Introduction
Pancreatic adenocarcinoma (PA) is one of the leading
causes of cancer-related mortality and has considerable economic
and social impact (1). The
lethality of the disease is largely caused by difficulties in early
detection, and most PA cases remain incurable by currently
available treatment strategies. A high proportion of PA patients
show locally advanced or metastatic tumor growth. Considerable
efforts have been made during the past decade to identify improved
systemic treatment options. However, clinical trials have not
identified a survival advantage for the majority of tested
therapies (2).
Pathological diagnosis is a key factor for the
effective and timely treatment of PA. Histological diagnosis of
cystic pancreatic lesions (CPLs) is often difficult, because of the
low sensitivity of fine needle aspiration biopsy and brush
cytology. Direct intracystic biopsy and pancreatic cystoscopy are
still the standard practice (3).
However, such biopsies are invasive and therefore difficult to
obtain. By consequence, certain candidate biomarkers for PA are
emerging. For instance, the intracellular anti-apoptotic protein
BAG3 was found to be expressed in pancreatic ductal adenocarcinoma
(PDAC) samples, and not in the surrounding non-neoplastic tissue or
in healthy pancreatic tissue. Furthermore, in a cohort of patients
who underwent R0 resection, the level of BAG3 inversely correlated
to patient survival. BAG3 protein was also present in the patients’
sera, and was thus proposed as a useful biomarker for PDAC
(4). In another example, Kosanam
et al performed Orbitrap(R) mass spectrometry proteomic
analysis of four PDAC tissues and adjacent benign tissues. A
significant elevation of LAMC2 was observed in the serum of
patients with PA (5). Although
several biomarkers for PA have been proposed (5–13),
no single marker has emerged as the test of choice. Further
research and better understanding of the biology of PA, improved
diagnostic techniques, and standardized interpretation are
essential steps to develop reliable biomarkers.
Compared to other types of biomarkers, an advantage
of serum biomarkers is that they can be measured by less invasive
methods. The soluble a proliferation-inducing ligand (sAPRIL)
protein is a member of the tumor necrosis factor (TNF) family,
comprising proteins that regulate B-cell maturation, survival, and
function (14).
Epithelial-mesenchymal transition (EMT) was shown to be upregulated
by tumour necrosis factor-α (TNF-α) and to relate to the recurrence
of certain types of cancer (15,16).
Thus, sAPRIL, which is often found overexpressed in a variety of
autoimmune diseases (17) may be
associated with MET. sAPRIL has been reported to stimulate tumor
cell growth (14), but the
function of sAPRIL in PA has not been reported to date. The serum
levels of APRIL have been widely studied in numerous patients in
order to explore its clinical significance (18–23).
sAPRIL is the active form of APRIL and serum sAPRIL was previously
detected in colorectal cancer (24). Here, we aimed to explore the
potential of using the serum sAPRIL level as a biomarker for the
prediction of PA occurrence and metastasis, which would contribute
to the early diagnosis of PA following surgery.
Materials and methods
Patient samples and cell lines
All protocols were approved by the ethics committee
of The General Hospital of Shenyang Military Region (Shenyang,
China). Written informed consent was obtained from all patients.
From March 2005 to March 2008, a total of 448 patients were
diagnosed with PA, and 400 of these subsequently underwent a
standardized Kausch-Whipple resection. Blood samples were obtained
preoperatively from five-year surviving patients and were processed
according to a standardized protocol. Serum samples were
immediately frozen in aliquots at −80°C, and the excised pancreatic
tissue samples were placed in liquid nitrogen and stored at −80°C
until use. PA diagnosis was performed histologically in all
patients. None of the patients received any additional therapy. The
final stages of pancreatic cancer were examined histopathologically
according to the general rules of the TNM classification system of
malignant tumors, defined by the International Union Against Cancer
(UICC) (25). The PA cell line
PanC-1 (American Type Culture Collection, Manassas, VA, USA) was
cultured in serum-free RPMI-1640 medium (Product no. R6504,
Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere
containing 5% CO2 at 37°C.
Immunohistochemistry
Immunohistochemistry was performed according to a
previously reported method (26).
Briefly, 4-mm-thick serial sections were performed on
paraffin-embedded pancreatic tissues and were deparaffinized. These
slices were placed in 10 mM citric acid buffer (pH 6.0) with 0.2%
Tween-20 and boiled in a microwave oven (6 min) to retrieve the
antigen. The slides were then rinsed and blocked in a 10%
H2O2 solution with methanol for 10 min.
