Introduction
Ultraviolet (UV) radiation induces several harmful
effects, including DNA damage, reactive oxygen species (ROS)
generation, cell cycle arrest, tumorigenesis, immunosuppression and
apoptosis in skin cells (1).
Therefore, photoprotection against UV-induced damage has an
important role in maintaining skin health. UV radiation is
classified as UVA, UVB and UVC. Although UVC is the most cytotoxic
radiation, it is almost completely blocked by the ozone layer
(1). UVA is a longer-wavelength
radiation and is able to penetrate deep into skin (1). UVB is a shorter-wavelength radiation
that scarcely penetrates into the dermis layer, but is more
cytotoxic than UVA (1). UVB is
considered to cause direct damage to DNA and indirect damage
through the generation of ROS (1).
Keratinocytes form the outermost tissue layer of the body and are
therefore constantly exposed to UV radiation. Numerous studies have
reported deleterious effects of UVB radiation on keratinocytes,
including senescence, inflammation, cell death and epithelial
malignancy (2). At the molecular
level, UVB affects diverse signaling pathways in keratinocytes.
Mitogen-activated protein kinases (MAPKs), including extracellular
signal-regulated kinase (ERK), p38 MAPK, and c-Jun N-terminal
kinase (JNK), are among the major UVB response molecules in
keratinocytes (2). UVB activates
MAPKs to induce either apoptosis or inflammation (2). The nuclear factor kappa B (NF-κB)
signaling pathway is also involved in UVB-mediated responses in
keratinocytes (2). UVB-induced
activation of NF-κB pathways induces the expression of several
genes that regulate the cell cycle, apoptosis and inflammation
(2).
Recently, microRNAs (miRNAs) have been revealed to
have pivotal roles in differentiation, senescence, cell survival
and apoptosis in keratinocytes. Rivetti et al (3) demonstrated that miRNA-p63 feedback is
important for keratinocyte senescence. Hidebrand et al
(4) analyzed miRNA expression
during keratinocyte differentiation and identified a role of miRNAs
in skin development, whereas Yang et al (5) demonstrated that miR-21 promotes
keratinocyte migration and re-epithelialization during wound
healing. Of note, it was recently reported that miRNAs are involved
in UVB-mediated cellular and molecular responses in keratinocytes.
Zhou et al (6) demonstrated
that acute exposure of keratinocytes to UVB results in several
specific patterns of miRNA response. Furthermore, Guo et al
(7) revealed that protection of
HaCaT keratinocytes against UVB radiation is mediated by miR-23a
through the regulation of
topoisomerase-1/caspase7/serine/threonine-protein kinase 4 (STK4).
These results indicate that miRNAs are crucial regulators of
keratinocyte development and UVB response.
Arctiin is a lignin compound that has been purified
from several plants, including Arctium lappa and
Forsythiae fructus. Previous studies have demonstrated that
arctiin exerted a protective effect against lipopolysaccharide
(LPS)-induced inflammation and has anti-proliferative and
anti-microbial functions (8–11).
However, to the best of our knowledge, the photoprotective effect
of arctiin has not been studied. In the present study, it was
demonstrated that treatment with a low dose of arctiin suppresses
UVB-induced keratinocyte damage though specific changes in miRNA
expression.
Materials and methods
Cell culture and chemicals
HaCaT keratinocytes were purchased from Cell Line
Service (DKFZ, Eppelheim, Germany) and were maintained with
Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Life Technologies,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco, Life Technologies) and antibiotics. Arctiin, a lignin
isolated from Arctium lappa, was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich). Propidium iodide (PI), a
fluorescent nuclear and chromosome counterstain, was purchased from
BD Biosciences (San Jose, CA, USA).
Cytotoxicity assay
The cytotoxicity of arctiin on HaCaT cells was
determined using a water-soluble tetrazolium (WST)-1 based cell
viability assay (EZ-Cytox Cell Viability Assay kit; Itsbio, Seoul,
Korea). HaCaT cells were seeded in a 96-well culture dish
(3×103 cells/well) and grown for 24 h prior to the
treatment with various doses of arctiin for a further 24 h. At the
end of the treatment, the cells were incubated with 10 μl of the
reagent from the WST-1 assay kit for 30 min. Absorbance was
recorded at 450 nm using a microplate spectrophotometer (iMark
microplate reader; Bio-Rad, Hercules, CA, USA). The results are
presented as the percentage of the control values.
UVB protection assay
The UVB protective effect of arctiin in HaCaT
keratinocytes was determined using a previously described method
(12). Cells were pretreated with
different concentrations of arctiin for various durations, washed
with phosphate-buffered saline (PBS) and irradiated with UVB (50
mJ/cm2) without lids on the plates. Following
irradiation the cells were incubated for 24 h in growth media and
cell viability was measured using an EZ-Cytox Cell Viability Assay
kit (Itsbio). The results are presented as the percentage relative
to the control (mean ± standard deviation).
Cell cycle analysis using flow
cytometry
Following exposure to UVB, the cells were washed
twice with cold PBS and fixed by careful resuspension in cold 70%
ethanol. Following fixation, the cells were washed with cold PBS
and stained with PI solution (0.05 mg/ml PI, 2 mg/ml RNase A, 0.1%
Triton X-100 in PBS). Fluorescence intensity (FL-2H) was measured
by flow cytometry (FACScalibur; BD Biosciences) with Cell Quest
software (BD Biosciences).
In vitro scratch assay
Cell migration in vitro was measured using a
scratch assay. Cells were seeded densely into 60 mm culture dishes.
Confluent cells were treated with arctiin (5 μM) for 6 h and a
standardized scratch was established in the monolayer using a 20 μl
loading tip. Following scratch formation the cells were irradiated
with UVB (50 mJ/cm2) and incubated in growth media. The
extent of scratch closure was quantified by measuring the area of
the scratch prior to (0 h) and 48 h following wounding, using a
phase-contrast microscope (Olympus CKX41; Olympus, Tokyo,
Japan).
