
(50:50) supplemented with 8 μg/ml polybrene (Solarbio). After 24 h,
the medium was changed to RPMI-1640 supplemented with 10% FBS and
0.05 mM 2-mercaptoethanol and cells were cultured for further
assay. Cell transduction with the lentiviral vector encoding the
shRNA that targets mouse CCL5 was conducted following the
manufacturer’s instructions (Open Biosystems). Western blotting and
qPCR were performed to confirm the overexpression or knockdown, and
consequently identify the A20-CCL5+ and
A20-CCL5− subclones, respectively.
Animals and in vivo tests
All procedures were conducted under sterile
conditions, and the animal protocol for this experiment was
approved by the Animal Care and Use Committee of the Nanchang
University Medical College. The 90 BALB/c mice were randomly
divided into two groups of 45: The DM models and the controls. DM
models were established with intraperitoneal injection of
streptozocin according to a previous method (7,8) and
randomized into an A20-CCL5+, A20-CCL5− and
A20 group. Each group also existed in the normal mice (Table I). Tumor cells (2×107)
in 0.2 ml growth medium (RPMI-1640 medium supplemented with 10%
FBS) were subcutaneously injected into the left axillary fossa of
each mouse. Tumor growth was measured by calculating the tumor
volume. Mice were sacrificed by cervical dislocation 30 days
post-injection. Blood serum was collected to detect secreted CCL5
protein by ELISA and a skin incision was made to remove the
tumor.
 | Table IAssignment of animals into groups. |
Table I
Assignment of animals into groups.
| Group | Mouse type | Cells inoculated |
|---|
| A1 | DM |
A20-CCL5+ |
| A2 | DM |
A20-CCL5− |
| A3 | DM | A20 |
| B1 | Normal |
A20-CCL5+ |
| B2 | Normal |
A20-CCL5− |
| B3 | Normal | A20 |
Immunohistochemistry
Tissue specimens were collected from tumors in the
mice, fixed in formalin and embedded in paraffin (Solarbio). The
tissues were then cut into 3-μm sections and dried on capillary-gap
glass slides (Solarbio). Immunohistochemistry of the paraffin
sections was performed using the ChemMate™ EnVision™ Detection kit
(Dako, Carpinteria, CA, USA). The sections were dewaxed,
dehydrated, subjected to antigen retrieval and blocked (Solarbio)
with hydrogen peroxide for endogenous peroxidase activity. The
sections were then immunostained with CCL5 antibody (brown color)
with DAB substrate (Dako), and counterstained using hematoxylin and
eosin (Solarbio). Image analysis was accomplished to measure the
average integral optical density of the positive cells using IPP
software, version 6.0 (9).
ELISA
The concentration of mCCL5 in the blood serum was
measured using commercially available kits (KHC1031; Invitrogen)
according to the manufacturer’s instructions. The range of
sensitivity for mCCL5 was 0–2,000 pg/ml. The assay was performed
according to the manufacturer’s instructions. ELISA plates were
assessed at 450 nm using Curve Expert software (Hyams Development,
Chattanooga, TN, USA).
