Introduction
Traumatic optic neuropathy (TON) is a serious injury
of the optic nerve, which involves the disruption of visual
function. The injury associated with TON may be permanent or
temporary. Etiological factors associated with TON include head
trauma due to motor vehicle and bicycle accidents, falls or
assault. TON is a type of head trauma (1) and may be divided into direct and
indirect injuries. Direct optic nerve injury is less common, due to
the protection provided by the bony orbit (2). At present, the pathogenesis,
treatment and prognosis of TON remain to be elucidated. It has been
reported that injury may activate the immune system, which may
influence the prognosis of the disease (3).
Cluster of differentiation (CD) 4+ T
helper (Th) cells are divided into pro- and anti-inflammatory Th1
and Th2 cells, respectively, based on their secretory cytokines
(4). Interleukin (IL)-6 and
transforming growth factor (TGF)-β induce naïve CD4+ T
cells into Th17 cells. Th17 cells secrete IL-1 (5) and are involved in the pathology of
various autoimmune diseases, including neuromyelitis optica
(6), rheumatoid arthritis and
multiple sclerosis (7). High
concentrations of TGF-β induce the development of regulatory T
cells (Tregs) and alterations in the Th17/Treg ratio have been
reported in several autoimmune diseases. Th17 cells are involved in
immunological responses and induce inflammation (8). IL-17 primarily functions to activate
macrophages and promote the secretion of inflammatory cytokines,
including TNF-α and IL-1β (9).
In the present study, an animal model of TON was
established in rats using a fluid percussion brain injury (FPI)
device and the rate of Th17 cells in the spleen was detected. The
expression of IL-17 in the optic nerve and the levels of IL-17 in
the retina were also detected. It was hypothesized that IL-17 may
regulate inflammation in TON.
Materials and methods
Chemicals
Lymphocyte separation medium was purchased from
Haoyang Biological Manufacture Co., Ltd (Tianjin, China).
Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum were
purchased from Invitrogen Life Technologies, (Carlsbad, CA, USA).
CD4 and IL-17 monoclonal antibodies were obtained from BD
Biosciences (San Diego, CA USA).
Induction of TON and treatment
protocols
Sixty Sprague-Dawley rats (age, 4 weeks) were
purchased from the Military Medical Academy of China (Beijing,
China). Rats were randomly divided into normal, control and TON
groups. The normal and control groups consisted of 10 rats and the
experimental TON group consisted of 40 rats. The TON group was
divided into four subgroups (1, 7, 14 and 28 days) of 10 rats on
the basis of different observation times. Rats in the normal group
received no treatment and those in the control group received the
same treatment as the TON groups, but without the induction of TON.
In the rats in the experimental TON groups, the right eyes were
considered experimental eyes and the left eyes received no
treatment. The TON model was established in accordance with the
method described previously (10).
In brief, rats were anaesthetized using 10% chloral hydrate. The
conjunctivas were cut and FPI was used to induce trauma. A tube of
optic nerve was then inserted through the conjunctival incision and
placed ~2 mm into the conjunctival cleft. The hammer angle was 25°.
Subsequent to injury, the right eyes were treated with Levofloxacin
(Santen Pharmaceutical Co., Osaka, Janpan) each day.
Flash-visual evoked potential (F-VEP)
examination
F-VEP was performed on the eyes. Rats were
anesthetized using 10% chloral hydrate prior to examination. A RETI
visual electrophysiology system (Roland Consult, Brandenburg an der
Havel, Germany) with silver needle electrodes was used. The
recording and reference electrodes were inserted subcutaneously
into the rat occipital tuberosity (OZ position). The ground
electrode was inserted subcutaneously into the ear mastoid. Full
visual field white flash stimulation was applied, at a stimulation
frequency of 1.6 Hz and a band pass width of 30–100 Hz, for 250 ms
per rat. The waveform was superimposed 100 times. Each rat was
recorded three times with an interval of 10 min. The parameters
recorded were F-VEP latent period LP2 (the P2 wave response time,
ms) and amplitude N2-P2 (Δ between N2 wave trough and P2 wave peak,
μV). Three measurements were averaged for each parameter.
