Introduction
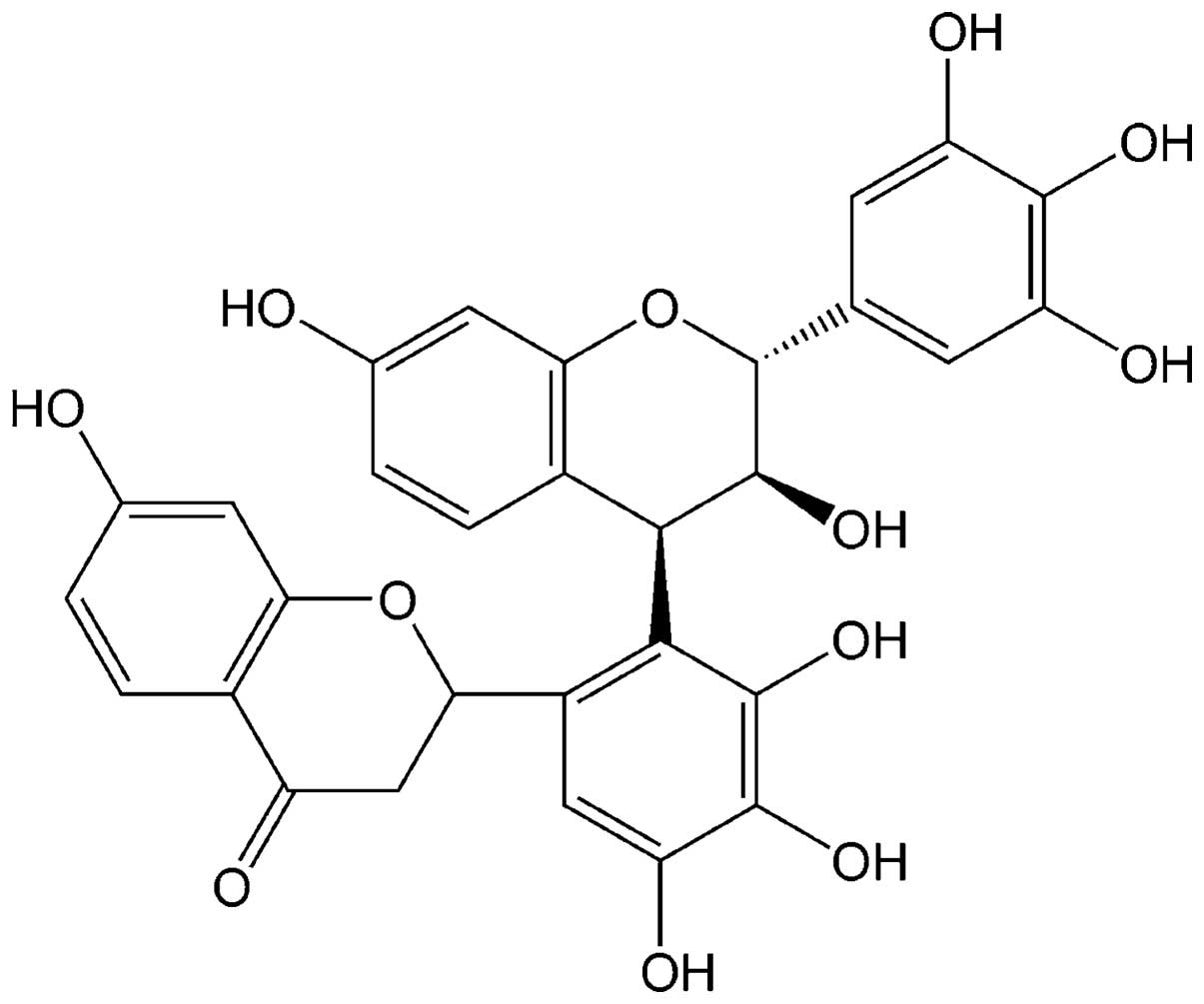
Robinetinidol-(4β,2′)-tetrahydroxy-flavone (RBF,
Fig. 1), is found in the bark of
the leguminous tree Acacia mearnsiid De Wild and has been
shown to exhibit anti-obesity properties (1). The mechanism by which RBF affects
obesity may be attributable to its effect on cholesterol synthesis
(2), since cholesterol is crucial
to the development of atherosclerosis (3). RBF may therefore prove useful in the
treatment of atherosclerosis.
A number of studies have demonstrated that
flavonoids, together with other polyphenols, such as luteolin and
quercetin, decreased cholesterol synthesis by direct inhibition of
squalene monooxygenase, a rate-limiting enzyme involved in
cholesterol synthesis (4–8). These results have suggested that
polyphenols may inhibit 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG-CoA) reductase, an important enzyme in the synthesis of
cholesterol (4,9–11).
However, RBF is an oligomeric condensed polyphenol, which may exert
additive effects, compared with those of individual polyphenols, on
cholesterol synthesis. In view of its directly and indirectly
influencing cholesterol synthesis, together with its nature and
safety characteristics, RBF may be useful as a therapeutic compound
for the prevention of atherosclerosis. However, the majority of
studies to date have been conducted in vitro, which is
significantly more simplistic than the in vivo process. In
addition, the mechanisms by which RBF reduces cholesterol have not
yet been established.