DNA repair assay
The effect on arctiin on UV-damaged plasmid was
determined using a luciferase system. The pGL3 luciferase vector
(Promega Corporation, Madison, WI, USA) was irradiated with UVC at
the dose rate of 200 J/m2/sec for 10 sec (total 2000
J/m2). The undamaged control vector and UV-damaged pGL3
vector were co-transfected with pSV-β-galactosidase (β-gal) plasmid
(Promega Corporation) into HaCaT cells. Following 24 h, the
luciferase activity was measured using a dual-luciferase reporter
assay system as recommended by the manufacturer (Promega
Corporation).
miRNA microarray assay
Total RNA was purified from HaCaT cells that were
UVB-irradiated with or without arctiin pretreatment using TRIzol
reagent (Life Technologies) according to the manufacturer’s
instructions. The quality and concentration of the RNAs were
analyzed using MaestroNano (Maestrogen, Las Vegas, NV, USA) and the
integrity of the RNAs was determined using an Agilent 2100
Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). A
microarray assay was performed with the SurePrint G3 Human V16
miRNA 8×60 K array (Agilent Technologies) as previously described
(12) and the results were
analyzed with GeneSpring GX software version 11.5 (Agilent
Technologies). Differentially expressed miRNAs were selected using
the criteria of a random variance t-test, P-value <0.05 and an
absolute fold change >2.
Bioinformatics analysis of miRNAs
Putative targets of each miRNA were identified using
a DNA Intelligent Analysis (DIANA)-microT web-based bioinformatics
program (http://diana.imis.athena-innovation.gr/DianaTools/index.php)
(13). The involvement of putative
target genes of each miRNA in the Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathways was determined using the web-based
bioinformatics tool Database for Annotation, Visualization and
Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov/home.jsp) (14).
Statistical analysis
All results were determined from three independent
experiments and analyzed with the unpaired Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Arctiin protects HaCaT cells against UVB
damage
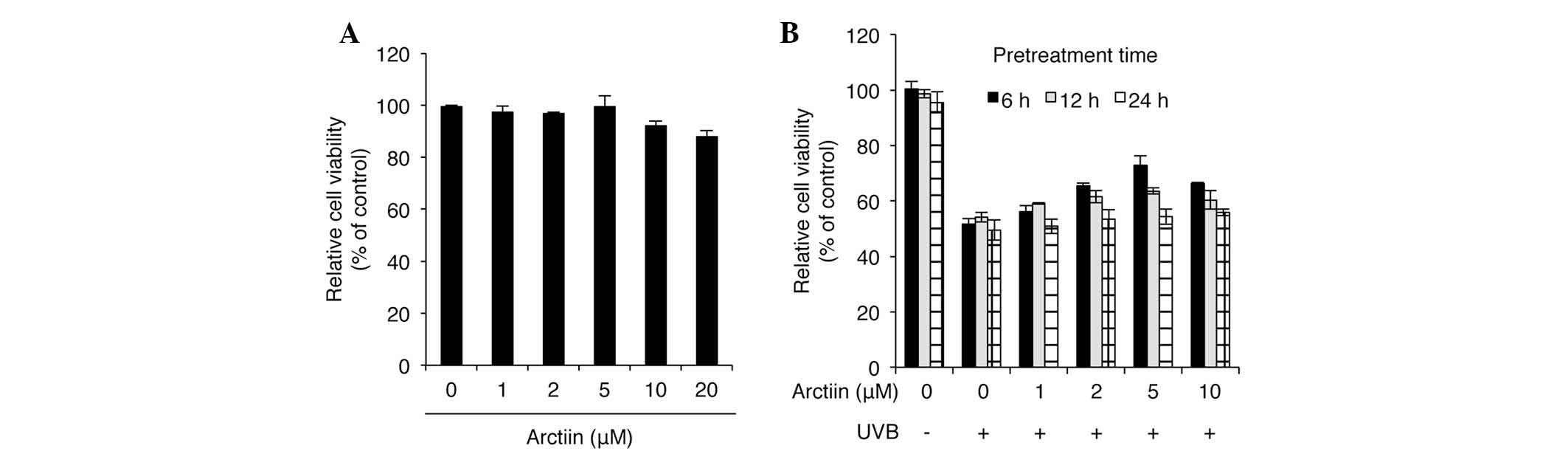
To determine the cytotoxicity of arctiin, a cell
viability assay was performed using water-soluble tetrazolium salts
(WSTs) following treatment of HaCaT cells with different doses of
arctiin for 24 h. Arctiin demonstrated no cytotoxicity at
concentrations of 1–5 μM and low cytotoxicity at 10 and 20 μM
(Fig. 1A). To investigate the UVB
protective effect of arctiin, HaCaT cells were pretreated with
arctiin at different doses and treatment durations prior to UVB (50
mJ/cm2) irradiation and a cytotoxicity assay were
performed. Significant UVB protection was observed in the cells
pretreated with 5 μM arctiin for 6 h (Fig. 1B). Although other pretreatment
conditions also demonstrated UVB protective activities, cell
viability was not significantly improved compared with
UVB-irradiated control cells. These results identify arctiin as a
novel UVB protective agent in keratinocytes.