Subsequently, the slices were incubated with mouse anti-human
sAPRIL monoclonal antibody (1:500 dilution; Product no. LS-C126875,
LifeSpan Biosciences, Inc., Seattle, WA, USA) overnight at 4°C.
They were then rinsed in phosphate-buffered saline, and incubated
for 1 h with secondary antibody labeled with
streptavidin-biotin-peroxidase (DAKO LSAB™2 kit; DakoCytomation,
Glostrup, Denmark). The bound complex was visualized using as a
chromogen liquid diaminobenzidine, and counterstained with
hematoxylin and eosin stain kit (Shanghai Haoran Bio Technologies
Co., Ltd., Shanghai, China).
sAPRIL expression construct
Genomic DNA from PA tissues was extracted using the
Genomic DNA Extraction kit (Takara, Dalian, China), following the
manufacturer’s instructions. The sAPRIL gene was amplified
from genomic DNA using the following primers: sense, 5′-GTG AGC TAG
CAT GCC AGC CTC ATC TCC AGG CCA CAT G-3′, and antisense, 5′-CTG AGA
ATT CTT ATA GTT TCA CAA ACC CCA GGA ATG-3′. The sAPRIL gene was
amplified on an Applied Biosystems® Thermal Cycler 2720
(Invitrogen Life Technologies, Carlsbad, CA, USA) using the
following parameters: One cycle of an initial denaturation step at
95°C for 4 min; followed by 25 cycles consisting of 30 sec at 94°C,
30 sec at 55°C and 1 min at 72°C; a final extension step of 10 min
at 72°C, which generated a 750-bp product. The PCR product was
cloned into the NheI-EcoRI sites of the pcDNA3.1
vector (TOPO TA® Expression Kit, Applied Biosystems
China Ltd., Beijing, China), giving the plasmid named as
pcDNA3.1-sAPRIL. This plasmid was then introduced and expressed in
E. coli cells, isolated using the MiniBEST Plasmid
Purification kit ver.4.0 (Takara Biotechnology Co., Ltd., Dalian,
China) and the cloned sequence of the sAPRIL gene was
verified by sequencing.
Construct for sAPRIL gene silencing
The pTZU6+1 expression vector was a gift from the
Shandong Provincial Institute of Parasitic Diseases (Jining,
Shandong, China). The sAPRIL coding and reverse
complementary sequences were amplified from genomic DNA with the
following primers: sense, 5′-TCG ACC TGG GTG AGT ACT GCT CTC CTT
GGG GAG AGC AGT ACT CAC CCA GTT TTT T-3′, and antisense, 5′-CTA GAA
AAA ACT GGG TGA GTA CTG CTC TCC CCA AGG AGA GCA GTA CTC ACC CAG-3′.
PCR was performed as described above. SalI and XbaI
restriction sites were incorporated on either end of the oligos for
cloning into the pTZU 6+1 vector, providing the
pTZU6+1-shRNA-sAPRIL plasmid.
Transfection of the PanC-1 PA cell
line
PanC-1 cells (2×105/plate) were
transfected with the pcDNA3.1-sAPRIL and the pTZU6+1-sAPRIL
vectors. Non-transfected cells served as the control group.
Transfection was performed in 50% confluent cells using 9 μl of
Lipofectamine 2000™ (Applied Biosystems Trading Co. Ltd., Shanghai,
China). Following a 40-h transfection, neomycin-resistant clones
were selected following addition of 500 μg/ml G418 (product no.
1453C082; Beijing Jingke Hongda Biotechnology Co., Ltd., Beijing,
China) in the medium. Resistant colonies were either pooled or
cloned.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from pancreatic tissues with
the RNeasy Mini Kit (Qiagen, Shanghai, China) according to the
manufacturer’s instructions. cDNA was synthesized from 1 mg of
total RNA with the T-Primed First-Strand kit for RT-PCR (GE
Healthcare Life Sciences, Shanghai, China). The level of
sAPRIL mRNA was evaluated by qPCR. The primer sequences for
gene amplification were the following: sAPRIL forward, 5′-AGT CCT
GCA TCT TGT TCC AG-3′, and reverse, 5′-AGA GAA ACT CTA TTC CGA
TG-3′; GAPDH forward, 5′-CCC TTC ATT GAC CTC AAC TAC-3,′ and
reverse, 5′-CCA CCT TCT TGA TGT CAT CAT-3′. GAPDH was used for
normalization. The qPCR was performed applying an SYBR Green I
reaction mix on a Real-Time Thermal Cycler (qTOWER 2.2; Analytik
Jena AG, Jena, Germany). Data analyses were performed based on the
standard curve and ΔΔCT method.