The cholesterol biosynthetic pathway is highly
regulated, and is subject to transcriptional, translational and
post-translational modulation (12). HMG-CoA is a pivotal rate-limiting
enzyme within the cholesterol biosynthetic pathway (13). The activity of HMG-CoA reductase is
subject to post-translational modification through its degradation
and its inactivation by phosphorylation (12,14).
Adenosine monophosphate (AMP)-kinase is the principal kinase
involved in the phosphorylation-mediated inactivation of HMG-CoA
reductase, and is itself activated by phosphorylation (15–16).
The present study investigated whether RBF inhibits
cholesterol synthesis in hypercholesterolemic rats and MC-RH-7777
hepatoma cells, and the possible mechanisms underlying this
effect.
Materials and methods
Materials
Acacia mearnsiid De Wild bark was obtained
from Guangxi Wuming Tannin Technology Co., Ltd. (Nanning, China).
Lipoprotein-free calf serum, AICAR, 1,1-dimethylbiguanide
hydrochloride (metformin) and protease inhibitor cocktail were
obtained from Sigma (St. Louis, MO, USA). HALT phosphatase
inhibitor and a bicinchoninic acid (BCA) protein assay kit were
obtained from Peking Luqiao Bio-tech Co. (Beijing, China).
Dulbecco’s modified Eagle’s media (DMEM),
penicillin-streptomycin-glutamine, trypsin and fetal bovine serum
(FBS) were obtained from Qi’ao Bio-tech Co. (Shanghai, China).
Radiochemicals (14C-acetate at 56 mCi/mmol,
14C-mevalonate at 65 mCi/mmol and
14C-hydroxymethylglutaryl-CoA at 55 mCi/mmol) were
obtained from American Radiolabeled Chemicals (St. Louis, MO,
USA).
Extraction, isolation and identification
of RBF compound
Extracts of Acacia mearnsiid De Wild were
obtained via aqueous acetone (1:1) immersion of bark drillings (10
kg) for seven days. The acetone was evaporated and the aqueous
solution was freeze dried, yielding a yellow-brown powder (554 g)
which was partitioned into four 80-g samples between a
butan-2-ol-water-hexane (5:4:1) mixture in a 20-tube, 100
cm3 under phase, Craig countercurrent assembly (Shanghai
Chemical Machinery Plant Co., Ltd. Shanghai, China). Qualitative
paper chromatographic analysis produced five main fractions.
Subsequent column chromatography of fraction 3 (52 mg) on Sephadex
LH-20 (5×170 cm column, flow rate 20 cm3/30 min) yielded
12 fractions. Sub-fraction Fr-3–5 (1020 mg) was further purified
into five main fractions via semi-preparation chromatography. The
most active fraction, Fr-3-5-2 (Rf 0.34, 43 mg), underwent further
purification. Acacia mearnsiid De Wild bark tannins
contained 93% RBF by high performance liquid chromatography, and
contained M+, 700.2518 (theoretical molecular mass of
C39H40O12, 700.2520), in the
peracetate mass spectrum (Thermo Fisher Scientific Inc., Waltham,
MA, USA). Purified RBF was stored at 4°C.
Animals and treatment groups
Male Sprague-Dawley rats (weight, 200±25 g; Dossy
Biological Technology Co., Ltd., Chengdu, China) were housed in
groups of five under standard environmental conditions (23±10°C,
55±5% humidity and a 12 h light/dark cycle) and had free access to
water and a standard diet, which consisted of AIN-93G, (Beijing
Lianlixin Biological Technology Co., Ltd.) containing 200 g/kg
casein lactic, 3 g/kg L-cystine, 397 g/kg corn starch, 132 g/kg
maltoes dextrin, 100 g/kg sucrose, 50 g/kg cellulose, 70 g/kg
soybean oil, 35 g/kg mineral mix, 10 g/kg vitamin mix and 2.5 g/kg
choline bitartrate. All animal experimentation was performed in
strict accordance with the recommendations set out in the Guide for
the Care and Use of Laboratory Animals of the National Institutes
of Health. The protocol was approved by the Animal Care and Use
Committee of the Sichuan University (Chengdu, China; permit no.
24). All surgery was performed under sodium pentobarbital
anesthesia and all efforts were made to minimize suffering.
The forty rats were randomly assigned to four
groups: A negative control group (NC group); a positive control
group, fed a high-fat diet (HF group); a low dose RBF group (60
mg/kg/day); and a high dose RBF group (120 mg/kg/day). The rats of
the NC group were fed with a standard diet and orally treated with
0.3% Tween-80 solution (10 ml/kg). The rats of the HF and RBF
groups were fed with a high fat diet, comprising 7.5% pork oil
(Chengdu Zhiwei Food Co., Ltd., Chengdu, China), 1% cholesterol
(Sigma Chemical Co., St. Louis, MO, USA), 10% egg yolk powder
(Chengdu Aimeiai Food Co., Ltd., Chengdu, China), 0.3% chocolate
(COFCO Le Conte Food Co., Ltd., Shenzhen, China), 0.2%
propylthiouracil (Sigma Chemical Co., St. Louis, MO, USA) and 81%
standard diet, and gavaged with 0.3% Tween-80 solution (Sigma
Chemical Co., St. Louis, MO, USA) or RBF solution, respectively,
daily for six weeks.