Statistical analysis
SPSS software version 20.0 (IBM, Armonk, NY, USA)
was used for statistical analyses. Numerical values are expressed
as the mean ± standard deviation. Statistical significance was
calculated using the Student’s t-test for paired samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
hCCL5 mRNA expression in different cell
lines with normal culture
The mRNA expression of hCCL5 in normal B and DLBCL
cell lines was examined by qPCR. All the three DLBCL cell lines
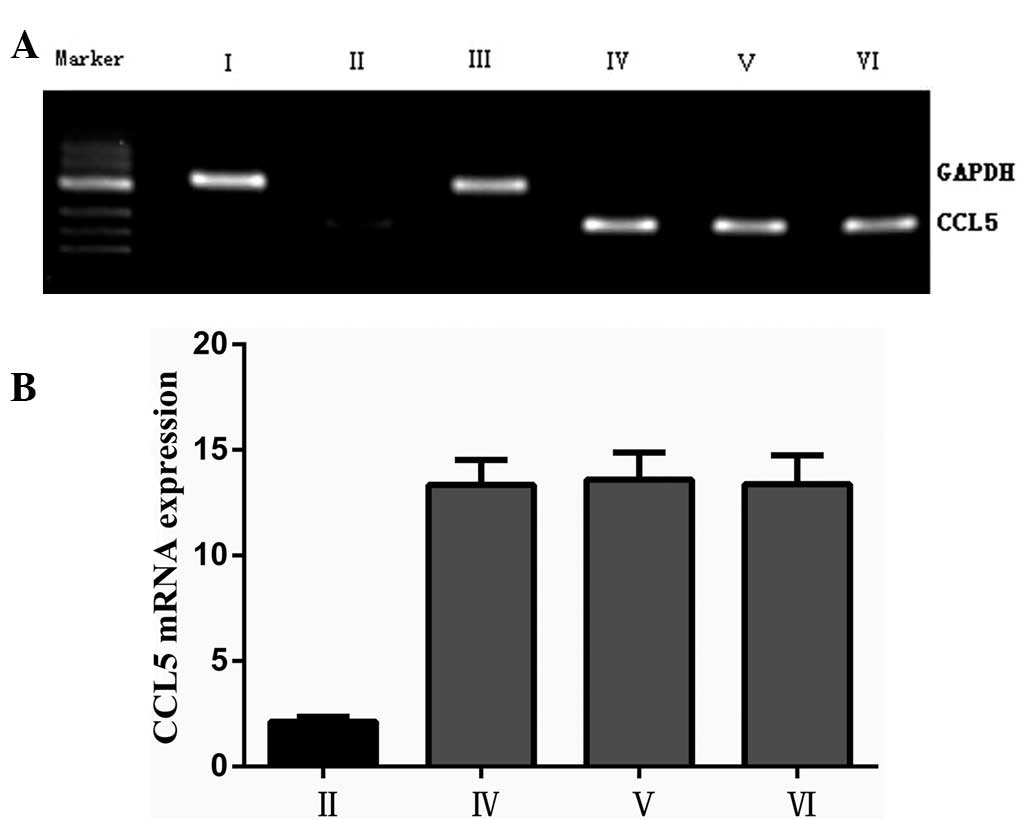
(Ly1, Ly8 and Ly10) expressed CCL5 mRNA (Fig. 1A). Compared with those in the
normal B cells, the CCL5 mRNA expression levels in the three cell
lines were increased significantly (P<0.05; Fig. 1B).
CCL5 mRNA expression in different cell
lines with 5 or 30 mmol/l Glu culture
All cell lines were collected following culture in 5
or 30 mmol/l Glu for 2 weeks. The CCL5 mRNA expression in the cell
lines was examined by qPCR. All the human cell lines (Ly1, Ly8 and
Ly10) expressed hCCL5 mRNA(Fig.
2A), and the A20 cells expressed mCCL5 mRNA (Fig. 2C). Compared with those in the cells
cultured with 5 mmol/l Glu, the CCL5 mRNA expression levels in the
cell lines cultured with 30 mmol/l Glu were increased significantly
(P<0.05; Fig. 2B and D).
Histology of tumors in BALB/c mice
Subcutaneous tumor models in BALB/c mice were
successfully established. Tumor growth of the DM groups (A1–3) was
higher than that of the normal mouse groups (B1–3, respectively)
(Fig. 3). The time frames of
tumorigenesis in the DM mice (A1–3) were shorter than those in the
normal mouse groups (B1–3). Mice were sacrificed 30 days after
injection and the skin was dissected to reveal the tumors. Tumors
were round or oval, and the tumor surface had a nodular appearance.
They were adherent to the skin of the capillary-rich surface, were
firm and tan-white, and presented no distant lymph node transfers.
The DM CCL5+ (A1) group tumor volumes were significantly
greater than those in the DM control group (A3) (P<0.01), but
there was no significant difference in tumor volume between
CCL5+ overexpression and control groups in the normal
mice (B1 and B3) (P=0.729) (Table
II). Using a microscope, it was possible to observe the size of
the tumor cells in the tumor tissues. The cells had large nuclei
that were irregular in size and shape and the nuclear staining was
uneven, with scant cytoplasm. A small number of pathological
mitotic figures and small lymphocytes were observed. With
hematoxylin and eosin staining, the A20 tumors were observed to
have diffuse homogeneous infiltrate consisting of large and
cohesive tumor cells with moderate cytoplasm and pleomorphic nuclei
(Fig. 4A). Immunohistochemical
staining results demonstrated that the expression levels of CCL5 in
DM mouse cells were higher those in normal mouse cells, and
CCL5+ mouse cells displayed more staining than
CCL5− cells (Fig.