Estimation of growth rate of PanC-1
cells
A clean hemocytometer and cover slip were prepared.
In order to count accurately, the single cells were obtained by
pipetting up and down in a tube. Subsequently, 50 μl of the cell
suspension was placed over the counting chambers and the entire
hemocytometer was filled. The cells viewed under a microscope
(magnification, ×100). Cells were counted on both sides of the
chambers. Each area of the grid was 0.0001 ml and all cells were
counted within four areas. The growth rate of transfected and
non-transfected PanC-1 cells was estimated at each 24-h interval
according to the cell concentration.
Western blot analysis
The extracellular proteins were isolated from cell
culture supernatants of PanC-1 cells. Firstly, the culture solution
was centrifuged at 900 × g for 5 min to collect the supernatants
and cells separately. Secondly, the collected supernatants were
further centrifuged at 10,000 × g for 1 h to remove the remaining
debris. The supernatants were filtered through 0.2 μm pore filters.
Finally, the supernatants were concentrated between 1 ml and 100 μl
using 2-ml Amicon Ultra centrifugal filtration units (UFC201024,
10,000 NMWL, EMD Millipore Headquarters, Billerica, MA, USA). The
intracellular proteins were isolated from cells by subjecting to
three freeze-thaw cycles, each consisting of 5 min of freezing at
−80°C and 10 min of thawing at 37°C in PBS buffer. The extracted
proteins from frozen tissue samples and cultured cells were
subjected to western blot analysis using the mouse anti-human
monoclonal antibody targeting sAPRIL (Shanghai Institute of
Biochemistry and Cell Biology, CAS, Shanghai, China). The antibody
targeting β-actin was purchased from Abcam (Beijing, China) and
used as the loading control. The non-transfected and transfected
cell lines and pancreatic tissues were homogenized in RIPA buffer
(150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, 0.1%
SDS, 50 mM Tris-HCl, pH 8.0) supplemented with the serine protease
inhibitor phenylmethanesulfonyl fluoride (Yaxin Biotechnology Co.,
Ltd., Shanghai, China). Total protein was extracted from the cell
lysate using cell lysis buffer (50 mM HEPES pH 7.0, 150 mM NaCl, 1
mM EDTA, 1 mM EDTA, 1 mM DTT and 0.1% Triton X-100). The protein
concentration was determined using a BCA assay kit (Thermo
Scientific, Rockford, IL, USA) according to the manufacturer’s
instructions. The proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skim milk in TBST (10 mM
Tris, pH 7.5, 100 mM NaCl and 0.1% Tween-20) and incubated with the
anti-sAPRIL antibody overnight at 4°C. The membrane was then
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody (1:3,000, GE Healthcare, Piscataway, NJ, USA).
Immunoreactive bands were visualized using an enchanced
chemiluminescence kit (GE Healthcare, Shanghai, China) and were
quantified by densitometry with the Image J software 1.45 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were carried out using the
appropriate tests. The long-term disease outcome and the prognostic
sAPRIL factor were analyzed by multivariate and univariate analyses
using SPSS software package (SPSS 13.0; SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Features of the five-year PA survivor
patients
Among 400 patients with PA, the five-year
postoperative mortality was 70% and the morbidity was 20%. A good
outcome, without recurrence or metastasis, was observed in only 10%
of the subjects. According to the TNM classification system
(25), six different tissue
samples were obtained from the five-year survivors and marked as
normal (healthy) pancreatic tissues, TNM stage I, IIA, IIB, III and
IV. The protein levels of sAPRIL gradually and significantly
(P<0.01) increased from normal pancreatic tissues to PA tissues
at TNM stage IV (Table I).
