Introduction
The management of various cartilage lesions remains
a challenge for surgeons, due to the limited regenerative capacity
of cartilage tissues. A number of echniques have been developed for
the treatment of cartilage defects during the past decade, aiming
to address this complex issue. These techniques, including
autologous cartilage implantation and autologous chondrocyte
implantation, have been used to repair cartilage defects. More
recently, tissue engineering has been reported as a promising
approach to repair cartilage defects. This technique involves
harvesting a high number of cells from the human body and seeding
them on a biodegradable scaffold, and then implanting these
scaffolds to cover the defective areas. Therefore, the emergence of
cartilage tissue engineering techniques provides potential
solutions for the clinical repair of cartilage defects. In
addition, seeding of chondrocytes on biodegradable scaffolds in
order to allow formation of a three-dimensional cartilage tissue
for implantation is a primary approach in cartilage tissue
engineering aiming to repair cartilage defects. Cao et al
(1) used a chondrocyte-polymer
composite to produce tissue-engineered cartilage in the shape of a
human ear. Luo et al (2)
reconstructed a segmental tracheal defect by pedicled
tissue-engineered trachea in rabbits.
A variety of biodegradable materials has been
explored for eventual use as scaffolds in cartilage tissue
engineering, including collagen gels and sponges, hyaluronic acid
matrices, and poly(α-hydroxyesters), such as polyglycolic acid
(PGA), polylactic acid (PLA) and their copolymers. Wu et al
(3) were able to form mature
cartilage tissue by using poly(hydroxybutyrate-co-hydroxyvalerate)
(PHBV), and improved the PHBV scaffold with bioglass to produce
cartilage tissue with improved biomechanical and biochemical
properties. Xue et al (4)
reported that chondrogenesis of bone marrow-derived stromal cells
seeded on PGA promotes regeneration of cartilage. However, the
natural and artificial synthetic material used in these studies are
still exogenous, often causing inflammatory and immune reactions.
Therefore, research on biodegradable scaffolds produced by
autologous material is receiving increasing attention.
Platelet-rich plasma (PRP) is ideal for use as a biomedical
material for tissue engineering due to its reduced immunogenicity
and improved biocompatibility, in addition to its high content in
growth factors.
Numerous studies have used PRP as a scaffold for
bone tissue engineering. El Backly et al (5) used platelet-rich plasma to enhance
the osteoconductive properties of a hydroxyapatite-β-tricalcium
phosphate scaffold for late healing of critical-size rabbit
calvarial defects. Jiang et al (6) repaired calvarial defects in rabbits
using platelet-rich plasma as the scaffold, which carried bone
marrow stromal cells.
However, up to now, few studies have investigated
the utility of autologous platelet-rich plasma gel (APG) in
cartilage tissue engineering. Therefore, in this study, APG was
prepared and studied. The chondrocytes were seeded on the scaffolds
and cultured in vitro for one week, followed by in
vivo implantation for six weeks, to observe the growth and
proliferation of the chondrocytes and the engineered cartilage
tissue in PRP. The level of growth factors, cell proliferation in
the scaffold, extracellular matrix production, as well as
biomechanical properties of the neocartilage were examined, to
evaluate the value of the PRP scaffold in cartilage
regeneration.
Materials and methods
Ethics statement
All experimental procedures conducted on animals in
this study were approved by the Ethics Committee of the Nanjing
Medical University.
Blood collection and cell isolation
New Zealand white rabbits (2–3 months old), obtained
from the Animal Center of Nanjing Medical University (Nanjing,
China) were used to isolate PRP and articular cartilage. Animals
were first anesthetized by intramuscular injection of sodium
pentobarbital (30 mg/kg) and ketamine hydrochloride (30 mg/kg).
Blood (50 ml) was collected from the posterior auricular vein with
a 14-G needle using a 50-ml syringe treated with an anti-coagulant
citrate dextrose solution (Sigma-Aldrich, St. Louis, MO, USA).
Following collection of peripheral blood, the animals were
sacrificed by intravenously injecting supersaturated
pentobarbital.