4).
 | Table IITumor growth of DM and normal BALB/c
mouse groups following A20 cell injection. |
Table II
Tumor growth of DM and normal BALB/c
mouse groups following A20 cell injection.
| Group | Mouse type | Cells inoculated | n | % | Time (day) | Weight (g) | Volume
(mm3) |
|---|
| A1 | DM |
A20-CCL5+ | 14 | 93.3 | 7.0±0.85 | 2.11±0.42 |
1612.40±191.94a |
| A2 | DM |
A20-CCL5− | 9 | 60.0 | 9.5±2.80 | 1.12±0.14 | 998.00±99.85a |
| A3 | DM | A20 | 10 | 66.6 | 9.0±1.80 | 1.67±0.22 | 1367.20±139.25 |
| B1 | Normal |
A20-CCL5+ | 3 | 20.0 | 12.0±1.30 | 1.33±0.42 |
1030.00±343.95b |
| B2 | Normal |
A20-CCL5− | 3 | 20.0 | 14.0±2.50 | 1.25±0.40 |
970.00±274.18b |
| B3 | Normal | A20 | 7 | 46.6 | 12.0±4.20 | 1.30±0.43 | 1025.83±237.74 |
DM and normal serum expression levels of
CCL5 protein
Serum samples were collected from each group, and
expression levels of the CCL5 protein in the serum were measured by
ELISA. The results demonstrated that diabetic BALB/c mice secreted
significantly higher levels of soluble CCL5 protein compared with
those of normal mice (P<0.05). CCL5+ tumor of DM vs.
CCL5− tumor of DM (223.78±3.78 vs. 98.25±3.05;
P<0.01) CCL5+ tumor of normal vs. CCL5−
tumor of normal (118.07±1.33 vs. 121.69±2.91; P=0.138) (Table III).
 | Table IIICCL5 protein levels in the peripheral
blood of different groups (pg/ml). |
Table III
CCL5 protein levels in the peripheral
blood of different groups (pg/ml).
| Group | Normal with
tumor | Normal no
tumor | DM with tumor | DM no tumor |
|---|
|
A20-CCL5+ | 118.07±1.33b | 88.11±5.32 | 223.78±3.78a | 180.84±3.98 |
|
A20-CCL5− | 121.69±2.91b | 62.34±3.42 | 98.25±3.05a | 147.54±3.78 |
| A20 | 106.64±3.14 | 79.23±3.67 | 179.40±3.42 | 159.55±3.90 |
Discussion
Data from the current study demonstrated that CCL5
expression levels in high-sugar cultivated human and mouse DLBCL
are higher than those of cells cultured in low-sugar medium. ELISA
detection indicated that protein levels of CCL5 in DM mouse BALB/c
serum are higher than those in normal mice. This may be due to a
complicated mechanism of secretion of CCL5, which is caused by high
blood sugar. However, according to previous studies there is a
close association between diabetes and the impaired islet function;
and lack of islet function is associated with the chemical
absorption of CCL5 by lymphocytes (10). It has also been demonstrated that
long-term insulin resistance can directly promote the secretion of
CCL5 in the body (11). On the
other hand, the high sugar levels in the serum of patients with DM
may lead to elevated CCL5 levels in the body, which are associated
with the expression of MCP-1, TNF-α, IL-6 and AGE. This implies
that the body exhibits different degrees of inflammation and that
the chemokine CCL5 is an important factor in inflammation (12). In addition, the risk of diabetes
has been demonstrated to be closely associated with the levels of
CCL5 protein in the body; and increased levels of CCL5 may directly
lead to a greater risk of suffering from diabetes (13).
In the present study, CCL5 expression levels in
human DLBCL cell lines were demonstrated to be significantly higher
than those in normal B cells. CCL5 may be involved in the
occurrence and development of tumors, and a number of studies have
reported that it is secreted in tumor tissue by tumor cells or
inflammatory cells. CCL5 is also important in the occurrence and
development of malignant tumors, as it can be involved in the
growth of blood vessels in the tumor tissues (14). Another study indicated that CCL5
may directly affect tumor cells through paracrine signaling and
promote the growth of the tumor, so the majority of tumor cells can
directly secrete CCL5 (15). CCL5
can also adjust the expression and secretion of normal T cells. For
example, CCL5 at the tumor site can promote the formation of tumor
blood vessels and biological activity by aggregating and activating
matrix metalloproteinases, in particular MMP-9, in tumor cells in
order to increase the rate of growth and metastasis of tumors
(16).