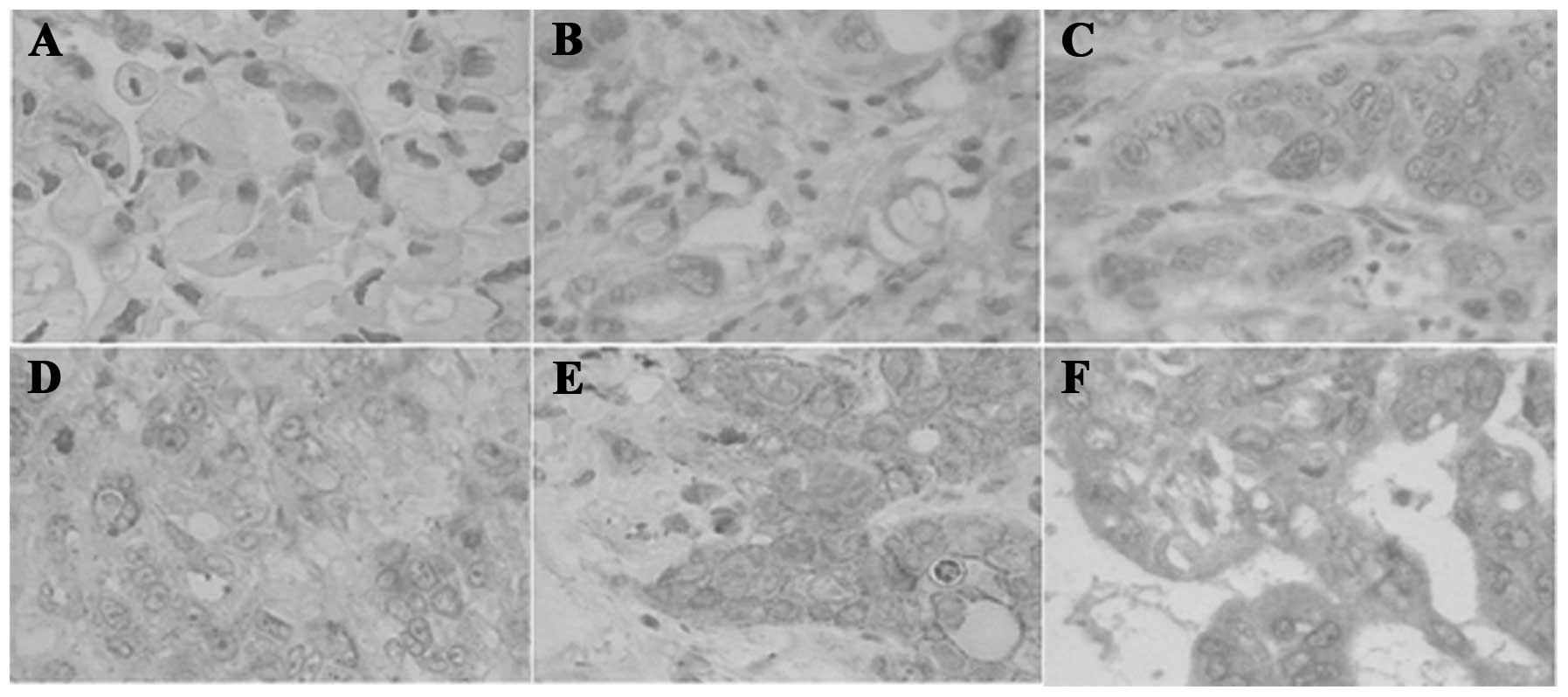
Immunohistochemical analysis confirmed that the protein level of
sAPRIL increased from the normal pancreatic (Fig. 1A) to the PA tissues (Fig. 1B–F). sAPRIL was highly expressed at
the advanced stages of PA (Fig. 1E and
F). The factor analysis indicated that the serum sAPRIL level
is a suitable prognostic marker for the development of PA.
According to the univariate analysis results, tumor size (>3 vs.
<3 cm), the presence of lymph node metastasis, the TNM
classification stage (I, IIA, IIB, III and IV) and the type of
tissues significantly correlated to the level of sAPRIL (Table I).
 | Table IPrognostic factors of the five-year
survivors of pancreatic adenocarcinoma (PA) following surgery. |
Table I
Prognostic factors of the five-year
survivors of pancreatic adenocarcinoma (PA) following surgery.