The articular cartilages were removed from the knees
and hip joints of the rabbits and were cut into small pieces as
described in (3). Chondrocytes
were released from the cartilage slices by digesting with
collagenase II (0.2% w/v; Invitrogen Life Technologies, Carlsbad,
CA, USA). The isolated cells were then cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal calf
serum (FCS; Gibco Life Technologies, Carlsbad, CA, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, Irvine, UK).
Cells were incubated at 37°C in a 5% CO2 incubator, and
the medium was changed every 3 days.
Preparation of PRP
PRP was isolated from fresh rabbit blood (~50 ml) as
previously described (7). Briefly,
the blood was treated with anti-coagulant and was separated into
the plasma and the hemocyte (erythrocytes and leukocytes)
fractions, and then separated into PRP by continuous two-step
sedimentation (Fig. 1A). PRP was
clotted by adding a 10% (v/v) thrombin solution in CaCl2
(1,000 U/ml thrombin in 100 mM CaCl2) to yield a final
thrombin concentration of 100 U/ml. Soluble PRP was released from
the clotted preparations by centrifugation at 1,500 g for 5
min.
Quantification of growth factors
The level of transforming growth factor-β (TGF-β),
insulin-like growth factor (IGF), epidermal growth factor (EGF) and
platelet-derived growth factor (PDGF)-AB in whole blood, PRP and
activated PRP (aPRP) was determined using corresponding
enzyme-linked immunosorbent assay (ELISA) kits according to the
manufacturer’s instructions (R&D Systems, Inc., Minneapolis,
MN) as in (8).
Cell cultures with different
concentrations of PRP and cell proliferation assay
Cell cultures in DMEM fetal calf serum-free medium
(SFM) containing different concentrations of PRP (0, 5, 10, 20 and
30%) were used to test the effect of PRP on the proliferation of
chondrocytes. Briefly, the chondrocytes at passage 2 were
precultured in DMEM SFM for 24 h. The chondrocytes were then
cultured under five different conditions: 0% PRP (SFM without PRP),
5% PRP (5% PRP in SFM), 10% PRP (10% PRP in SFM), 20% PRP (20% PRP
in SFM), and 30% PRP (30% PRP in SFM). The proliferation of the
chondrocytes was assessed with a cell counting kit (CCK)-based
colorimetric assay as in (9),
following the manufacturer’s instructions (CCK-8 kit; Rockville,
MD, USA). The optical density (OD) of the samples was measured on a
DU800 spectrophotometer (Beckman Coulter, Brea, CA, USA).
Formation of chondrocyte/PRP
composites
After subculturing twice, the chondrocyte suspension
at passage 2 was collected by trypsin digestion and centrifuged at
352 × g for 5 min. The chondrocytes were washed 2 times with
phosphate-buffered saline (PBS) to remove the residual serum, and
cells were counted and resuspended in PRP, at a density of
5.0×107 cells/ml. To activate PRP, 0.15 ml of a
CaCl2 and Gibco® bovine thrombin mixture
(1,000 U/ml in 100 mg/ml CaCl2; Thermo Fisher
Scientific, Waltham, MA, USA) was added to the cell/PRP suspension.
Seven days later, the composites were subcutaneously implanted into
BALB-c-nu nude mice (4 weeks old, 20 g), obtained from the Animal
Center of Nanjing Medical University. The composites were harvested
at 6 weeks post-implantation.
Scanning electron microscopy (SEM) of in
vitro-engineered tissue
The attachment, proliferation and matrix production
of the cells on the scaffolds were examined using light microscopy
(Eclipse TS100 microscope; Nikon, Tokyo, Japan) and SEM (XL-30;
Philips, Amsterdam, The Netherlands).
Evaluation of in vivo-engineered
tissue
Gross observation of in
vivo-engineered tissue
The composites were harvested at 6 weeks after
implantation and were recorded by image analysis.