Flow cytometry
A splenocyte suspension was generated from rat
spleen samples and the lymphocytes were isolated using lymphocyte
separation medium. Lymphocytes were collected and washed twice with
DMEM and transferred onto 24-well plates at a concentration of
1×107 cells/ml. A total of 50 ng/ml phorbol ester and 1
μg/ml ionomycin were added to the suspension. Following 5 h of
culture at 37°C, lymphocytes were collected. Cells were incubated
using fluorescein isothiocyanate (FITC)-conjugated monoclonal
antibodies against rat CD4+ and
R-phycoerythrin-conjugated monoclonal antibodies against rat IL-17
for 30 min at room temperature. Impurities were then filtered from
the cells and the number of Th17 cells in spleen was detected using
a flow cytometer (BD Bioscieces, San Diego, CA, USA).
Immunohistochemistry
IL-17 staining was performed on rat retinas using a
monoclonal antibody against IL-17 at a dilution of 1:100 and an
Immunocruz® staining system (Santa Cruz Biotechnology,
Inc., CA, USA) according to the manufacturer’s instructions.
Western blot analysis
Optic nerve and tissue lysates were homogenized and
proteins were extracted from the optic nerve according to the
manufacturer’s instructions (Sigma-Aldrich, Munich, German). A
total of 20 μg protein was electrophoresed using 12% SDS-PAGE and
transferred to polyvinylidene fluoride (PVDF) membranes. Following
blocking with 25 ml 5% non-fat milk and washing with Tris-buffered
saline with Tween-20 solution three times, PVDF membranes were
probed overnight at 4°C with anti-IL-17 and anti-β-actin