| | sAPRIL level | |
|---|
| |
| |
|---|
| Prognostic
factors | Number | Light density | Protein
concentration (ng/ml) | P-value |
|---|
| Age |
| <60 | 45 | 0.0024±0.0022 | 13.1±10.2 | 0.64/0.46 |
| ≥60 | 35 | 0.0026±0.0033 | 14.2±11.9 | |
| Gender |
| Male | 50 | 0.0027±0.0032 | 13.8±5.0.. | 0.88/0.57 |
| Female | 30 | 0.0023±0.0029 | 14.0±4.5.. | |
| Tumor size |
| <3 cm | 47 | 0.0021±0.0013 | 6.1±1.8 | 0.57/0.82 |
| ≥3 cm | 33 | 0.0039±0.0033 | 28.4±6.7.. | |
| TNM stage |
| I + IIA | 41 | 0.0005±0.0002 | 5.7±1.5 | 0.001/0.006 |
| IIB + III +
IV | 39 | 0.0043±0.0034 | 30±7.1 | |
| Lymphoid knot
transfer |
| Yes | 57 | 0.0045±0.0032 | 31±8.3 | 0.001/0.006 |
| No | 23 | 0.0005±0.0002 | 5.9±1.7 | |
| Number of lymphoid
knot transfer |
| <3 | 31 | 0.0036±0.0025 | 8±7.7 | 0.005/0.007 |
| ≥3 | 26 | 0.0112±0.0060 | 25±10.4 | |
| Type of
tissues |
| Tumor | 80 | 0.0028±0.0021 | 14.8±12.1 | 0.001/0.004 |
| Healthy | 80 | 0.0009±0.0010 | 4.3±3.2 | |
The serum sAPRIL level is associated with
the development of PA
Pancreatic biopsies of five-year survivors were
collected by cystoscopy and stored at −80°C. A total of 160 tissue
samples (n=80 for normal pancreatic tissues, n=18 for TNM stage I,
n=10 for TNM stage IIA, n=8 for TNM stage IIB, n=20 for TNM stage
III, and n=24 for TNM stage IV) were examined by a pathologist
experienced with PA at the Departments of Hepatobiliary and
Pancreas Surgery and Pathology, The General Hospital of Shenyang
Military Region (Shenyang, China). To examine the clinical
relevance of the sAPRIL level, we investigated whether the sAPRIL
mRNA and protein levels are associated with the PA stage of the
patients. From TNM stage I to IV, the mRNA levels of sAPRIL
gradually increased in the PA tissues (Fig. 2A). The mRNA level of sAPRIL
remained low in the adjacent healthy pancreatic tissues (Fig. 2A). Similar to the mRNA, the protein
levels of sAPRIL increased with the development of PA (Fig. 2B). The sAPRIL protein was not
detected in the adjacent healthy pancreatic tissues (Fig. 2B). To explore a non-invasive method
for the diagnosis of PA, we also examined the serum sAPRIL levels
at different stages of the disease. As expected, the serum sAPRIL
levels gradually increased from stages I to IV (Fig. 2C).
The growth rate of PA cells is increased
upon transfection with the sAPRIL expression vector
We enquired whether sAPRIL is expressed at the mRNA
and protein level in PA cell lines transfected with the
sAPRIL expression vector using RT-qPCR (Fig. 3A) and western blot analysis
(Fig. 3B). Furthermore, we
investigated the possibility that sAPRIL is secreted from PA cells.
Western blot analysis of the secreted protein fraction showed that
the sAPRIL protein is present in the medium and is increased when
the PA cells were transfected with the sAPRIL expression
vector (Fig. 3C). These results
indicated that the PA PanC-1 cells may secrete sAPRIL. Compared to
the PA non-transfected PanC-1 cells, the growth rate of the
transfected cells increased up to 27% (P<0.01) after a three-day
culture (Fig. 3D).
The growth rate of PA cells is decreased
when cells are transfected with sAPRIL shRNA
The enhanced growth rate of the cells with an
increased serum level of sAPRIL suggests that this protein may be
involved in cancer progression. We therefore examined whether gene
silencing of sAPRIL affects the growth rate of PA PanC-1
cells. RT-qPCR and western blot analysis showed that sAPRIL is
present in the PanC-1 cells that were not transfected and absent in
the cell lines transfected with the sAPRIL shRNA (Fig. 4A and B). Similarly, the protein
sAPRIL was present in the medium of the cultured non-transfected
PanC-1 cells and absent in the medium of the cells transfected with
sAPRIL shRNA (Fig. 4C).
Compared to the non-transfected cells, the growth rate of the cells
decreased up to 19% (P<0.01) when they were transfected with the
sAPRIL shRNA after a three-day culture (Fig. 4D).
Discussion
Detection of PA, particularly at early stages,
remains a great challenge. Recently, serum biomarkers have drawn
increasing attention in the search for less invasive tools for the
diagnosis of numerous types of cancer (27–32).