Arctiin pretreatment antagonizes
UVB-mediated HaCaT cell death
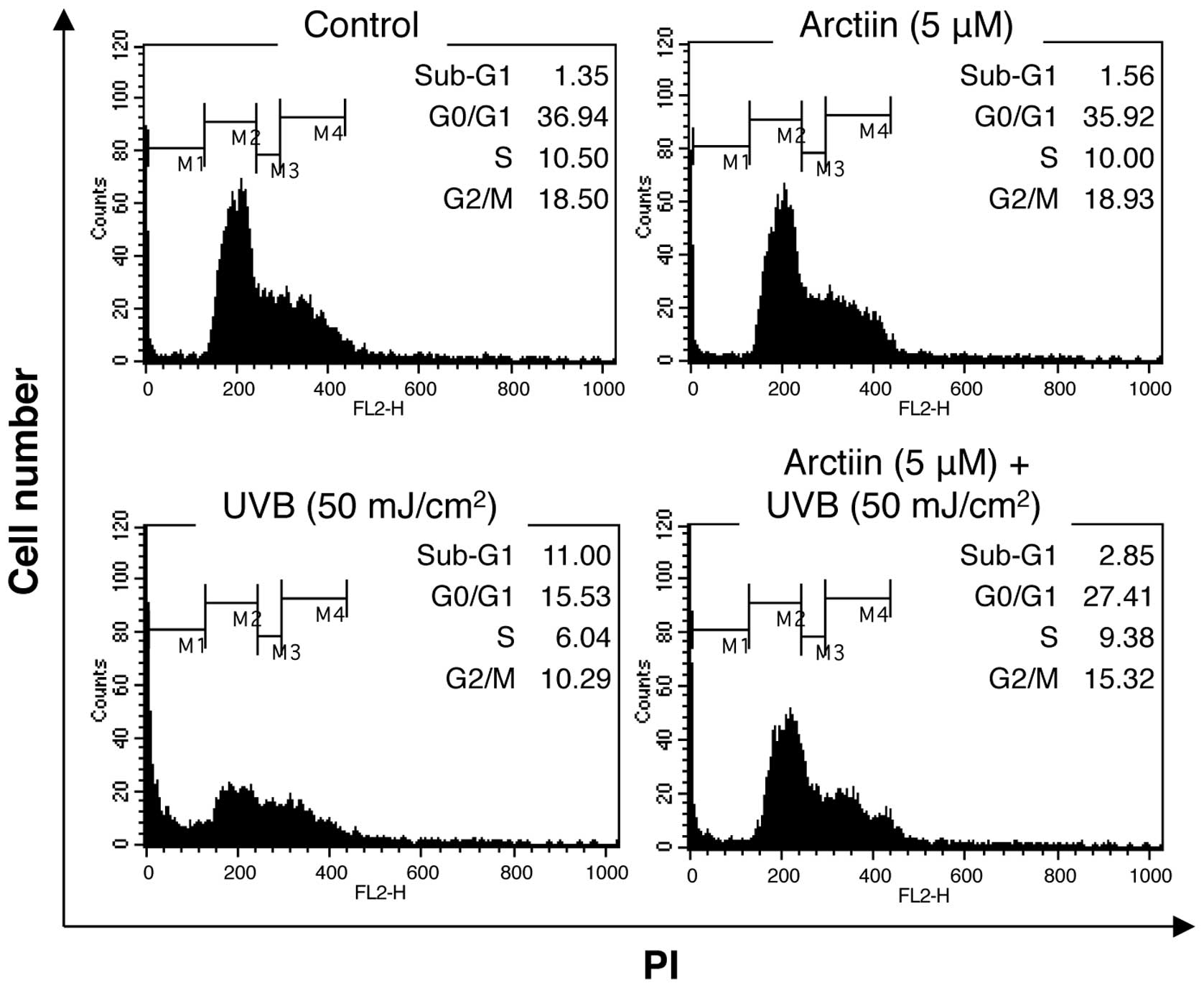
To determine the protective effect of arctiin on
UVB-irradiated HaCaT cells, the cell cycle distribution was
examined by PI staining and flow cytometry. UVB irradiation (50
mJ/cm2) increased the cell population in the sub-G1
phase, indicating the induction of cell death (Fig. 2). Pretreatment with arctiin prior
to UVB treatment markedly decreased the population of cells in
sub-G1 phase, suggesting that arctiin affects the UVB-mediated
cellular mechanisms that induce cell death (Fig. 2).
Arctiin pretreatment rescues UVB-induced
deficiencies in wound healing and DNA repair
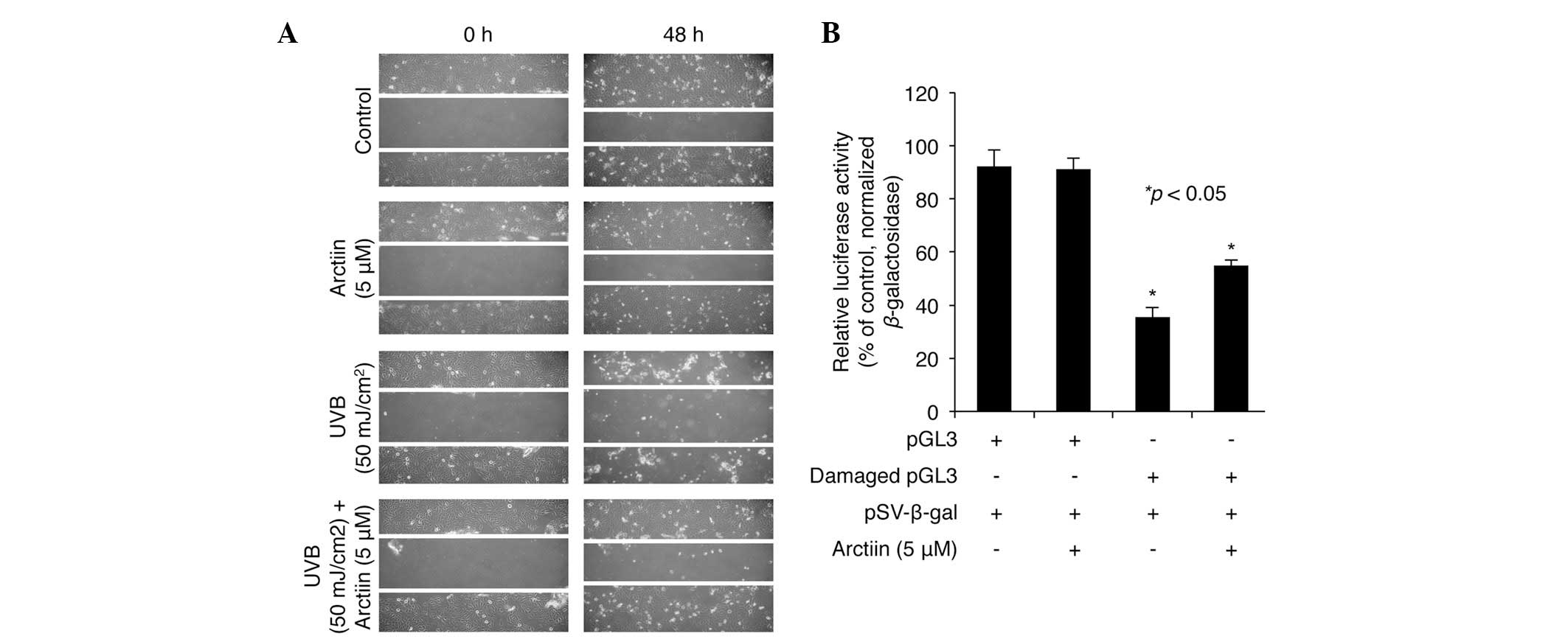
To examine whether arctiin pretreatment induced UVB
resistance through the regulation of the cell migration associated
with wound healing, a series of scratch assays were performed in
the HaCaT cells. Firstly, marked closure of the scratch was
demonstrated in the untreated control cells following 48 h
incubation with DMSO vehicle (Fig.