antibodies, diluted 1:500 and 1:1,000, respectively. Subsequent to
washing, membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G secondary
antibodies diluted 1:5,000 for 1 h at room temperature. Proteins
were then detected using an enhanced chemiluminescence system
(Millipore, Billerica, MA, USA) and X-ray film (Thermo, Rockford,
IL, USA).
Statistical analysis
All statistical analyses were performed using the
SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). Baseline characteristics for all groups are presented as the
mean ± standard deviation. Differences between groups were analyzed
using Student’s paired t-test or analysis of variance, as
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
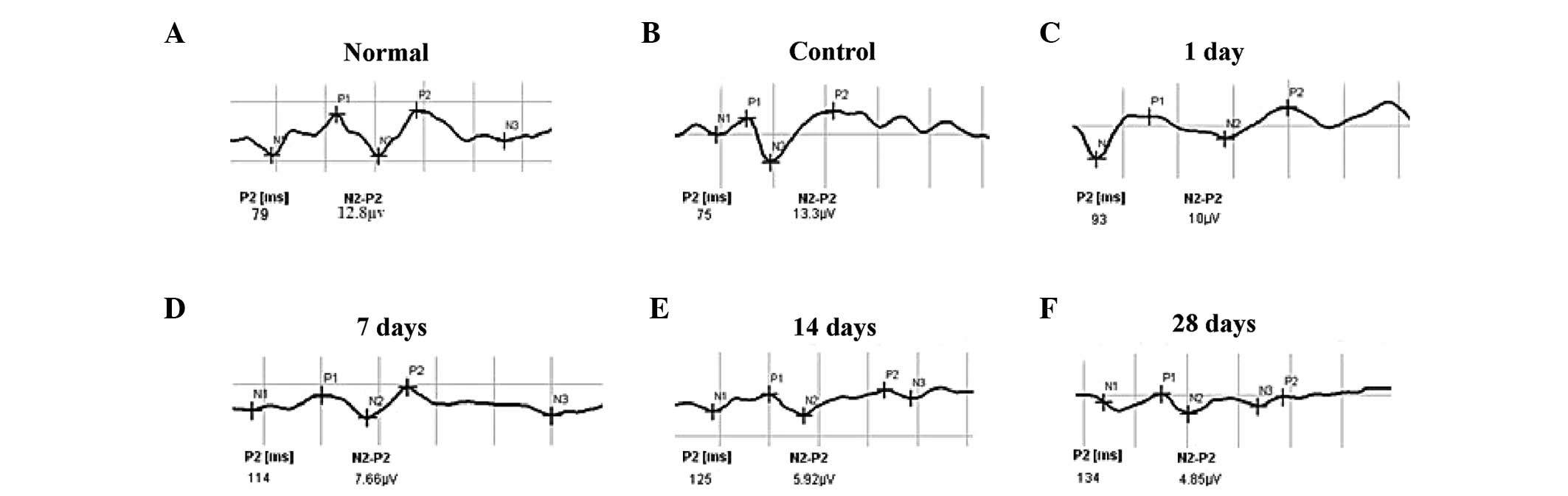
F-VEP examination of the TON model
To determine the establishment of the TON rat model,
F-VEP examinations were performed. Rats in all groups were observed
to have NPN waveforms, which the P2 and N2–P2 waveforms were
observed markedly. If the latent period of P2 was prolonged and the
amplitude of N2–P2 was reduced, these means optic nerve was
injured. Following optic nerve injury, the latent period was
markedly prolonged and the amplitude was significantly reduced
until ~14 days, following which the waveforms stablized (Fig. 1). No significant difference was
identified in the latent period between the rats in the normal and
control groups (P=0.829). However, the differences in latent period
and amplitude between the rats in the control and experimental TON