Serum biomarkers for the diagnosis of PA have been also widely
reported (33–37). For instance, CYFRA 21-1 levels were
found to be significantly associated with the PA stage and may
serve as a valuable tool for the treatment and prognosis of
advanced PA (37). The aberrant
expression of microRNAs (miRNAs) in various tissues is also related
to various types of cancer. miRNAs are present in the serum and the
plasma of humans. Furthermore, the levels of miRNAs in the serum
are stable, reproducible, and consistent in the same individuals
(38). Thus, serum miRNA may also
be suitable as a biomarker for the diagnosis of cancer. In a recent
study, seven miRNAs showed different levels of expression in PA
compared to those observed in the control group. The 7 miRNA-based
biomarker assay showed high sensitivity to distinguish the
different stages of PA, and was thus proposed as a novel
non-invasive diagnostic tool (33). Although numerous serum biomarkers
have been reported, none has been applied in the clinic to date.
Thus, it is still necessary to find novel biomarkers for the early
diagnosis of PA.
Surgery is the primary option for the treatment of
cancer, but the survival rate is still low due to recurrence and
metastasis (39). Thus, early
diagnosis is of high importance. EMT plays a critical role in cell
invasion, migration and metastasis (40), which is closely related to the
recurrence and metastasis of cancer after surgery. sAPRIL is a
member of the TNF family (41,42)
and may be the inducer of MET (43–46).
This prompted us to explore the functions of sAPRIL in PA in this
study. sAPRIL was found to be highly expressed in PA tissues, but
expressed at low levels in healthy pancreatic tissues adjacent to
the cancerous cells. These findings were supported by the mRNA and
protein level detection of sARPIL by RT-qPCR and western blot
analysis, respectively. We also found that the serum sAPRIL level
is higher in PA patients compared to the controls.
The high growth rate of cells transfected with the
sARPIL expression vector, along with the high expression of sAPRIL
in PA cells, suggest that sARPIL promotes the development of PA
(Fig. 3D). To confirm this
finding, sAPRIL shRNA was used. The results showed that the
inhibition of sAPRIL expression inhibits growth of the PA
cell line PanC-1 (Fig. 4D).
Therefore, sAPRIL can be considered as a novel enhancer of PA
progression. The elevated level of serum sAPRIL offers a
convenient, less invasive tool for the early diagnosis of PA. To
further understand the molecular mechanism underlying the increased
serum expression of sAPRIL, the relationship between sAPRIL and EMT
will be investigated in future work.
In conclusion, we demonstrated that serum levels of
sAPRIL, which appeared to be secreted by PA cells, show good
predictive value for the prognosis of PA. Further research to
explore whether sAPRIL promotes the EMT in PA cells after long-term
surgery is however urgently needed. Overall, sAPRIL is a promising
target to develop for therapeutic treatment of PA.
References
|
1
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of apoptosis by
capsaicin in pancreatic cancer cells is mediated through ROS
generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aparicio JR, Martínez J, Niveiro M, et al:
Direct intracystic biopsy and pancreatic cystoscopy through a
19-gauge needle EUS (with videos). Gastrointest Endosc.
72:1285–1288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Falco A, Rosati A, Festa M, et al: BAG3 is
a novel serum biomarker for pancreatic adenocarcinomas. Am J
Gastroenterol. 108:1178–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosanam H, Prassas I, Chrystoja CC, et al:
Laminin, gamma 2 (LAMC2): a promising new putative pancreatic
cancer biomarker identified by proteomic analysis of pancreatic
adenocarcinoma tissues. Mol Cell Proteomics. 12:2820–2832. 2013.
View Article : Google Scholar
|
|
6
|
Wang Y, Kuramitsu Y, Ueno T, et al:
Proteomic differential display identifies upregulated vinculin as a
possible biomarker of pancreatic cancer. Oncol Rep. 28:1845–1850.
2012.PubMed/NCBI
|
|
7
|
Wang T, Wentz SC, Ausborn NL, et al:
Pattern of breast cancer susceptibility gene 1 expression is a
potential prognostic biomarker in resectable pancreatic ductal
adenocarcinoma. Pancreas. 42:977–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tempero MA, Klimstra D, Berlin J, et al:
Changing the way we do business: recommendations to accelerate
biomarker development in pancreatic cancer. Clin Cancer Res.