Histological evaluation
Following implantation for 6 weeks, representative
in vivo-formed cartilaginous tissue was fixed in
neutral-buffered formalin, embedded in paraffin and sectioned (5-μm
thick sections). The cross-sections were stained with hematoxylin
and eosin (H&E) and safranine O (Sigma-Aldrich, St. Louis, MO,
USA).
Five-micrometer cryosections were used for
immunostaining with the collagen type II monoclonal mouse
anti-rabbit antibody (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). The samples were immersed in PBS containing 1% goat serum
at 4°C overnight, in order to block the non-specific reactions.
Subsequently, the sections were incubated in PBS containing 1%
bovine serum albumin (BSA) and anti-collagen type II antibody
(1:100 working dilution) at 25°C for 4 h. After washing with PBS
three times, the samples were incubated in PBS containing 3% BSA.
Finally, the samples were incubated in PBS containing 1% BSA and
horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody
(1:150 working dilution; Santa Cruz Biotechnology, Inc.) at 25°C
for 4 h, followed by color development with diaminobenzidine
tetrahydrochloride (DAB; Santa Cruz Biotechnology, Inc), as in
(3).
Quantitative analysis of in vivo
cartilage formation
After 6 weeks of in vivo culture, the
glycosaminoglycan (GAG) content (10,11)
and the total collagen content were analyzed according to
previously described methods (12). Briefly, the protein solutions of
the specimens were prepared. The specific binding of Alcian Blue
and polysulfated molecules of GAGs in cartilage were acheived by
adding a series of reagents. All GAGs were precipitated
specifically in guanidine-HCl by using a low pH in combination with
detergent and high salt concentration. The precipitate was
dissolved in a mixture of guanidine-HCl and propanol. The optical
density (OD) of the samples was measured, and a linear standard
curve between 0.5 and 20 mg was generated by adding known amounts
of proteoglycans.
Biomechanical analysis
A biomechanical analyzer (Instron, Norwood, MA, USA)
was used for the biomechanical tests. As previously described
(3), a constant compressive strain
rate of 1 mm/min was applied, until a maximal force of 100 N was
achieved, and thus a force-displacement curve was obtained. The
compressive modulus of the tested tissue was calculated based on
the force-displacement curve.
Statistical analysis
All data were expressed as means ± standard
deviation (SD), with n=6. The differences were analyzed with a
one-way analysis of variance (ANOVA), using SPSS for Windows
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Cell cultures and chondrocyte-APG
composite formation
After 3 days of primary culture, cells reached
85–90% confluence. Following two additional subculturing rounds,
the cells maintained their morphology, and chondrocytes at passage
2 were used for cartilage engineering. The chondrocyte-APG
composite had an ivory-whitish appearance (Fig. 1B and D). When observed by SEM, the
chondrocytes grew well in the APG scaffold, and the scaffold
exhibited a macroporous structure with interconnected open pores,
of size varying from 30 to 300 μm (Fig. 1C).
Quantification of growth factors
The results of ELISA assays showed that the level of
TGF-β1 increased from 3.10±0.43 ng/ml in whole blood to 5.9±0.67
ng/ml in PRP and 13.55±1.53 ng/ml in aPRP (Fig. 2A). The concentration of IGF showed
an increase from 14.50±1.63 ng/ml in whole-blood to 45.03±4.67
ng/ml in PRP and 110.06±12.53 ng/ml in aPRP (Fig. 2B). The concentration of PDGF-AB
showed an increase from 145.02±16.3 pg/ml in whole blood to
263.00±26.71 pg/ml in PRP and 503.00±53.00 pg/ml in aPRP (Fig. 2C). The concentration of EGF showed
an increase from 135.12±16.3 pg/ml in whole blood to 235.30±25.67
pg/ml in PRP and 460.23±50.98 pg/ml in aPRP (Fig. 2D).
The effect of different concentrations of
PRP on cell proliferation
The result of the CCK assay showed that different
concentrations of PRP have different effects on cell proliferation.
Cell proliferation in the presence of different concentrations of
PRP (0, 5, 10, 20 and 30%) gradually increased, and reached a peak
at 20% PRP (Fig. 3).