groups were found to be statistically significant (Table I). These findings suggest that the
TON model was established successfully in the rats in the
experimental groups.
 | Table IChanges in latent period and amplitude
in flash-visual evoked potential examination. |
Table I
Changes in latent period and amplitude
in flash-visual evoked potential examination.
| Group | Latent period
(ms) | P-valuea | Amplitude (μV) | P-valueb |
|---|
| Normal | 78.80±8.52 | | 12.23±1.74 | |
| Control | 79.70±9.79 | | 13.45±2.21 | |
| TON 1 day | 93.60±12.50 | <0.001 | 10.41±1.75 | <0.001 |
| TON 7 days | 112.00±9.46 | <0.001 | 7.50±1.34 | <0.001 |
| TON 14 days | 126.30±10.48 | <0.001 | 5.89±1.29 | <0.001 |
| TON 28 days | 129.30±8.23 | <0.001 | 5.61±1.09 | <0.001 |
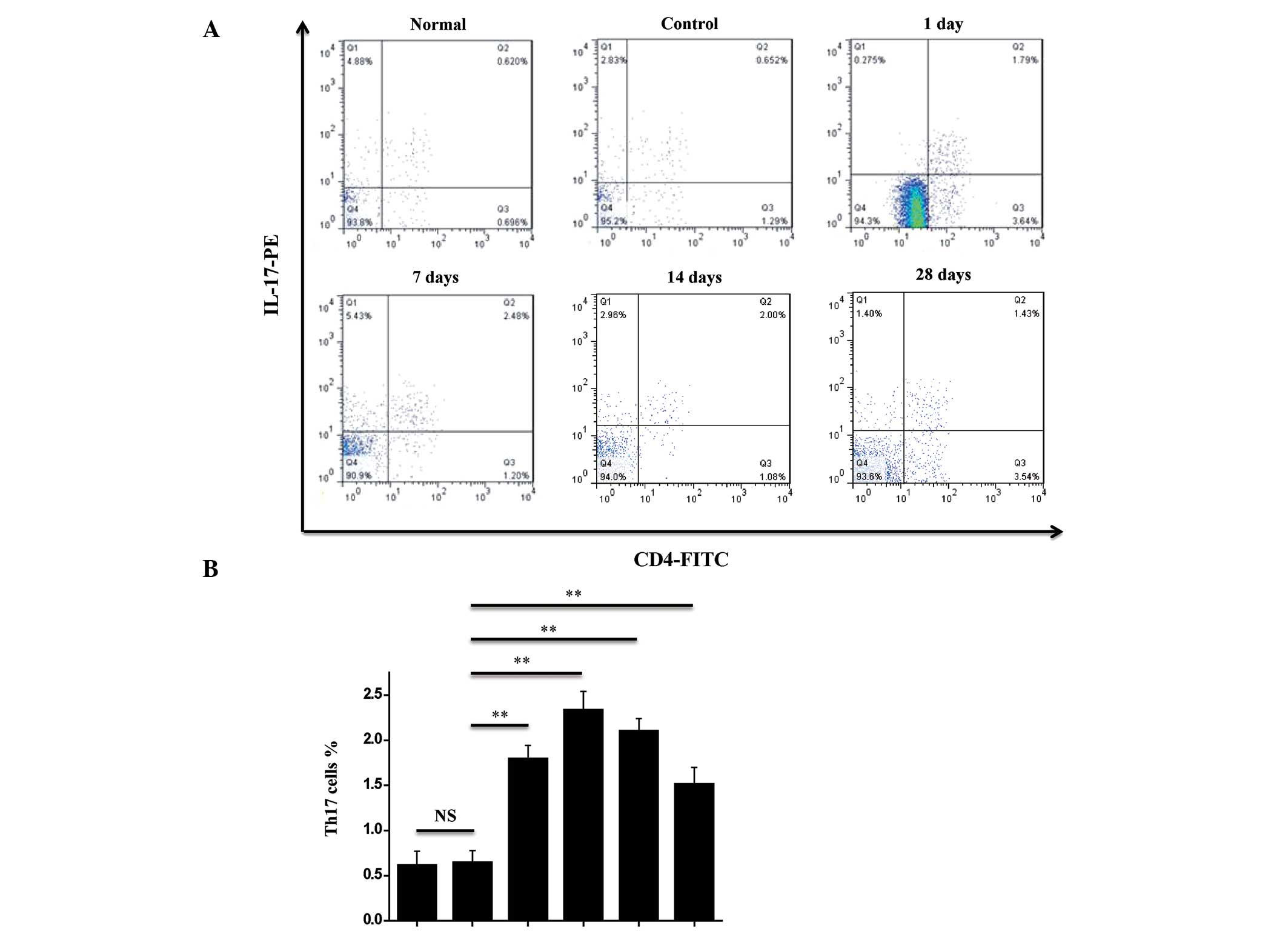
Changes in Th17 cells in the spleens of
rats with TON
In order to investigate the changes in the Th17
cells in the spleens of the rats with TON, flow cytometry was
performed. Th17 cells were identified using FITC-conjugated
monoclonal antibodies against rat CD4+ and PE-conjugated
monoclonal antibodies against rat IL-17. As shown in Fig. 2A, the ratio of Th17 cells in the
spleens of the rats in the control group was 0.94±0.13%, which
increased one day following injury and peaked seven days following
injury (Fig. 2A and B). The ratio
of Th17 cells in the spleens of the TON rats was observed to
decrease at 14 and 28 days following injury, but remained higher
than that in the rats in the control group (Fig. 2). These findings suggest that the
ratio of Th17 cells in the spleen was increased in the TON
rats.