3A, top panel). A similar extent of cell migration was observed
in the cells treated with arctiin (5 μM; Fig. 3A, second panel). However,
UVB-irradiated cells demonstrated only a weak change in the extent
of cell migration between 0 and 48 h (Fig. 3A, third panel). Of note, the
UVB-mediated defect in cell migration was rescued by pretreatment
with arctiin to a level of scratch closure similar to that in the
control cells, suggesting that arctiin protects against the
UVB-induced loss of migration in HaCaT cells (Fig. 3A, bottom panel).
To further examine the effect of arctiin on the
repair of UV-induced DNA damage in HaCaT cells, a DNA repair assay
was performed using the luciferase system and a damaged pGL3
luciferase plasmid that had been irradiated with UVC (2000
mJ/cm2). The control or damaged pGL3 were co-transfected
into HaCaT cells with the β-gal plasmid, followed by treatment with
arctiin for 24 h. In the absence of arctiin, the luciferase
activity of damaged pGL3 was ~40% of that measured for the
undamaged control pGL3 (Fig. 3B).
However, the decrease in luciferase activity for the damaged
plasmid was significantly reduced in the cells treated with arctiin
following transfection, implying that arctiin enhances DNA repair
in HaCaT cells (Fig. 3B).
UVB protective functions of arctiin are
associated with changes in miRNA expression profiles in HaCaT
cells
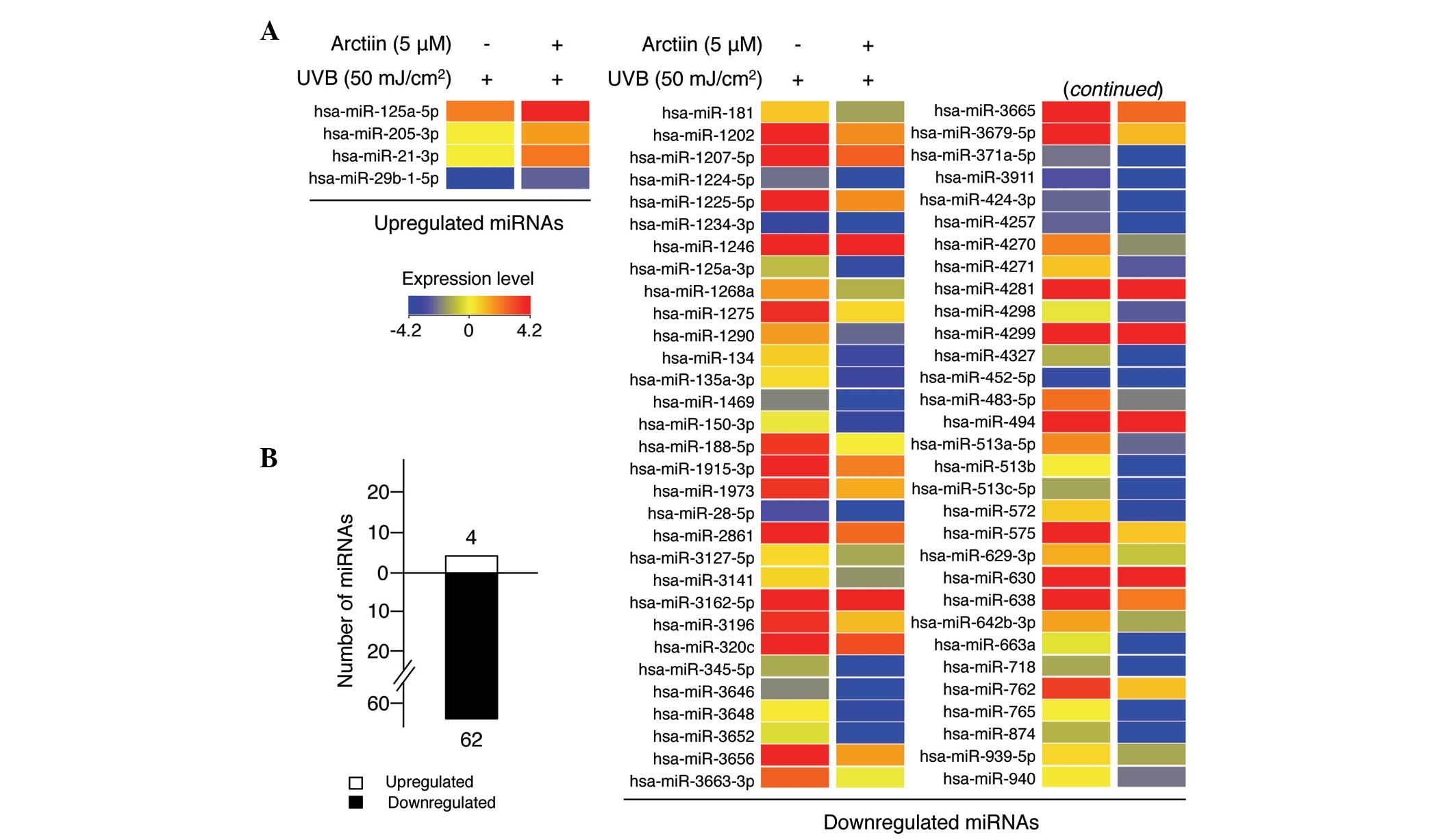
Next, the present study focused on exploring whether
the UVB protective function of arctiin in HaCaT cells was
associated with changes in miRNA expression profiles by miRNA
microarray analysis. UVB radiation following arctiin pretreatment
altered the expression levels of several miRNAs compared with UV
treatment alone (Fig. 4A). Four
miRNAs were significantly upregulated and 62 miRNAs were
significantly downregulated >2-fold (Fig. 4B), implying that arctiin modulates
the expression of several miRNAs to protect cells from UVB-induced
HaCaT cell damage. The full list of dysregulated miRNAs is shown in
Table I. miR-21-3p was the most
highly upregulated (3.85-fold) and miR-513c-5p was the most
downregulated (13.32-fold) miRNA during the arctiin-mediated UVB
protective response in HaCaT cells.
 | Table IChanges in miRNA expression in
UVB-irratiated HaCaT cells pretreated with arctiina. |
Table I
Changes in miRNA expression in
UVB-irratiated HaCaT cells pretreated with arctiina.
| Gene name | Fold change | Regulation | Chr |
|---|
| hsa-miR-125a-5p | 2.74 | Up | chr19 |
| hsa-miR-205-3p | 2.51 | Up | chr1 |
| hsa-miR-21-3p | 3.85 | Up | chr17 |
| hsa-miR-29b-1-5p | 2.81 | Up | chr7 |
| hsa-miR-1181 | −2.38 | Down | chr19 |
| hsa-miR-1202 | −3.40 | Down | chr6 |
| hsa-miR-1207-5p | −3.81 | Down | chr8 |
| hsa-miR-1224-5p | −2.70 | Down | chr3 |
| hsa-miR-1225-5p | −4.11 | Down | chr16 |
| hsa-miR-1234-3p | −2.90 | Down | chr8 |
| hsa-miR-1246 | −4.23 | Down | chr2 |
| hsa-miR-125a-3p | −2.86 | Down | chr19 |
| hsa-miR-1268a | −3.12 | Down | chr15 |
| hsa-miR-1275 | −3.94 | Down | chr6 |
| hsa-miR-1290 | −4.59 | Down | chr1 |
| hsa-miR-134 | −4.14 | Down | chr14 |
|
hsa-miR-135a-3p | −3.92 | Down | chr3 |
| hsa-miR-1469 | −5.69 | Down | chr15 |
| hsa-miR-150-3p | −3.28 | Down | chr19 |
| hsa-miR-188-5p | −4.11 | Down | chrX |
|
hsa-miR-1915-3p | −2.80 | Down | chr10 |
| hsa-miR-1973 | −2.63 | Down | chr4 |
| hsa-miR-28-5p | −2.20 | Down | chr3 |
| hsa-miR-2861 | −4.08 | Down | chr9 |
|
hsa-miR-3127-5p | −2.00 | Down | chr2 |
| hsa-miR-3141 | −2.33 | Down | chr5 |
|
hsa-miR-3162-5p | −3.08 | Down | chr11 |
| hsa-miR-3196 | −2.96 | Down | chr20 |