19:538–540. 2013. View Article : Google Scholar
|
|
9
|
Ray P, Sullenger BA and White RR: Further
characterization of the target of a potential aptamer biomarker for
pancreatic cancer: cyclophilin B and its posttranslational
modifications. Nucleic Acid Ther. 23:435–442. 2013. View Article : Google Scholar
|
|
10
|
Ray P, Rialon-Guevara KL, Veras E,
Sullenger BA and White RR: Comparing human pancreatic cell
secretomes by in vitro aptamer selection identifies cyclophilin B
as a candidate pancreatic cancer biomarker. J Clin Invest.
122:1734–1741. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ono M, Kamita M, Murakoshi Y, et al:
Biomarker discovery of pancreatic and gastrointestinal cancer by
2DICAL: 2-dimensional image-converted analysis of liquid
chromatography and mass spectrometry. Int J Proteomics.
2012:8974122012.PubMed/NCBI
|
|
12
|
Lyn-Cook BD, Yan-Sanders Y, Moore S,
Taylor S, Word B and Hammons GJ: Increased levels of NAD(P)H:
quinone oxidoreductase 1 (NQO1) in pancreatic tissues from smokers
and pancreatic adenocarcinomas: A potential biomarker of early
damage in the pancreas. Cell Biol Toxicol. 22:73–80. 2006.
View Article : Google Scholar
|
|
13
|
Lau C, Kim Y, Chia D, et al: Role of
pancreatic cancer-derived exosomes in salivary biomarker
development. J Biol Chem. 288:26888–26897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahne M, Kataoka T, Schröter M, et al:
APRIL, a new ligand of the tumor necrosis factor family, stimulates
tumor cell growth. J Exp Med. 188:1185–1190. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu
YH, Sung JS, Cha TL and Sun GH: Tumor-derived tumor necrosis
factor-alpha promotes progression and epithelial-mesenchymal
transition in renal cell carcinoma cells. Cancer Sci. 99:905–913.
2008. View Article : Google Scholar
|
|
16
|
Takahashi E, Nagano O, Ishimoto T, Yae T,
Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S and Koga H: Tumor
necrosis factor-α regulates transforming growth factor-β-dependent
epithelial-mesenchymal transition by promoting
hyaluronan-CD44-moesin interaction. J Biol Chem. 285:4060–4073.
2010.
|
|
17
|
Dillon SR, Harder B, Lewis KB, et al:
B-lymphocyte stimulator/a proliferation-inducing ligand
heterotrimers are elevated in the sera of patients with autoimmune
disease and are neutralized by atacicept and B-cell maturation
antigen-immunoglobulin. Arthritis Res Ther. 12:R482010. View Article : Google Scholar
|
|
18
|
Xin G, Cui Z, Su Y, Xu LX, Zhao MH and Li
KS: Serum BAFF and APRIL might be associated with disease activity
and kidney damage in patients with anti-glomerular basement
membrane disease. Nephrology (Carlton). 18:209–214. 2013.
View Article : Google Scholar
|
|
19
|
Pollard RP, Abdulahad WH, Vissink A, et
al: Serum levels of BAFF, but not APRIL, are increased after
rituximab treatment in patients with primary Sjogren’s syndrome:
data from a placebo-controlled clinical trial. Ann Rheum Dis.
72:146–148. 2013.
|
|
20
|
Gheita TA, Raafat H, Khalil H and Hussein
H: Serum level of APRIL/BLyS in Behcet’s disease patients: clinical
significance in uveitis and disease activity. Mod Rheumatol.
23:542–546. 2013.PubMed/NCBI
|
|
21
|
Kiyama K, Kawabata D, Hosono Y, et al:
Serum BAFF and APRIL levels in patients with IgG4-related disease
and their clinical significance. Arthritis Res Ther. 14:R862012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Chen L, Ding W, et al: Serum
APRIL, a potential tumor marker in pancreatic cancer. Clin Chem Lab
Med. 49:1715–1719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tecchio C, Nichele I, Mosna F, et al: A
proliferation-inducing ligand (APRIL) serum levels predict time to
first treatment in patients affected by B-cell chronic lymphocytic
leukemia. Eur J Haematol. 87:228–234. 2011. View Article : Google Scholar
|
|
24
|
Ding W, Wang J, Wang F, et al: Serum
sAPRIL: a potential tumor-associated biomarker to colorectal
cancer. Clin Biochem. 46:1590–1594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 28. 7th edition.