Cell culture
McA-RH-7777 rat hepatoma cell lines, obtained from
Shanghai Maisha Bioscience Co. (Shanghai, China), were cultured in
DMEM supplemented with 100 U/ml penicillin and 100 lg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA) containing 10%
FBS. The cells were incubated at 37°C in a 5% CO2
atmosphere.
Assessment of liver index
At the end of the treatment period, rats were kept
for overnight fasting and then given the final gavage. Rats were
weighed and anesthetized with 36 mg/kg sodium pentobarbital
(Sigma-Aldrich) by intraperitoneal injection to collect blood
samples from the aorta ventralis 1.5 h after the gavage. Following
blood collection from rats sacrificed by anoxia, the livers were
removed and weighed in order to calculate the liver index (liver
weight/body weight). The plasma was separated by centrifugation at
1,000 × g for 15 min at 4°C. Plasma samples were stored at −80°C
until required for analysis.
Biochemical analysis of serum
Serum total cholesterol (TC) and triglycerides (TG)
were measured in a spectrophotometer (DU800, Beckman Coulter,
Pasadena, CA, USA) using commercial kits (Jiancheng Institute of
Biotechnology, Nanjing, China) according to the manufacturer’s
instructions (CHODPAP and GPO-PAP methods). The optical density of
the samples was measured on a spectrophotometer at a wavelength of
546 nm. Total levels TC and TG in the serum were calculated by
dividing absorption of the tested sample by that of the standard
sample and multiplying by the TG and TC content of the standard
sample. Serum low density lipoprotein cholesterol (LDL-C) levels
were evaluated according the manufacturer’s instructions
(polyethylene sulfate precipitation method) to evaluate the
difference between cholesterol in the supernatant and total
cholesterol.
Determination of cholesterol synthesis in
hepatoma cells
Following culture for 48 h in six-well plates,
hepatoma cells were treated with 14C-acetate or
14C-mevalonate for 3 h. The RBF concentration (1, 2, 3,
4 or 5 mM) was included in incubations with
14C-mevalonate in order to inhibit the activity of
HMG-CoA reductase. The medium was removed and cells were washed
three times with phosphate-buffered saline (PBS), harvested by
trypsin, resuspended in 20 mM Tris buffer (pH 7.4, containing 0.1%
Triton X-100; Sigma-Aldrich) and lysed by a sonication three times
for 5 sec (Shanghai Bilon Instruments Co., Shanghai, China) on a
medium setting. Lipids were extracted into 5 ml chloroform/methanol
(2:1). The medium was reduced to near dryness using Savant Speed
Vac SC100 apparatus (Selby Anax, Adelaide, Australia). Dried
extracts were resuspended in 50 μl chloroform/methanol and resolved
by silica thin-layer chromatography (Whatman, Plc., Little
Chalfont, UK) in petroleum ether/ethyl ether/acetic acid (60:40:1).
Radiolabeled cholesterol was identified by comparison with
standards visualized by iodine-vapor stain and quantified by
electronic autoradiography (Packard Instant Imager, PerkinElmer
Inc., Waltham, MA, USA).
Isolation and assay of HMG-CoA reductase
activity from rat livers
Microsomal fractions were prepared from livers of
the rats maintained on rat standard diet containing 5%
cholestyramine for seven days. HMG-CoA reductase was solubilized
from the microsomal fractions according to the method described by
Heller and Shrewsbury (17) and
purified through the second ammonium sulfate precipitation step
described by Kleinsek et al (18). The enzyme preparation was stored at
−80°C in 100 μl aliquots until required for analysis. Prior to use,
the enzyme was activated at 37°C for 30 min. The assay was adapted
from methods described in the study by Beg et al (19). In brief, the reaction mixture
contained 100 μl of 0.14 M potassium phosphate buffer, pH 6.8; 0.18
M KCl; 3.5 mM EDTA, pH 7.0; 10 mM dithiothreitol; 0.1 mg/ml bovine
serum albumin; 0.04 μCi of 14C-HMG-CoA; and 8 μg
partially purified enzyme (specific activity 14 nmol/min/mg) with
or without RBF. Following incubation for 5 min at 37°C, the
reaction was initiated with 0.2 mM NADPH and terminated with 20 μl
of 5 M HCl. Following an additional incubation for 30 min at 37°C
to allow for complete lactonization of the product mevalonate, the
mixture was passed through a column with an internal diameter of
0.5 cm and a length of 5 cm, which contained 100–200 mesh Bio-Rex,
chloride form (Bio-Rad, Hercules, CA, USA). Any unreacted
14C-HMG-CoA was adsorbed onto this mesh. The product of
14C-mevalonolactone was eluted into scintillation vials
with 3 ml distilled water. After the addition of 15 ml Aquasol II
(Sigma-Aldrich), the radioactivity of the samples was measured.