| hsa-miR-320c | −2.75 | Down | chr18 |
| hsa-miR-345-5p | −10.76 | Down | chr14 |
| hsa-miR-3646 | −2.75 | Down | chr20 |
| hsa-miR-3648 | −4.46 | Down | chr21 |
| hsa-miR-3652 | −8.13 | Down | chr12 |
| hsa-miR-3656 | −4.21 | Down | chr11 |
|
hsa-miR-3663-3p | −3.31 | Down | chr10 |
| hsa-miR-3665 | −3.01 | Down | chr13 |
|
hsa-miR-3679-5p | −4.16 | Down | chr2 |
|
hsa-miR-371a-5p | −2.26 | Down | chr19 |
| hsa-miR-3911 | −3.00 | Down | chr9 |
| hsa-miR-424-3p | −4.00 | Down | chrX |
| hsa-miR-4257 | −4.71 | Down | chr1 |
| hsa-miR-4270 | −4.41 | Down | chr3 |
| hsa-miR-4271 | −3.72 | Down | chr3 |
| hsa-miR-4281 | −3.08 | Down | chr5 |
| hsa-miR-4298 | −2.31 | Down | chr11 |
| hsa-miR-4299 | −3.21 | Down | chr11 |
| hsa-miR-4327 | −3.48 | Down | chr21 |
| hsa-miR-452-5p | −2.72 | Down | chrX |
| hsa-miR-483-5p | −5.72 | Down | chr11 |
| hsa-miR-494 | −3.35 | Down | chr14 |
|
hsa-miR-513a-5p | −5.23 | Down | chrX |
| hsa-miR-513b | −4.68 | Down | chrX |
|
hsa-miR-513c-5p | −13.32 | Down | chrX |
| hsa-miR-572 | −5.49 | Down | chr4 |
| hsa-miR-575 | −4.10 | Down | chr4 |
| hsa-miR-629-3p | −2.33 | Down | chr15 |
| hsa-miR-630 | −4.37 | Down | chr15 |
| hsa-miR-638 | −4.40 | Down | chr19 |
|
hsa-miR-642b-3p | −3.03 | Down | chr19 |
| hsa-miR-663a | −5.28 | Down | chr20 |
| hsa-miR-718 | −4.48 | Down | chrX |
| hsa-miR-762 | −2.73 | Down | chr16 |
| hsa-miR-765 | −6.70 | Down | chr1 |
| hsa-miR-874 | −3.09 | Down | chr5 |
| hsa-miR-939-5p | −2.01 | Down | chr8 |
| hsa-miR-940 | −2.39 | Down | chr16 |
Following this, the biological mechanisms by which
altered miRNA expression mediates the UVB protection effect were
investigated. miRNAs target mRNAs by directly interacting with
complementary sequences, so in the present study, putative target
genes of each miRNA were analyzed using the DIANA bioinformatics
tool. The resultant target genes were then analyzed using the
bioinformatics database DAVID. The results generated by the KEGG
pathway-based enrichment analysis program in DAVID indicated that
these miRNAs may be involved in the regulation of pathways in
cancer, the cell cycle, Wnt and MAPK signaling pathways (Tables II and III). Targets of the majority of
upregulated miRNAs had biological functions in pathways involved in
cancer, the cell cycle and Wnt signaling as described in Table II, whereas the majority of the
targets of downregulated miRNAs were involved in the regulation of
the actin cytoskeleton, cancer pathways, and MAPK, Axon, ErbB and
insulin signaling pathways (Table
III). Previous studies have reported that these pathways are
affected by UVB radiation (2,15–17).
In summary, these results identified the cellular processes
involved in arctiin-mediated UVB protection.
 | Table IIFunctional annotation chart for
miRNAs that were upregulated in arctiin-pretreated UVB-irradiated
HaCaT keratinocytes. |
Table II
Functional annotation chart for
miRNAs that were upregulated in arctiin-pretreated UVB-irradiated
HaCaT keratinocytes.
| miRNA (Homo
sapiens) | Putative target
genes | KEGG pathway | Genes involved in
the term | % of involved
genes/total genes | P-value |
|---|
| miR-125a-5p | 162 | Pathways in
cancer | 8 | 4.9 |
3.60×10−2 |
| | Cell cycle | 4 | 2.5 |
1.20×10−1 |
| miR-205-3p | 944 | Pathways in
cancer | 19 | 2 |
2.50×10−1 |
| | MAPK signaling
pathway | 17 | 1.8 |
1.70×10−1 |
| | Wnt signaling
pathway | 15 | 1.6 |
9.20×10−3 |
| miR-21-3p | 210 | CAMs | 7 | 3.3 |
4.70×10−3 |
| | Ubiquitin mediated
proteolysis | 6 | 2.9 |
2.30×10−2 |
| | Long-term
potentiation | 5 | 2.4 |
8.60×10−3 |
| | Oocyte meiosis | 5 | 2.4 |