Evaluation of in vivo-engineered
tissue
Gross evaluation of the in
vivo-engineered composites
The chondrocyte-APG composites maintained their
original size and presented a cartilage-like appearance following
in vivo implantation for 6 weeks.
Histology and
immunohistochemistry
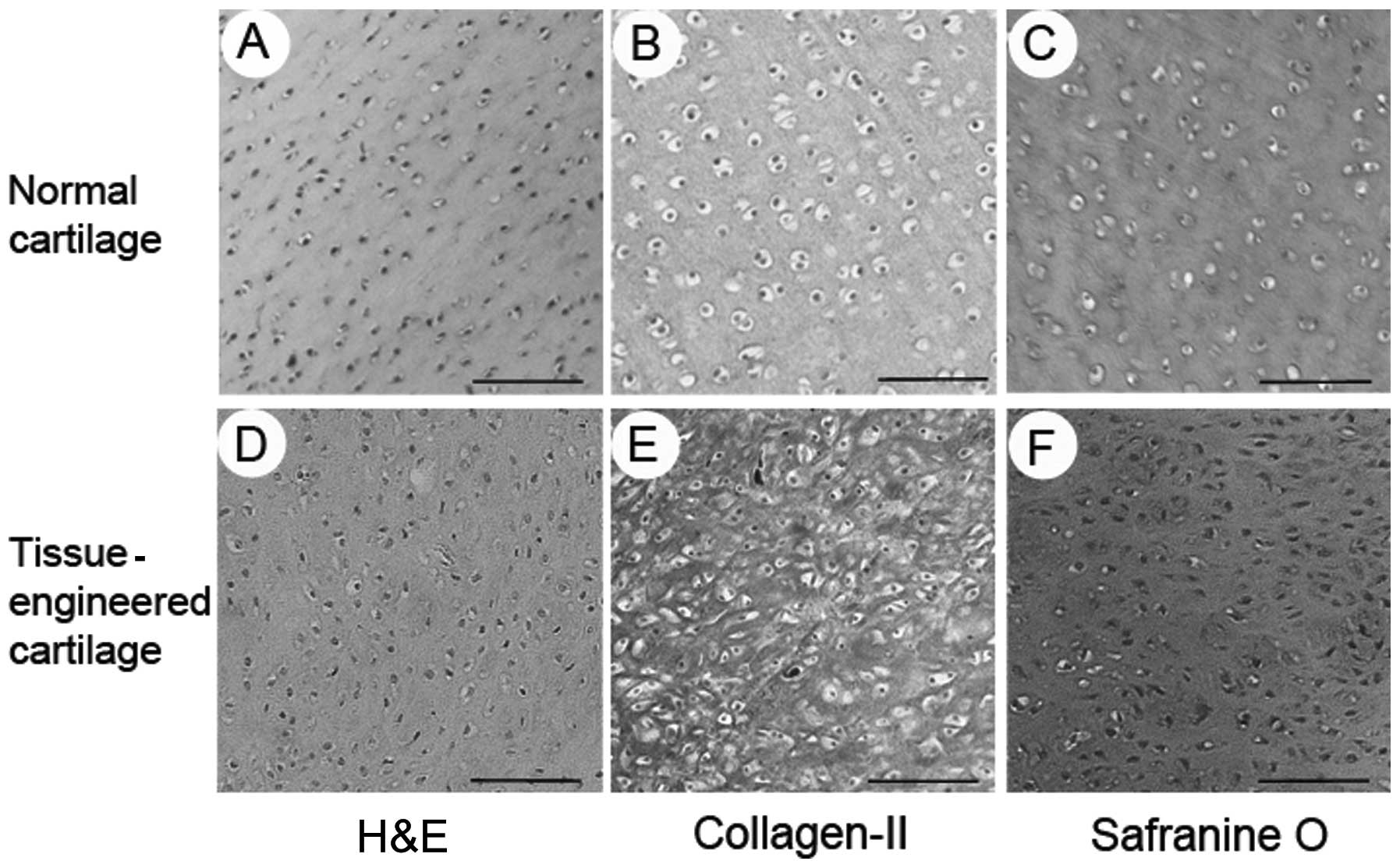
Histology and immunohistochemistry revealed the
formation of tissue-engineered cartilage. Formation of the
cartilage-like tissue was observed, with an obvious lacuna
structure and positive staining for safranine O and type II
collagen (Fig. 4).