Wiley-Blackwell; West Sussex, UK: pp. 231–234. 2009
|
|
26
|
Takano S, Yoshitomi H, Togawa A, et al:
Apolipoprotein C-1 maintains cell survival by preventing from
apoptosis in pancreatic cancer cells. Oncogene. 27:2810–2822. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blumenstein B, Saad F, Hotte S, et al:
Reduction in serum clusterin is a potential therapeutic biomarker
in patients with castration-resistant prostate cancer treated with
custirsen. Cancer Med. 2:468–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Du X, Xue C, et al: Quantification
of serum SOX2 DNA with FQ-PCR potentially provides a diagnostic
biomarker for lung cancer. Med Oncol. 30:7372013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harima Y, Ikeda K, Utsunomiya K, et al:
Apolipoprotein C-II is a potential serum biomarker as a prognostic
factor of locally advanced cervical cancer after chemoradiation
therapy. Int J Radiat Oncol Biol Phys. 87:1155–1161. 2013.
View Article : Google Scholar
|
|
30
|
Toiyama Y, Hur K, Tanaka K, et al: Serum
miR-200c is a novel prognostic and metastasis-predictive biomarker
in patients with colorectal cancer. Ann Surg. 259:735–743. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wang X, Lin S, et al:
Identification of kininogen-1 as a serum biomarker for the early
detection of advanced colorectal adenoma and colorectal cancer.
PLoS One. 8:e705192013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biskup K, Braicu EI, Sehouli J, et al:
Serum glycome profiling: a biomarker for diagnosis of ovarian
cancer. J Proteome Res. 12:4056–4063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu R, Chen X, Du Y, et al: Serum microRNA
expression profile as a biomarker in the diagnosis and prognosis of
pancreatic cancer. Clin Chem. 58:610–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eguchi H, Ishikawa O, Ohigashi H, et al:
Serum REG4 level is a predictive biomarker for the response to
preoperative chemoradiotherapy in patients with pancreatic cancer.
Pancreas. 38:791–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dutta SK, Girotra M, Singla M, et al:
Serum HSP70: a novel biomarker for early detection of pancreatic
cancer. Pancreas. 41:530–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brand RE, Nolen BM, Zeh HJ, et al: Serum
biomarker panels for the detection of pancreatic cancer. Clin
Cancer Res. 17:805–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boeck S, Wittwer C, Heinemann V, et al:
Cytokeratin 19-fragments (CYFRA 21-1) as a novel serum biomarker
for response and survival in patients with advanced pancreatic
cancer. Br J Cancer. 108:1684–1694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Ba Y, Ma L, et al:
Characterization of microRNAs in serum: a novel class of biomarkers
for diagnosis of cancer and other diseases. Cell Res. 18:997–1006.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sugiura T, Uesaka K, Mihara K, et al:
Margin status, recurrence pattern, and prognosis after resection of
pancreatic cancer. Surgery. 154:1078–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kitamura K, Seike M, Okano T, et al:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther.
13:444–453. 2014.PubMed/NCBI
|
|
41
|
Mackay F and Ambrose C: The TNF family
members BAFF and APRIL: the growing complexity. Cytokine Growth
Factor Rev. 14:311–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lascano V, Zabalegui LF, Cameron K, et al:
The TNF family member APRIL promotes colorectal tumorigenesis. Cell
Death Differ. 19:1826–1835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Watanabe T, Takahashi A, Suzuki K, et al:
Epithelial-mesenchymal transition in human gastric cancer cell
lines induced by TNF-α-inducing protein of Helicobacter
pylori. Int J Cancer. 134:2373–2382. 2014.
|
|
44
|
Wang H, Fang R, Wang XF, et al:
Stabilization of Snail through AKT/GSK-3β signaling pathway is
required for TNF-α-induced epithelial-mesenchymal transition in
prostate cancer PC3 cells. Eur J Pharmacol. 714:48–55.
2013.PubMed/NCBI
|
|
45
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transition (EMT) induced by TNF-α requires
AKT/GSK-3β-mediated stabilization of snail in colorectal cancer.
PLoS One. 8:e566642013.
|
|
46
|
Techasen A, Namwat N, Loilome W, et al:
Tumor necrosis factor-α (TNF-α) stimulates the
epithelial-mesenchymal transition regulator Snail in
cholangiocarcinoma. Med Oncol. 29:3083–3091. 2012.
|