4.20×10−2 |
| miR-29b-1-5p | 265 | Insulin signaling
pathway | 5 | 1.9 |
8.50×10−2 |
| | Cell cycle | 4 | 1.5 |
2.00×10−1 |
| | Wnt signaling
pathway | 4 | 1.5 |
2.90×10−1 |
| | Jak-STAT signaling
pathway | 4 | 1.5 |
3.00×10−1 |
 | Table IIIFunctional annotation chart for
miRNAs that were downregulated in arctiin-pretreated UVB-irradiated
HaCaT keratinocytes.a |
Table III
Functional annotation chart for
miRNAs that were downregulated in arctiin-pretreated UVB-irradiated
HaCaT keratinocytes.a
| miRNA (Homo
sapiens) | Putative target
genes | KEGG pathway | Genes involved in
the term | % of involved
genes/total genes | P-value |
|---|
| miR-513c-5p | 142 | Tight junction | 4 | 2.8 |
3.30×10−2 |
| |
Phosphatidylinositol signaling system | 3 | 2.1 |
5.80×10−2 |
| | CAMs | 3 | 2.1 |
1.50×10−1 |
| miR-345-5p | 94 | Axon guidance | 3 | 3.2 |
8.20×10−2 |
| | Regulation of actin
cytoskeleton | 3 | 3.2 |
1.90×10−1 |
| | Type II diabetes
mellitus | 2 | 2.1 |
1.60×10−1 |
| | Arginine and
proline metabolism | 2 | 2.1 |
1.80×10−1 |
| | VEGF signaling
pathway | 2 | 2.1 |
2.50×10−1 |
| | TGF-β signaling
pathway | 2 | 2.1 |
2.80×10−1 |
| miR-3652 | 195 | Axon guidance | 5 | 2.6 |
3.30×10−2 |
| | CAMs | 5 | 2.6 |
3.50×10−2 |
| | Insulin signaling
pathway | 5 | 2.6 |
3.80×10−2 |
| | ErbB signaling
pathway | 4 | 2.1 |
4.80×10−2 |
| | MAPK signaling
pathway | 4 | 2.1 |
4.70×10−1 |
| miR-765 | 548 | Cytokine-cytokine
receptor interaction | 11 | 2 |
2.00×10−1 |
| miR-483-5p | 32 | Focal adhesion | 2 | 6.2 |
1.50×10−1 |
| miR-1469 | 2 | - | - | - | - |
| miR-572 | 6 | - | - | - | - |
| miR-663a | 33 | - | - | - | - |
| miR-513a-5p | 980 | MAPK signaling
pathway | 25 | 2.6 |
1.00×10−2 |
| | Pathways in
cancer | 24 | 2.4 |
1.30×10−1 |
| | Regulation of actin
cytoskeleton | 20 | 2 |
2.50×10−2 |
| miR-1290 | 593 | Pathways in
cancer | 17 | 2.9 |
4.00×10−2 |
| | Focal adhesion | 14 | 2.4 |
7.90×10−3 |
| | Insulin signaling
pathway | 13 | 2.2 |
7.60×10−4 |
| | Regulation of actin
cytoskeleton | 12 | 2 |
6.30×10−2 |
| | MAPK signaling
pathway | 12 | 2 |
1.90×10−1 |
| | ErbB signaling
pathway | 11 | 1.9 |
2.80×10−4 |
| miR-718 | 40 | - | - | - | - |
| miR-3648 | 13 | - | - | - | - |
| miR-4270 | 423 | Axon guidance | 8 | 1.9 |
5.50×10−3 |
| | Regulation of actin
cytoskeleton | 8 | 1.9 |
6.70×10−2 |
Discussion
Keratinocytes are the predominant cell type in the
outermost skin layer, the epidermis, and have a pivotal role in
primary protection against environmental insults, including UV
radiation. UV radiation is the major inducer of photoaging of
keratinocytes and may result in cellular aging, senescence,
apoptosis or cancer by inducing damage to intracellular molecules,
in particular DNA. In the present study, biochemical and genetic
analyses were utilized to identify the phytochemical arctiin as a
novel photoprotective agent against UVB-mediated keratinocyte
damage. The data revealed that arctiin itself had a low
cytotoxicity in HaCaT cells. Notably, arctiin pretreatment reduced
UVB-induced cytotoxicity and suppressed UVB-mediated cell death. It
was also demonstrated that arctiin enhanced wound healing and DNA
repair in UVB-exposed HaCaT keratinocytes.
Significant changes in miRNA expression profiles in
cells that were pretreated with arctiin prior to UVB irradiation
were observed, as compared with those in the cells that only
received UVB irradiation, with 66 miRNAs exhibiting altered
expression. Of note, almost all of the dysregulated miRNAs were
downregulated (62/66) and only four miRNAs were upregulated. This
expression pattern differs from the results of a previous study by