Collagen and GAG content, and
compressive modulus
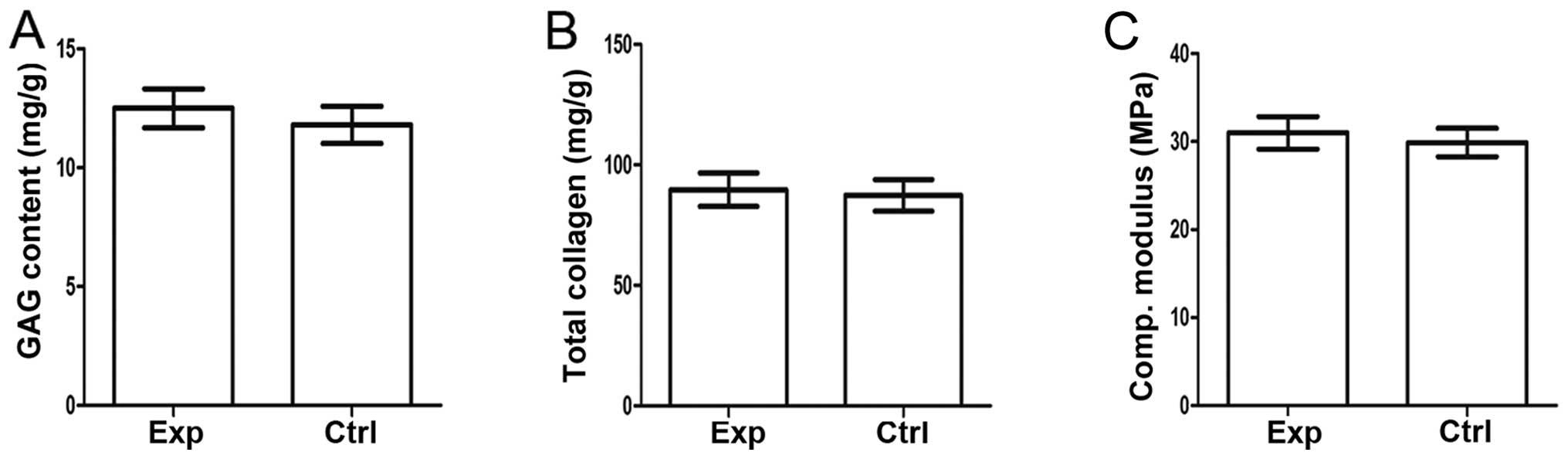
Quantitative analysis of the collagen and GAG
content demonstrated that there is no significant difference
(P>0.05) on extracellular matrix (ECM) contents between normal
and tissue-engineered cartilage (Fig.
5A and B).
The mechanical properties of the cartilage are of
importance, and the collagen and GAG content only partly reflect
these. Analysis of the compressive modulus further indicated that
there is no significant difference (P>0.05) between
tissue-engineered and normal cartilage (Fig. 5C).
Discussion
Cartilage defects have been a challenge for
surgeons. However, developments in cartilage tissue engineering in
the past few years have provided a means to repair cartilage
defects (13–15). Certain cases of tissue-engineered
cartilage implantation have been reported in the clinic (e.g.,
16). The scaffold, which acts as
a cell carrier, plays a significant role in the regeneration of the
cartilage. Numerous natural or synthetic materials have been
exploited as scaffolds during the past decade for cartilage tissue
engineering. However, an optimal biodegradable scaffold for
cartilage tissue engineering would ideally be less immunogenic than
the currently used ones, with improved biocompatibility and an
adequate degradation rate; in addition, the scaffold is expected to
promote attachment, proliferation and the activity of cells
(17).