Immunoblot analysis of HMG-CoA reductase
and AMP-kinase phosphorylation
For western blot analysis hepatoma cells were grown
in 6-cm plates for 3–5 days with a daily medium change. Cells were
treated with RBF or an AMP-kinase activator (AICAR or metformin, 1
mM final concentration) for 3 h in fresh media. Subsequent to the
incubation period, cells were washed once with ice-cold PBS and
removed with lysis buffer composed of, 0.25 M Tris buffer (pH 7.5),
and protease and phosphatase inhibitor cocktails at standard
concentrations. Lysates were centrifuged at 1,300 × g for 10 min at
4°C and the supernatant was stored at −80°C. Protein (30 μg,
identified by BCA analysis) was fractionated by SDS-gel (8%)
electrophoresis and transferred onto to a nitrocellulose membrane
(Whatman; Sigma-Aldrich) The membrane was blocked with 0.05%
Tween-20 and 5% non-fat milk. For immunodetection, a rabbit
antibody against total AMP-kinase (polyclonal anti-AMPK α-pan,
1:2,000; Upstate Biotechnology Inc., Charlottesville, VA, USA) or
phosphorylated AMP-kinase (anti-phospho-AMPKα, 1:500; Upstate
Biotechnology Inc.) were used overnight at 4°C. Antibody binding
was detected with a horseradish peroxidase (HRP)-conjugated
polyclonal secondary goat anti-rabbit antibody (1:1,000; Chengdu
Aoxin Biotechnology Co., Ltd., Chengdu, China) for 1 h at room
temperature. The chemiluminescent image (Supersignal West Pico
Chemiluminescent Substrate, Pierce Biotechnology, Rockford, IL,
USA) was captured by autoradiography. Band densitometric analysis
was conducted using Image J 1.416a (National Institues of Health,
Bethesda, MD, USA). In order to determine the degree of HMG-CoA
reductase phosphorylation, lysates were incubated for 1 h at 4°C
with 20 μl mouse monoclonal antibody against
phosphoserine/phosphothreonine/phosphotyrosine (51AB Biotech Co.,
Shanghai, China). Antibody conjugates were precipitated with 35 μl
of protein G Plus-Agarose from Calbiochem (La Jolla, CA, USA)
following Amersham Biosciences (Chalfont St. Giles, UK)
immunoprecipitation instructions. Immunoprecipitated protein was
released by boiling in a gel loading buffer, fractionated by
SDS-gel electrophoresis and transferred to a nitrocellulose
membrane. HMG-CoA reductase was detected and quantified via rabbit
polyclonal HMG-CoA reductase antibody (Upstate Biotechnology Inc.),
followed byincubation with an HRP-conjugated secondary goat
anti-rabbit antibody, as described above.
Statistical analysis
All values are expressed as the mean ± standard
deviation. The groups were compared using unpaired Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RBF inhibits cholesterol synthesis in
vivo
Following a six week hypercholesterolemic diet, the
serum TC in rats increased by 166%, from 52.55±2.53 to 140.05±6.77
mg/dl. Treatment with 120 mg/kg RBF significantly reduced
cholesterol levels in the treated groups, with serum cholesterol
being 25.9% lower than that in the positive control groups
(Table I).
 | Table IEffect of RBF on serum TG, TC, LDL-C
and HDL-C in hypercholesterolemic rats. |
Table I
Effect of RBF on serum TG, TC, LDL-C
and HDL-C in hypercholesterolemic rats.
| Group | Dose of RBF
(mg/kg) | TG (mg/dl) | TC (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) |
|---|
| NC | 0 | 27.17±2.01 | 52.55±2.53 | 11.09±2.46 | 39.66±2.71 |
| HF | 0 | 78.82±3.72a | 140.05±6.77a | 79.93±6.59a | 51.81±3.28 |
| RBF | 60 | 65.53±2.25b | 115.16±3.55 | 69.31±5.53 | 45.1±3.13 |
| RBF | 120 | 50.71±2.9c | 103.65±4.02c | 38.72±4.11c | 47.01±2.91 |
No changes in the serum levels of LDL cholesterol
and HDL cholesterol were observed in the sham groups fed with a
standard diet and treated with RBF, compared with the negative
control group. In the treatment groups, 120 mg/kg RBF reduced mean
serum LDL-C levels by 50.8%, from 79.93±6.59 to 38.72±4.11 mg/dl,
whereas no significant changes were detected in HDL-C levels
(51.81±3.28 to 47.01±2.91 mg/dl, P>0.05). RBF treatment
therefore resulted in a decrease in the LDL/HDL ratio of 66% in
hypercholesterolemic rats (Table
I), indicating that RBF had a greater effect on LDL-C than
HDL-C levels.