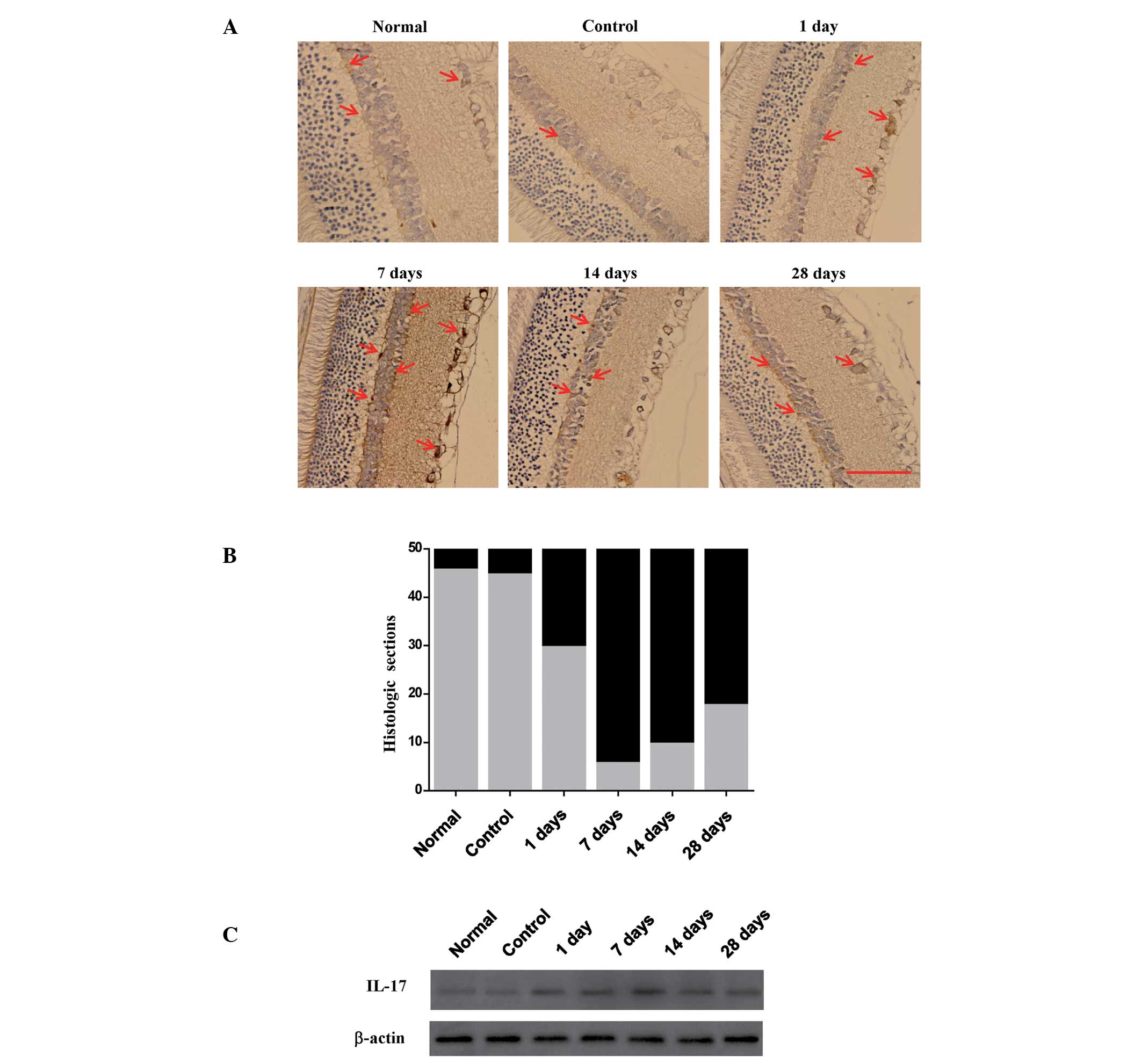
Changes in IL-17 in the retina and optic
nerve of rats with TON
Th17 cells secrete IL-17, which regulates
inflammation. Therefore, the levels of IL-17 in the retina were
analyzed. Immunohistochemistry revealed that the levels of IL-17
increased in the retina of the TON rats and peaked seven days
following the induction of injury. Results were classified as
either moderate (0% to <25%, gray area) or strong (>25%, dark
area) (Fig. 3B). Compared with the
seven day group, levels of IL-17 were observed to be decreased in
the TON rats in the 14 and 28 day groups (Fig. 3A and B), but remained higher than
that in the rats in the control group.