Autologous platelet-rich plasma gel (APG) is a
fraction of the plasma containing multiple growth factors, such as
TGF-β1, PDGF, EGF and IGF-1, and which can induce biological
changes in cell proliferation and matrix metabolism in a variety of
connective tissues (18,19). APG can be produced by centrifugal
separation of autologous whole-blood samples (20). Moreover, APG has an ideal
three-dimensional structure, reduced immunogenicity and
satisfactory biocompatibility. Scanning electron microscope
analysis in this study revealed that APG has a three-dimensional
nanostructure, and that cells grew well in APG. Due to the above
advantages, APG appears promising as a novel biodegradable scaffold
to be used in tissue engineering.
Despite the development of various natural or
synthetic biomaterials for cartilage tissue engineering, the
biological safety of scaffolds and the long-term biocompatibility
between chondrocytes and scaffolds, or between tissue-engineered
cartilage and acceptor tissue, still needs to be further
investigated. Most of the currently used biodegradable material has
been reported to cause certain inflammatory reactions, and in some
cases, it is even rejected following implantation via immune
reactions (21,22). Compared to other materials, APG is
an autologous material with reduced immunogenicity and enhanced
biocompatibility, and has been used in certain clinical cases.
Torrero et al (23) treated
30 patients (18–65 years old, diagnosed with Outerbridge grade
I–III chondropathy in the knee, pain for more than 3 months
following conservative treatment, and no bone axial defects)
affected by chondropathy of the knee with a single intrarticular
injection of PRP, and statistical analysis with ANOVA showed
significantly better (P<0.05) results in these patients in terms
of KOOS and VAS scores at 1, 3 and 6 months, in comparison to the
pre-injection values. Redaelli et al (24) revitalized face and neck with PRP
injection and also obtained satisfactory results.
In addition, PRP provides a high level of growth
factors such as TGF-β1, FGF etc. In the present study, we measured
the concentration of growth factors in the whole blood, PRP and
aPRP. The results showed higher concentrations of growth factors in
PRP than that in the whole blood. Moreover, the activated PRP in
APG showed the highest concentrations of growth factors among the
three groups. The reason for that is that the platelets collected
in PRP are activated by the addition of thrombin and calcium
chloride, which induces the release of these factors from the α
granules (25,26).
It was reported that most growth factors found in
PRP can enhance cell proliferation and/or matrix production
(18). TGF-β plays an important
role in tissue regeneration, cell differentiation and embryonic
development, and the application of TGF-β on the chondrocytes
induces the synthesis of type I relative to type II collagen
(27,28). Both IGF-I and IGF-II stimulate
chondrocyte proliferation, and maintain the chondrocyte phenotype
(29). We showed in this study
that PRP, a highly concentrated natural combination of growth
factors, has the ability to stimulate cell proliferation.
Furthermore, we studied the effect of different concentrations of
PRP on cell proliferation, and the result showed that cell
proliferation is dose- and time-dependently increased by PRP, with
the highest levels of cell proliferation observed at a dose of 20%
PRP. In addition to proliferation, the growth factors present in
PRP are known to accelerate epithelial regeneration, promote
angiogenesis, and ultimately improve wound healing in skin and
periodontal tissue (30).
In conclusion, PRP provides a good environment for
cell growth and proliferation, by supplementing the chondrocytes
with all the essential nutrients. In this study, the
chondrocyte-APG composite was formed on an ivory-whitish,
cartilage-like tissue in vitro and in vivo.
Histological and immunohistochemical examination of the engineered
cartilage showed that the majority of the cells formed lacuna-like
structures, with positive staining for safranine O and type II
collagen.