Diabetes itself is one of the inducing factors of
malignant tumors (1), as the high
blood glucose levels lead to the production of a large number of
free radicals and induce the accumulation of reactive oxygen
species (ROS). Excessive ROS production can promote multiple
glycolytic pathways and produce AGE and PkG, which cause DNA damage
and mutations and may result in malignant tumor growth (17). Insulin resistance can easily lead
to an increase in existing high insulin levels observed in
diabetes; and this build-up can speed up the growth of tumors
(18–20). As a result, the present study
established A20 cell lines with different levels of CCL5 expression
by slow virus transfection in order to identify whether CCL5 is
important in diabetes with malignant tumors. The animal tumor
models demonstrated that the DM tumor mice transfected with the
CCL5-overexpressing A20 cell line presented higher CCL5 protein
levels in the peripheral blood than those of the control mice. The
average maximum tumor volume and weight of DM mice was greater than
that of the normal mice, suggesting that high blood sugar levels in
the body can increase the occurrence and potentiate the development
of DLBCL. There was no significant difference in the tumor growth
capacity of the normal mouse model (group B3) and normal mouse
model with CCL5 knockdown (group B2). These results indicate that
the knockdown of CCL5 in DM may reduce and slow tumor growth, but
this effect of CCL5 knockdown is not observed in the normal,
non-diabetic mice. Therefore, this experiment indicates a key role
for the chemokine CCL5 in the diabetic mouse with DLBCL.
Diabetes decreases immunity, and the occurrence of
DLBCL has a complex association with immune function; DLBCL is more
likely to occur on the condition that overall immune function
decreases. Nevertheless, a large number of background inflammatory
cells infiltrate the DLBCL tumor tissue, suggesting that the immune
response occurs during the development of DLBCL to a certain
extent. Various studies have demonstrated that inflammatory cells
can be induced by certain chemokines (21), and CCL5 in the body has been noted
to attract T helper 2 and T regulatory cells into tumor and
adjacent tissues (22–24). In DLBCL, CCL5 triggers T cells to
release interleukin 2 and to promote the occurrence of DLBCL
(25). CCL5 can weaken the immune
surveillance function in the body, increasing the probability of
DLBCL occurrence (26).
Additionally, the low immunity combined with high CCL5 levels may
lead to the occurrence of DLBCL. The current study demonstrated
that CCL5 is important in the induction of DLBCL in DM mice.
Diabetes increases the levels of CCL5 protein in the body and in
return, the high expression levels of CCL5 accelerate the growth,
invasion and metastasis of tumors. The BALB/c mouse genome is
>90% homologous to humans, so mouse tumors present similarities
to those of humans with respect to organization, formation and
developmental processes (27).
Therefore, the present study may provide reliable theoretical basis
for the study of DLBCL in human diabetes. Unfortunately, in the
current study, it was not possible to collect a sufficient number
of tissue samples from patients with DM combined with DLBCL, thus
no analysis of human samples was conducted. In future, it will be
valuable to compare the CCL5 expression of DLBCL tumor tissues of
diabetic and non-diabetic patients in order to provide further
evidence that CCL5 is key in diabetic human patients with DLBCL.
Thus, the present study provides novel insight for the prevention
and control of DLBCL in DM.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grants 81160251, 81101434 and
81071659).
References
|
1
|
Renehan AG, Tyson M, Egger M, et al:
Body-mass index and incidence of cancer: a systematic review and
meta-analysis of prospective observational studies. Lancet.
371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitri J, Castillo J and Pittas AG:
Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of
observational studies. Diabetes Care. 31:2391–2397. 2008.
|
|
3
|
Nakajima K, Tanaka Y, Nomiyama T, et al:
RANTES promoter genotype is associated with diabetic nephropathy in
type 2 diabetic subjects. Diabetes Care. 26:892–898. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herder C, Peltonen M, Koenig W, et al:
Systemic immune mediators and lifestyle changes in the prevention
of type 2 diabetes: results from the Finnish Diabetes Prevention
Study. Diabetes. 55:2340–2346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann MH, Schreiber S, Vogelsang H and
Sigusch HH: Constitutive expression of MCP-1 and RANTES in the
human histiocytic lymphoma cell line U-937. Immunol Lett.