our group in which the titrated extract of Centella asiatica
(TECA) was demonstrated to protect keratinocytes against
UVB-induced damage through upregulation of 46 miRNAs and
downregulation of 36 miRNAs (12).
In addition, Zhou et al (6)
recently characterized UVB-responsive miRNAs in human keratinocytes
using microRNA microarray analysis and identified that the
expression of 44 miRNAs was up- or downregulated by >2-fold
compared with that in non-irradiated keratinocytes. There appears
to be no correlation between the UVB-responsive miRNAs and the
arctiin-induced UVB protective miRNAs; however, certain miRNAs
identified in the present study were also dysregulated in other
studies. miR-3652 and miR-494, which were downregulated by 8.31-
and 3.35-fold in the present study, were also downregulated in
keratinocytes with TECA-induced UVB protection (12). miR-125a-5p was previously reported
to be expressed at significantly lower levels in UVB-irradiated
mouse epidermis than in control epidermis (18), although the results of the present
study demonstrated that the miRNA was significantly upregulated
(2.74-fold). In addition, miR-1246 (4.23-fold downregulation in
this study) was reported to be a target of the p53 transcription
factor, which is significantly upregulated by UVB irradiation in
keratinocytes (19), and
miR-125a-3p (2.86-fold downregulation) has been demonstrated to
reduce cell proliferation and migration by targeting Fyn kinase,
which is activated by UVB irradiation in keratinocytes (20,21).
In summary, the microarray data of the present study suggested that
although the dysregulated miRNAs were specifically induced by
arctiin treatment, they may be involved in the regulation of
general UVB-mediated cellular transduction. Although miR-513c-5p
and miR-345-5p were the most highly dysregulated miRNAs in the
present study (13.32- and 10.76-fold downregulated, respectively),
their biological functions have not been reported. It is possible
that these miRNAs also regulate UVB-mediated cytotoxicity in
keratinocytes.
The biological significance of the dysregulated
miRNAs identified in the present study was further examined. For
this, the putative target genes of the miRNAs were predicted using
DIANA, a web-based bioinformatics program, and the target genes
involved in KEGG pathways were then analyzed using the DAVID
database. The results indicated that the MAPK signaling as the
pathway that was most affected by arctiin-induced UVB protection in
keratinocytes. miR-205-3p, miR-3652, miR-513a-5p and miR-1290 were
predicted to affect target genes involved in the MAPK pathway.
Indeed, MAPK activation is one of the main signaling pathways
activated by UVB exposure. In keratinocytes, UVB stimulates
immediate activation of JNK, p38 MAPK and ERK1/2 (2). Activated JNK and p38 MAPK mediate
both cell survival and death in UVB-irradiated keratinocytes
(2). In addition, it was
demonstrated that UVB-induced production of ROS (including
H2O2) activates p38 MAPK and JNK signaling in
keratinocytes, suggesting that UVB-induced ROS may affect signal
transduction through MAPK pathways (2). Furthermore, it has been suggested
that p38 MAPK and JNK have a pivotal role in the UVB-induced
inflammatory response by regulating cyclooxygenase-2 (COX-2)
activity in keratinocytes (2). Of
note, UV radiation does not regulate the mRNA levels of JNK, p38
MAPK and ERK1/2, but rather the levels of
post-translational modification by protein phosphorylation. This
indicates that the activation of MAPK proteins by UVB radiation is
not transcriptionally induced (2).