In addition, there was no significant difference
observed in the compressive modulus between the tissue-engineered
and the normal cartilage groups. The mechanical properties of
neocartilage tissue are determined by the content of the ECM
(31). Previous studies reported
that the ECM content (GAG and total collagen) in cartilage tissue
contributes to the improvement of mechanical strength (e.g.,
32). Our study also found that
the GAG and total collagen content in tissue-engineered cartilage
is similar to that in the normal cartilage tissue. Thus, the APG
scaffold supported the growth of cells, which maintained their
activity, fully expressed their phenotype, produced the
extracellular matrix, and preserved the characteristic spherical
morphology associated with the synthesis of type II collagen and
cartilage-specific proteoglycans.
In summary, the APG scaffolds provide a high level
of growth factors, which can efficiently promote chondrocyte
proliferation. The chondrocyte-scaffold composites formed
cartilage-like tissue of homogeneous structure, showing strong
mechanical properties and high cartilage matrix content. Overall,
these results suggest that the APG scaffolds may constitute a
suitable biodegradable scaffolds for cartilage tissue
engineering.
Acknowledgements
This study was supported by a research grant from
the National Natural Science Foundation of China (no.
81272108).
References
|
1
|
Cao Y, Vacanti JP, Paige KT, Upton J and
Vacanti CA: Transplantation of chondrocytes utilizing a
polymer-cell construct to produce tissue-engineered cartilage in
the shape of a human ear. Plast Reconstr Surg. 100:294–303.
1997.PubMed/NCBI
|
|
2
|
Luo X, Liu Y, Zhang Z, et al: Long-term
functional reconstruction of segmental tracheal defect by pedicled
tissue-engineered trachea in rabbits. Biomaterials. 34:3336–3344.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu J, Xue K, Li H, Sun J and Liu K:
Improvement of PHBV scaffolds with bioglass for cartilage tissue
engineering. PloS One. 8:e715632013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue K, Zhu Y, Zhang Y, Chiang C, Zhou G
and Liu K: Xenogeneic chondrocytes promote stable subcutaneous
chondrogenesis of bone marrow-derived stromal cells. Int J Mol Med.
29:146–152. 2012.PubMed/NCBI
|
|
5
|
El Backly RM, Zaky SH, Canciani B, et al:
Platelet rich plasma enhances osteoconductive properties of a
hydroxyapatite-β-tricalcium phosphate scaffold (Skelite™) for late
healing of critical size rabbit calvarial defects. J
Craniomaxillofac Surg. Aug 7–2013.(Epub ahead of print).
|
|
6
|
Jiang ZQ, Liu HY, Zhang LP, Wu ZQ and
Shang DZ: Repair of calvarial defects in rabbits with platelet-rich
plasma as the scaffold for carrying bone marrow stromal cells. Oral
Surg Oral Med Oral Pathol Oral Radiol. 113:327–333. 2012.
View Article : Google Scholar
|
|
7
|
Kanthan SR, Kavitha G, Addi S, Choon DS
and Kamarul T: Platelet-rich plasma (PRP) enhances bone healing in
non-united critical-sized defects: a preliminary study involving
rabbit models. Injury. 42:782–789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kilroy GE, Foster SJ, Wu X, et al:
Cytokine profile of human adipose-derived stem cells: expression of
angiogenic, hematopoietic, and pro-inflammatory factors. J Cell
Physiol. 212:702–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Björnsson S: Simultaneous preparation and
quantitation of proteoglycans by precipitation with alcian blue.
Anal Biochem. 210:282–291. 1993.PubMed/NCBI
|
|
11
|
Zhou G1, Liu W, Cui L, Wang X, Liu T and
Cao Y: Repair of porcine articular osteochondral defects in
non-weightbearing areas with autologous bone marrow stromal cells.
Tissue Eng. 12:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reddy GK and Enwemeka CS: A simplified
method for the analysis of hydroxyproline in biological tissues.
Clin Biochem. 29:225–229. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuhne M, John T, El-Sayed K, et al:
Characterization of auricular chondrocytes and auricular/articular
chondrocyte co-cultures in terms of an application in articular
cartilage repair. Int J Mol Med. 25:701–708. 2010.
|
|
14
|
Moon MH, Jeong JK, Lee YJ, Seol JW and
Park SY: Sphingosine-1-phosphate inhibits interleukin-1β-induced
inflammation in human articular chondrocytes. Int J Mol Med.