In order to further investigate the changes in IL-17
in the optic nerve, the expression of IL-17 was assessed using
western blot analysis. IL-17 was observed to be expressed in the
rats in all groups (Fig. 3C), but
was lower in the rats in the normal and control groups than those
in the TON groups. Following the induction of injury, the
expression of IL-17 was observed to increase initially, peak at
seven days then decrease. The differences in IL-17 expression
between the control and the TON groups was statistically
significant. These findings show that the pattern of IL-17
expression correlates with the trend in the ratio of Th17
cells.
Discussion
TON often occurs in patients with closed head
injury. Damage to the optic nerve includes immediate and secondary
damage in a proportion of retinal ganglion cells (11). It is difficult to treat the
immediate damage to the optic nerve (12) and it is important to suppress
secondary damage (13). A previous
study reported that patients with TON were treated with high doses
of intravenous steroids (14). In
the present study, the immunologic mechanism underlying TON was
investigated in order to identify a novel, efficient method for the
therapeutic treatment of TON.
In the present study, FPI was used to establish an
animal model of TON, which has advantages with regard to
experimental repeatability and stability (15). Following the generation of the
model, F-VEP revealed that the latent period was increased and the
amplitude was significantly reduced until 14 days following the
induction of injury. These findings show that the animal model was
successfully established.
The pathogenesis of TON is complex. Clinical and
experimental studies have revealed that optic nerve damage involves
numerous mechanisms, which have yet to be elucidated (12,16).
There is no proven effective treatment for TON. High dose
corticosteroids and decompressing surgery have been adopted in
clinical treatment (1,17). Therefore, it is important to
investigate the pathogenesis of TON in order to identify an
effective treatment. The immunologic response has been suggested to
be involved in the pathogenesis of TON. Kipnis et al
(18) observed certain beneficial
or destructive autoimmunity following injury. Furthermore,
immunization with myelin basic proteins emulsified in complete or
incomplete Freund’s adjuvant was found to protect optic nerve
neurons from secondary degeneration following crush injury. Fisher
et al (19) reported that
IL-6 inhibited neuronal cell survival. Whether immune-derived
factors are beneficial or harmful to nerve recovery depends on the
phenotype of the immune cells and the timing of the interaction
with the damaged neural tissue. Based on these reports, in the
present study, it was hypothesized that the ratio of Th17 cells
increases in the TON model.
In the present study, dynamic changes in the level
of IL-17 were observed in the retina and optic nerves of TON rats,
which correlated with the trend in the ratio of Th17 cells in the
spleen. The levels of Th17 cells and IL-17 initially increased upon
induction of TON, peaking at 7 days and decreasing at 14 and 28
days subsequent to the induction of injury. Th17 cells are highly
auto-pathogenic and induce tissue inflammation and autoimmune
diseases (20). Th17 cells secrete
IL-17, -22 and -21 and express the IL-23 receptor (21). IL-17 is a cytokine with numerous
pro-inflammatory functions and is likely to be involved in either
the causation or progression of inflammatory diseases and
transplant rejection in humans (22). Peng et al (23) identified that it is possible to
detect Th17 cells among activated auto-reactive and bystander T
cells and that Th17 cells may have a key role in the pathogenesis
of experimental autoimmune uveoretinitis. The results of the
present study are consistent with those of previous reports, which
suggest that Th17 cells and IL-17 may induce or promote
inflammation in TON.
In conclusion, the present study has shown the
changes in Th17 cells and IL-17 in TON rats. Furthermore, the
findings suggest that the ratio of Th17 cells in the spleen and the
expression of IL-17 in the retina and optic nerve increased in rats
with TON. These findings demonstrate that Th17 cells may
participate in and promote the progression of TON.
Acknowledgements
This study was supported by the Tianjin application
infrastructure and cutting-edge technology research program of
China (grant no. 10JCZDJC20300)
References
|
1
|
Yu Wai Man P and Griffiths PG: Surgery for
traumatic optic neuropathy. Cochrane Database Syst Rev.
6:CD0050242013.
|
|
2
|
Miliaras G, Fotakopoulos G, Asproudis I,
Voulgaris S, Zikou A and Polyzoidis K: Indirect traumatic optic
neuropathy following head injury: report of five patients and
review of the literature. J Neurol Surg A Cent Eur Neurosurg.
74:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz M: Optic nerve crush: protection
and regeneration. Brain Res Bull. 62:467–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I
Definition according to profiles of lymphokine activities and
secreted proteins 1986. J Immunol. 175:5–14. 2005.
|
|
5
|
Bettelli E, Carrier Y, Gao W, et al:
Reciprocal developmental pathways for the generation of pathogenic
effector TH17 and regulatory T cells. Nature. 441:235–238. 2006.
View Article : Google Scholar
|
|
6
|
Li Y, Wang H, Long Y, Lu Z and Hu X:
Increased memory Th17 cells in patients with neuromyelitis optica
and multiple sclerosis. J Neuroimmunol. 234:155–160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ortega C, Fernández S, Estévez OA, Aguado
R, Molina IJ and Santamaria M: IL-17 producing T Cells in celiac
disease: angels or devils? Int Rev Immunol. 32:534–543. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dragon S, Saffar AS, Shan L and Gounni AS:
IL-17 attenuates the anti-apoptotic effects of GM-CSF in human
neutrophils. Mol Immunol. 45:160–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan H, Li F and Zhang L: A new and
reliable animal model for optic nerve injury. Curr Eye Res.
37:941–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Negi A: New insights into the study of
optic nerve diseases. Nihon Ganka Gakkai Zasshi. 117:187–210.
2013.(In Japanese).
|
|
12
|
Ropposch T, Steger B, Meço C, et al: The
effect of steroids in combination with optic nerve decompression
surgery in traumatic optic neuropathy. Laryngoscope. 123:1082–1086.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi W, Wang HZ, Song WX, Yang WL, Li WY
and Wang NL: Axonal loss and blood flow disturbances in the natural
course of indirect traumatic optic neuropathy. Chin Med J (Engl).
126:1292–1297. 2013.PubMed/NCBI
|
|
14
|
Yu Wai Man P and Griffiths PG: Steroids
for traumatic optic neuropathy. Cochrane Database Syst Rev.
6:CD0060322013.
|
|
15
|
Thompson HJ, Lifshitz J, Marklund N, et
al: Lateral fluid percussion brain injury: a 15-year review and
evaluation. J Neurotrauma. 22:42–75. 2005.PubMed/NCBI
|
|
16
|
Bodanapally UK, Kathirkamanathan S,
Geraymovych E, et al: Diagnosis of traumatic optic neuropathy:
application of diffusion tensor magnetic resonance imaging. J
Neuroophthalmol. 33:128–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinsapir KD and Goldberg RA: Traumatic
optic neuropathy: an evolving understanding. Am J Ophthalmol.
151:928–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kipnis J, Mizrahi T, Yoles E, Ben-Nun A
and Schwartz M: Myelin specific Th1 cells are necessary for
post-traumatic protective autoimmunity. J Neuroimmunol. 130:78–85.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fisher J, Mizrahi T, Schori H, et al:
Increased post-traumatic survival of neurons in IL-6-knockout mice
on a background of EAE susceptibility. J Neuroimmunol. 119:1–9.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basu R, Hatton RD and Weaver CT: The Th17
family: flexibility follows function. Immunol Rev. 252:89–103.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dardalhon V, Korn T, Kuchroo VK and
Anderson AC: Role of Th1 and Th17 cells in organ-specific
autoimmunity. J Autoimmun. 31:252–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Afzali B, Lombardi G, Lechler RI and Lord
GM: The role of T helper 17 (Th17) and regulatory T cells (Treg) in
human organ transplantation and autoimmune disease. Clin Exp
Immunol. 148:32–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng Y, Han G, Shao H, Wang Y, Kaplan HJ
and Sun D: Characterization of IL-17+ interphotoreceptor
retinoid-binding protein-specific T cells in experimental
autoimmune uveitis. Invest Ophthalmol Vis Sci. 48:4153–4161.
2007.PubMed/NCBI
|