RBF inhibits cholesterol synthesis in
vitro
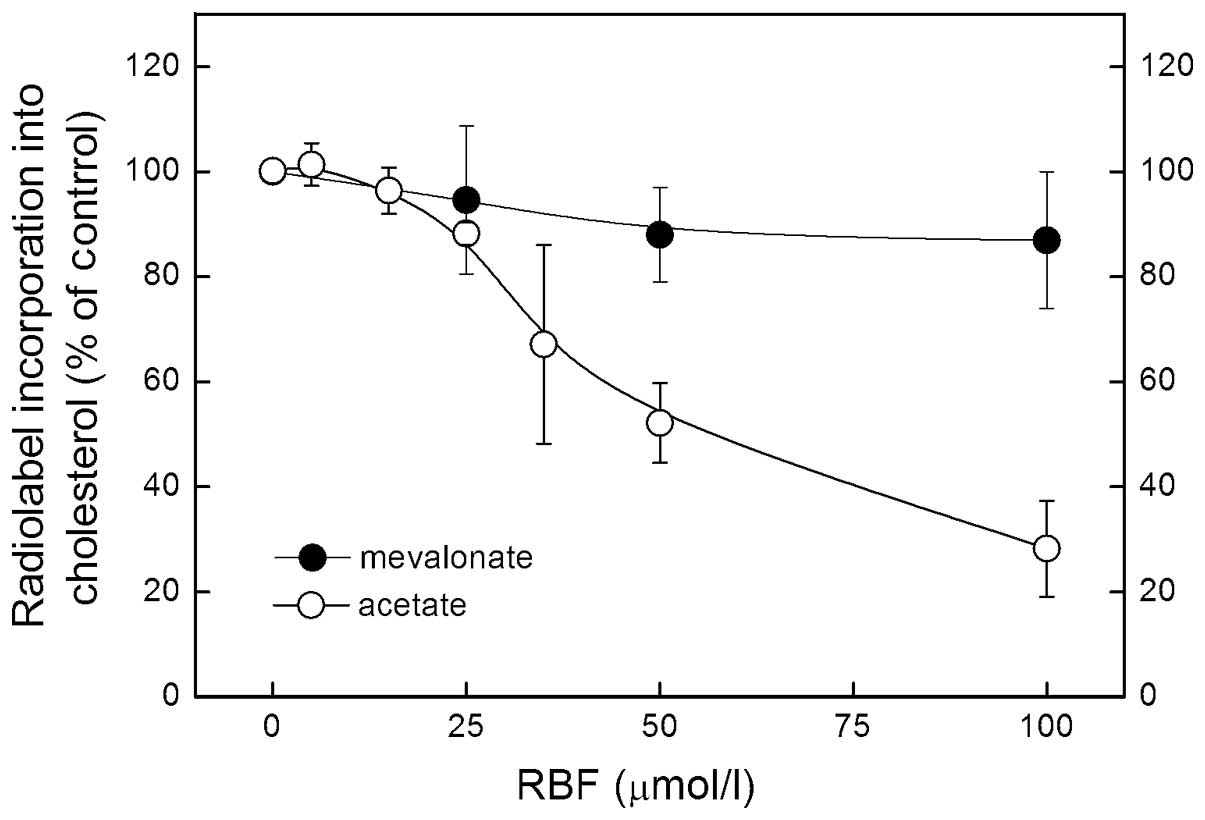
In a 3 h assay, addition of RBF to McARH7777 rat
hepatoma cell cultures decreased the incorporation of
14C-acetate and 14C-mevalonate into
cholesterol (Fig. 2). RBF-mediated
inhibition of 14C-acetate incorporation into cholesterol
was evident at 35 μg/ml and reached 72% at 100 μg/ml. By contrast,
even at the highest concentration of RBF tested, inhibition of
14C-mevalonate incorporation did not exceed 15%. As
mevalonate is the product of HMG-CoA reductase, these results
suggest that RBF acts at or above the level of HMG-CoA
reductase.
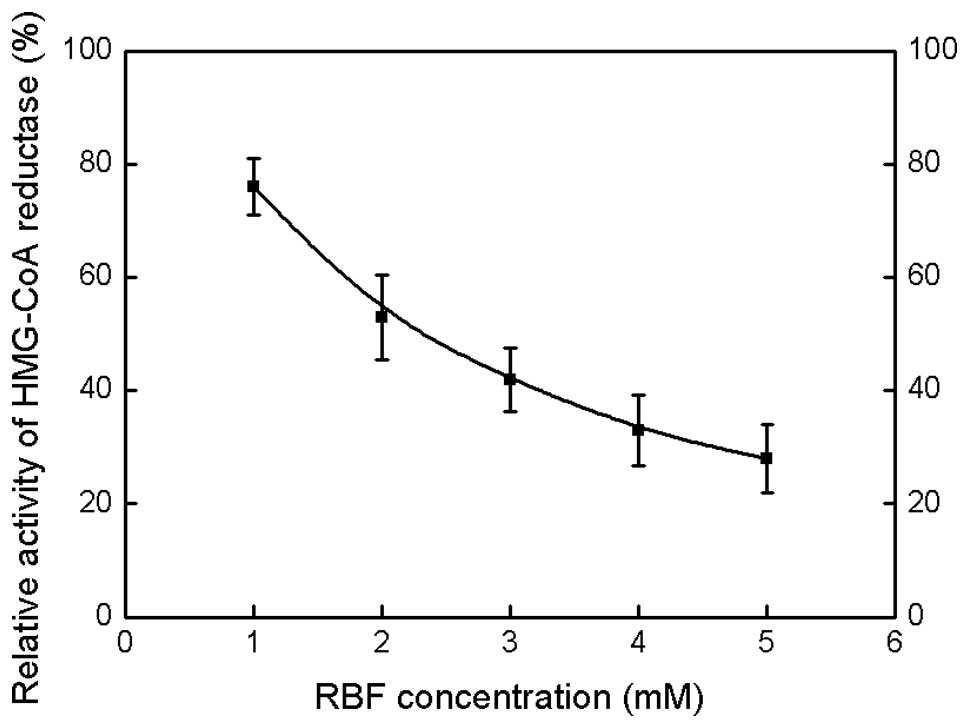
RBF inhibits HMG-CoA reductase activity
in microsomal fractions
In order to detect the effect of RBF on the activity
of HMG-CoA reductase, this rate-limiting enzyme in cholesterol
biosynthesis was also investigated. As shown in Fig. 3, RBF inhibits HMG-CoA reductase
activity in a dose-dependent manner and the concentration of RBF
required for 50% inhibition of enzymatic activity was ~3 mM.