76:111–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Zhang Y, Wu ZQ and Zhao T: Analysis
of CCL5 expression in classical Hodgkin’s lymphoma L428 cell line.
Mol Med Rep. 4:837–841. 2011.
|
|
7
|
Liu FR, Zhong QL, Liu DW, et al:
Establishment of type 1 diabetic BALB/c mouse models and detection
of CD4+CD25+regulatory T cells. Guangdong Med
J. 32:427–429. 2011.(In Chinese).
|
|
8
|
Zhong QL, Liu FR, Liu DW, et al:
Expression of β-catenin and cyclin D1 in epidermal stem cells of
diabetic rats. Mol Med Rep. 4:377–381. 2011.
|
|
9
|
Kraan MC, Smith MD, Weedon H, et al:
Measurement of cytokine and adhesion molecule expression in
synovial tissue by digital image analysis. Ann Rheum Dis.
60:296–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carvalho-Pinto C, García MI, Gómez L, et
al: Leukocyte attraction through the CCR5 receptor controls
progress from insulitis to diabetes in non-obese diabetic mice. Eur
J Immunol. 34:548–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bogdanski P, Pupek-Musialik D, Dytfeld J,
et al: Influence of insulin therapy on expression of chemokine
receptor CCR5 and selected inflammatory markers in patients with
type 2 diabetes mellitus. Int J Clin Pharmacol Ther. 45:563–567.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ando H, Kurita S and Takamura T: The
specific p38 mitogen-activated protein kinase pathway inhibitor
FR167653 keeps insulitis benign in nonobese diabetic mice. Life
Sci. 74:1817–1827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura K, Yamagishi S, Adachi H, et al:
Serum levels of sRAGE, the soluble form of receptor for advanced
glycation end products, are associated with inf1ammatory markers in
patients with type 2 diabetes. Mol Med. 13:185–189. 2007.
View Article : Google Scholar
|
|
14
|
Kulbe H, Levinson NR, Balkwill F and
Wilson JL: The chemokine network in cancer - much more than
directing cell movement. Int J Dev Biol. 48:489–496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adler EP, Lemken CA, Katchen NS and Kurt
RA: A dual role for tumor-derived chemokine RANTES (CCL5). Immunol
Lett. 90:187–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brownlee M: The pathobiology of diabetes
complications: a unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodwin PJ, Ennis M, Pritchard KI, et al:
Fasting insulin and outcome in early-stage breast cancer: results
of a prospective cohort study. J Clin Oncol. 20:42–51. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saydah SH, Loria CM, Eberhardt MS and
Brancati FL: Abnormal glucose tolerance and the risk of cancer
death in the United States. Am J Epidemiol. 157:1092–1100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richardson LC and Pollack LA: Therapy
insight: Influence of type 2 diabetes on the development, treatment
and outcomes of cancer. Nat Clin Pract Oncol. 2:48–53. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mueller CG, Boix C, Kwan WH, et al:
Critical role of monocytes to support normal B cell and diffuse
large B cell lymphoma survival and proliferation. J Leukoc Biol.
82:567–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skinnider BF and Mak TW: The role of
cytokines in classical Hodgkin lymphoma. Blood. 99:4283–4297. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aldinucci D, Lorenzon D, Cattaruzza L, et
al: Expression of CCR5 receptors on Reed-Steinberg cells and
Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor
cell growth and microenvironmental interactions. Int J Cancer.
122:769–776. 2008. View Article : Google Scholar
|
|
24
|
Fischer M, Juremalm M, Olsson N, et al:
Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and
its possible role in the recruitment of mast cells into
lymphomatous tissue. Int J Cancer. 107:197–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Appay V and Rowland-Jones SL: RANTES: a
versatile and controversial chemokine. Trends Immunol. 22:83–87.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun N, Yang G, Zhao H, et al: Multidose
streptozotocin induction of diabetes in BALB/c mice induces a
dominant oxidative macrophage and a conversion of THl to TH2
phenotypes during disease progression. Mediators Inflamm.
2005:202–209. 2005. View Article : Google Scholar
|
|
27
|
Bernardi R, Grisendi S and Pandolfi PP:
Modelling haematopoietic malignancies in the mouse and
therapeutical implications. Oncogene. 21:3445–3458. 2002.
View Article : Google Scholar
|