However, recent studies have demonstrated that post-transcriptional
regulators, including miRNAs, may regulate MAPK activity. Although
the miRNAs do not directly regulate mRNA levels of MAPKs, upstream
or downstream members of MAPK signaling pathways are
post-transcriptionally regulated by miRNAs. Zhang et al
(22) demonstrated that miR-451
regulates p38 MAPK signaling by targeting of Ywhaz, and Antoon
et al (23) demonstrated
that increased p38 MAPK activity results in part from differential
miRNA expression. Therefore, arctiin-induced UVB protective miRNAs
may regulate members of MAPK signaling pathways, rather than the
mRNAs of MAPKs themselves.
In conclusion, the present study suggested that
arctiin may be a novel photoprotective agent that confers
resistance to UVB-mediated keratinocyte damage by altering miRNA
expression profiles. In particular, the MAPK signaling pathway
appears to be a major target of the miRNAs that are affected by
arctiin. Further studies are required to determine the exact
mechanisms by which arctiin induces a UVB protective effect in
keratinocytes.
Acknowledgements
The authors are grateful to all other members of
Coreana Cosmetics Co., Ltd. for their support. This paper was
supported by the KU Research Professor Program of Konkuk University
and grant from the Ministry of Science, ICT and Future Planning
(no. 20110028646) of the Republic of Korea.
References
|
1
|
Wondrak GT, Jacobson MK and Jacobson EL:
Endogenous UVA-photosensitizers: mediators of skin photodamage and
novel targets for skin photoprotection. Photochem Photobiol Sci.
5:215–237. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muthusamy V and Piva TJ: The UV response
of the skin: a review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivetti di Val Cervo P, Lena AM, Nicoloso
M, et al: p63-microRNA feedback in keratinocyte senescence. Proc
Natl Acad Sci USA. 109:1133–1138. 2012.PubMed/NCBI
|
|
4
|
Hildebrand J, Rütze M, Walz N, et al: A
comprehensive analysis of microRNA expression during human
keratinocyte differentiation in vitro and in vivo. J Invest
Dermatol. 131:20–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Wang J, Guo SL, et al: miR-21
promotes keratinocyte migration and re-epithelialization during
wound healing. Int J Biol Sci. 7:685–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou BR, Xu Y, Permatasari F, et al:
Characterization of the miRNA profile in UVB-irradiated normal
human keratinocytes. Exp Dermatol. 21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Z, Zhou B, Liu W, et al: miR-23a
regulates DNA damage repair and apoptosis in UVB-irradiated HaCaT
cells. J Dermatol Sci. 69:68–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee S, Shin S, Kim H, et al:
Anti-inflammatory function of arctiin by inhibiting COX-2
expression via NF-κB pathways. J Inflamm (Lond).
8:162011.PubMed/NCBI
|
|
9
|
Hirose M, Yamaguchi T, Lin C, et al:
Effects of arctiin on PhIP-induced mammary, colon and pancreatic
carcinogenesis in female Sprague-Dawley rats and MeIQx-induced
hepatocarcinogenesis in male F344 rats. Cancer Lett. 155:79–88.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi K, Narutaki K, Nagaoka Y, Hayashi
T and Uesato S: Therapeutic effect of arctiin and arctigenin in
immunocompetent and immunocompromised mice infected with influenza
A virus. Biol Pharm Bull. 33:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuzaki Y, Koyama M, Hitomi T, et al:
Arctiin induces cell growth inhibition through the down-regulation
of cyclin D1 expression. Oncol Rep. 19:721–727. 2008.PubMed/NCBI
|
|
12
|
An IS, An S, Choe TB, et al: Centella
asiatica protects against UVB-induced HaCaT keratinocyte damage
through microRNA expression changes. Int J Mol Med. 30:1349–1356.
2012.
|
|
13
|
Maragkakis M, Reczko M, Simossis VA, et
al: DIANA-microT web server: elucidating microRNA functions through
target prediction. Nucleic Acids Res. 37:W273–W276. 2009.
View Article : Google Scholar
|
|
14
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI
|
|
15
|
Misovic M, Milenkovic D, Martinovic T,
Ciric D, Bumbasirevic V and Kravic-Stevovic T: Short-term exposure
to UV-A, UV-B, and UV-C irradiation induces alteration in
cytoskeleton and autophagy in human keratinocytes. Ultrastruct
Pathol. 37:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madson JG and Hansen LA: Multiple
mechanisms of Erbb2 action after ultraviolet irradiation of the
skin. Mol Carcinog. 46:624–628. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada T, Hasegawa S, Inoue Y, et al:
Wnt/β-catenin and kit signaling sequentially regulate melanocyte
stem cell differentiation in UVB-induced epidermal pigmentation. J
Invest Dermatol. 133:2753–2762. 2013.
|
|
18
|
Zhou BR, Xu Y and Luo D: Effect of UVB
irradiation on microRNA expression in mouse epidermis. Oncol Lett.
3:560–564. 2012.PubMed/NCBI
|
|
19
|
Henseleit U, Zhang J, Wanner R, Haase I,
Kolde G and Rosenbach T: Role of p53 in UVB-induced apoptosis in
human HaCaT keratinocytes. J Invest Dermatol. 109:722–727. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ninio-Many L, Grossman H, Shomron N,
Chuderland D and Shalgi R: microRNA-125a-3p reduces cell
proliferation and migration by targeting Fyn. J Cell Sci.
126:2867–2876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Z, Cho YY, Ma WY, Choi HS, Bode AM and
Dong Z: Regulation of ultraviolet B-induced phosphorylation of
histone H3 at serine 10 by Fyn kinase. J Biol Chem. 280:2446–2454.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Luo X, Ding S, et al:
microRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and
suppresses the mesangial hypertrophy in early diabetic nephropathy.
FEBS Lett. 586:20–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antoon JW, Nitzchke AM, Martin EC, et al:
Inhibition of p38 mitogen-activated protein kinase alters microRNA
expression and reverses epithelial-to-mesenchymal transition. Int J
Oncol. 42:1139–1150. 2013.
|