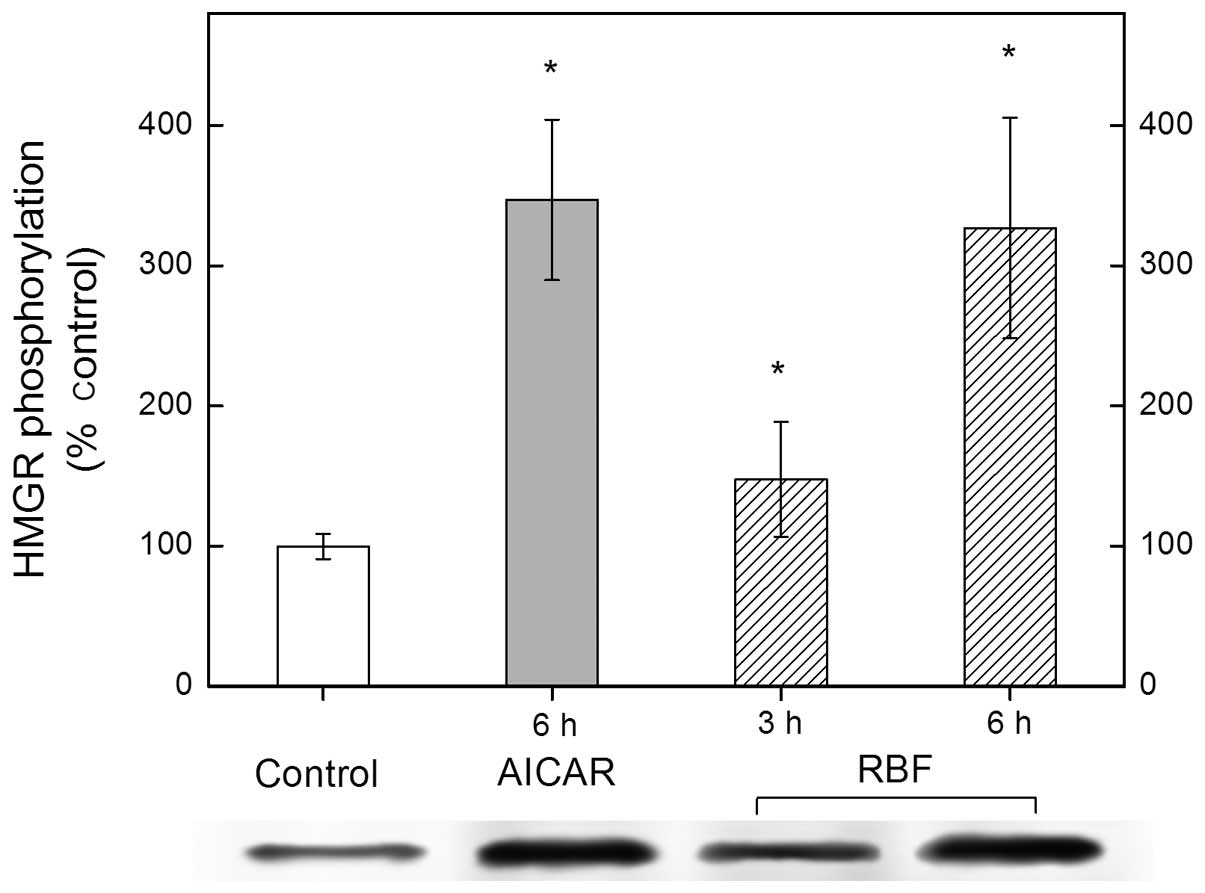
RBF inactivates HMG-CoA
reductase-activated AMP-kinase via phosphorylation in vitro
To investigate the mechanism underlying the
inactivation of HMG-CoA by RBF, the phosphorylation of HMG-CoA
reductase in hepatoma cells was measured with and without the
addition of RBF. As shown in Fig.