30:1451–1458. 2012.
|
|
15
|
Schubert T, Anders S, Neumann E, et al:
Long-term effects of chondrospheres on cartilage lesions in an
autologous chondrocyte implantation model as investigated in the
SCID mouse model. Int J Mol Med. 23:455–460. 2009.
|
|
16
|
Sharma B, Fermanian S, Gibson M, et al:
Human cartilage repair with a photoreactive adhesive-hydrogel
composite. Sci Transl Med. 5:167ra1662013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vinatier C, Bouffi C, Merceron C, et al:
Cartilage tissue engineering: towards a biomaterial-assisted
mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 4:318–329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akeda K, An HS, Okuma M, et al:
Platelet-rich plasma stimulates porcine articular chondrocyte
proliferation and matrix biosynthesis. Osteoarthritis Cartilage.
14:1272–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staudenmaier R, Froelich K, Birner M, et
al: Optimization of platelet isolation and extraction of autogenous
TGF-beta in cartilage tissue engineering. Artif Cells Blood Substit
Immobil Biotechnol. 37:265–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu W, Zhang J, Dong Q, Liu Y, Mao T and
Chen F: Platelet-rich plasma - a promising cell carrier for
micro-invasive articular cartilage repair. Med Hypotheses.
72:455–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arora R, Milz S, Sprecher C, Sitte I,
Blauth M and Lutz M: Behaviour of ChronOS Inject in metaphyseal
bone defects of distal radius fractures: tissue reaction after 6–15
months. Injury. 43:1683–1688. 2012.PubMed/NCBI
|
|
22
|
Pascual G, Rodriguez M, Sotomayor S,
Perez-Kohler B and Bellon JM: Inflammatory reaction and neotissue
maturation in the early host tissue incorporation of polypropylene
prostheses. Hernia. 16:697–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torrero JI, Aroles F and Ferrer D:
Treatment of knee chondropathy with platelet rich plasma.
Preliminary results at 6 months of follow-up with only one
injection. J Biol Regul Homeost Agents. 26(2 Suppl 1): S71–S78.
2012.
|
|
24
|
Redaelli A, Romano D and Marciano A: Face
and neck revitalization with platelet-rich plasma (PRP): clinical
outcome in a series of 23 consecutively treated patients. J Drugs
Dermatol. 9:466–472. 2010.PubMed/NCBI
|
|
25
|
Borrione P, Gianfrancesco AD, Pereira MT
and Pigozzi F: Platelet-rich plasma in muscle healing. Am J Phys
Med Rehabil. 89:854–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu W, Wang J and Yin J: Platelet-rich
plasma: a promising product for treatment of peripheral nerve
regeneration after nerve injury. Int J Neurosci. 121:176–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Centrella M, McCarthy TL and Canalis E:
Effects of transforming growth factors on bone cells. Connect
Tissue Res. 20:267–275. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Centrella M, McCarthy TL and Canalis E:
Platelet-derived growth factor enhances deoxyribonucleic acid and
collagen synthesis in osteoblast-enriched cultures from fetal rat
parietal bone. Endocrinology. 125:13–19. 1989. View Article : Google Scholar
|
|
29
|
McCarthy TL, Centrella M and Canalis E:
Regulatory effects of insulin-like growth factors I and II on bone
collagen synthesis in rat calvarial cultures. Endocrinology.
124:301–309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Canalis E, McCarthy TL and Centrella M:
Effects of platelet-derived growth factor on bone formation in
vitro. J Cell Physiol. 140:530–537. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bastiaansen-Jenniskens YM, Koevoet W, de
Bart AC, et al: Contribution of collagen network features to
functional properties of engineered cartilage. Osteoarthritis
Cartilage. 16:359–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mikic B, Isenstein AL and Chhabra A:
Mechanical modulation of cartilage structure and function during
embryogenesis in the chick. Ann Biomed Eng. 32:18–25. 2004.
View Article : Google Scholar : PubMed/NCBI
|