4, phosphorylated HMG-CoA reductase increased by 47% and 227%
after 3 and 6 h of RBF treatment, respectively. To further
elucidate the pathways involved in RBF downregulation of HMG-CoA
reductase activity, it was determined whether the activation by RBF
of AMP-kinase via phosphorylation affected this process. As shown
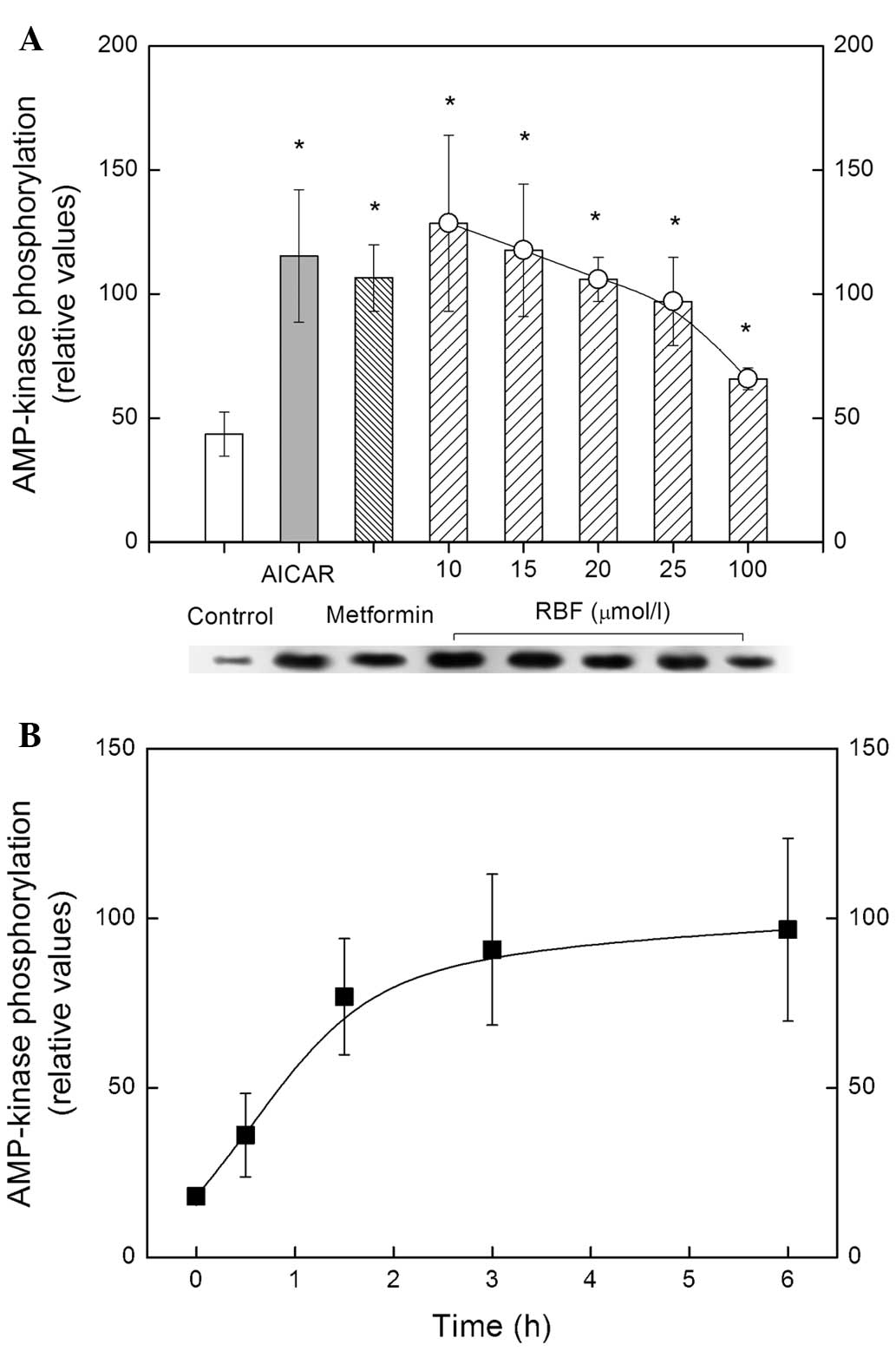
in Fig. 5, RBF administration
tripled AMP-kinase phosphorylation, with the maximal effect at 10
μg/ml, indicating that its potency in terms of AMP-kinase
activation may be on par with AICAR or metformin, which are known
to activate AMP kinase by phosphorylation (21). It may be noted in Fig. 5B that AMP-kinase activation after
RBF administration rapidly increased from 0–3 h, and then
maintained a slight elevation over time (Fig. 5B).
Discussion
The present study evaluated the cholesterol-lowering
effect of RBF in rats. RBF treatment significantly decreased serum
TC, indicating a potential role for RBF in the treatment of
atherosclerosis. This effect was replicated in rat hepatoma cells,
in which a 72% decrease of cholesterol synthesis at 100 μg/ml RBF
was observed. These results suggest that inhibition of cholesterol
synthesis may contribute to the effect of RBF on HMG-CoA in
vitro and in vivo.
To investigate the mechanism of inhibition of RBF on
cholesterol synthesis, the effect of RBF on the incorporation into
cholesterol of radiolabeled acetate and radiolabeled mevalonate in
rat hepatoma cell culture was measured. The results of this
experiment suggested that the inhibition of cholesterol synthesis
appears to be mediated primarily at the level of HMG-CoA reductase,
since inhibition of the incorporation of radiolabeled acetate was
markedly higher than that of radiolabeled mevalonate, the product
of HMG-CoA reductase. This finding is consistent with the study by
Singh et al (9) on black
tea extracts, which also predominantly consist of a diverse mixture
of polymerized polyphenols. Furthermore, treatment of RBF in
microsomal preparations was found to directly inactivate HMG-CoA
reductase, indicating that RBF may suppress cholesterol synthesis
via inactivation of HMG-CoA reductase, the rate-controlling enzyme
in cholesterol biosynthesis (12,14).
To further investigate the mechanism of action of
RBF on cholesterol synthesis, the exact effect of RBF on HMG-CoA
reductase phosphorylation was examined. Previous studies have
demonstrated that AMP-kinase serves as the principal kinase
involved in the phosphorylation-mediated inactivation of HMG-CoA
reductase, and is itself activated by phosphorylation (15–16).
Results from the present study showed that RBF caused a pronounced
increase in the quantity of phosphorylated HMG-CoA reductase and
AMP kinase, suggesting that RBF may activate AMP kinase by
mediating its phosphorylation, in turn leading to phosphorylation
and thus inactivation of HMG-CoA reductase. This effect may
contribute to the suppression of cholesterol synthesis (20).
Together with previous studies (9,21),
the current study demonstrates that RBF may suppress cholesterol
synthesis via inhibition of HMG-CoA reductase activity.
Furthermore, RBF may be a candidate for the development of
therapies for atherosclerosis. Further investigation is required to
determine whether RBF can be absorbed from the human gut in a
sufficient quantity to suppress hepatic cholesterol synthesis.
Acknowledgements
This study was financially supported by the China
National ‘12.5’ Foundation (grant no. 2011BAJ07B04) and the Open
Foundation from the State Key Laboratory of Oral Diseases Sichuan
University (grant no. SKLODSCUKF2012-03) and the National Natural
Science Foundation of China (grant no. 20972105).
References
|
1
|
Ikarashi N, Toda T, Okaniwa T, et al:
Anti-obesity and anti-diabetic effects of acacia polyphenol in
obese diabetic KKAy mice fed high-fat diet. Evid Based Complement
Alternat Med. 2011:9520312011.PubMed/NCBI
|
|
2
|
Ståhlberg D, Rudling M, Angelin B, et al:
Hepatic cholesterol metabolism in human obesity. Hepatology.
25:1447–1450. 1997.
|
|
3
|
Kaul D: Molecular link between
cholesterol, cytokines and atherosclerosis. Mol Cell Biochem.
219:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gebhardt R: Variable influence of
kaempferol and myricetin on in vitro hepatocellular cholesterol
biosynthesis. Planta Med. 69:1071–1074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamura T, Inoue N, Ozawa M, et al:
Peanut-skin polyphenols, procyanidin A1 and
epicatechin-(4β→6)-epicatechin-(2β→O→7, 4β→8)-catechin, exert
cholesterol micelle-degrading activity in vitro. Biosci Biotechnol
Biochem. 77:1306–1309. 2013.
|
|
7
|
Friedlander EJ, Caras IW, Lin LF and Bloch
K: Supernatant protein factor facilitates intermembrane transfer of
squalene. J Biol Chem. 255:8042–8045. 1980.PubMed/NCBI
|
|
8
|
Brito PM, Devillard R, Nègre-Salvayre A,
et al: Resveratrol inhibits the mTOR mitogenic signaling evoked by
oxidized LDL in smooth muscle cells. Atherosclerosis. 205:126–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh DK, Banerjee SD and Porter TD: Green
and black tea extracts inhibit HMG-CoA reductase and activate AMP
kinase to decrease cholesterol synthesis in hepatoma cells. J Nutr
Biochem. 20:816–822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bursill CD and Roach PD: Modulation of
cholesterol metabolism by the green tea polyphenol
(−)-epigallocatechin gallate in cultured human liver (HepG2) cells.
J Agric Food Chem. 54:1621–1626. 2006.
|
|
11
|
Berrougui H, Cloutier M, Isabelle M and
Khali AL: Phenolic-extract from argan oil (Argania spinosa
L.) inhibits human low-density lipoprotein (LDL) oxidation and
enhances cholesterol efflux from human THP-1 macrophages.
Atherosclerosis. 184:389–396. 2006.PubMed/NCBI
|
|
12
|
Espenshade PJ and Hughes AL: Regulation of
sterol synthesis in eukaryotes. Annu Rev Genet. 41:401–427. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mokashi V, Singh DK and Porter TD:
Supernatant protein factor stimulates HMG-CoA reductase in cell
culture and in vitro. Arch Biochem Biophys. 433:474–480. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beg ZH, Stonik JA and Brewer HB Jr:
Modulation of the enzymic activity of 3-hydroxy-3-methylglutaryl
coenzyme A reductase by multiple kinase systems involving
reversible phosphorylation: a review. Metabolism. 36:900–917. 1987.
View Article : Google Scholar
|
|
15
|
Carling D, Clarke PR, Zammit VA and Hardie
DG: Purification and characterization of the AMP-activated protein
kinase. Copurification of acetyl-CoA carboxylase kinase and
3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J
Biochem. 186:129–136. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heller RA and Shrewsbury MA:
3-Hydroxy-3-methylglutaryl coenzyme A reductase from rat liver. Its
purification, properties, and immunochemical studies. J Biol Chem.
251:3815–3822. 1976.PubMed/NCBI
|
|
18
|
Kleinsek DA, Ranganathan S and Porter JN:
Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
from rat liver. Proc Natl Acad Sci USA. 74:1431–1435. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beg ZH, Stonik JA and Brewer HB Jr:
Purification and characterization of 3-hydroxy-3-methylglutaryl
coenzyme A reductase from chicken liver. FEBS Lett. 80:123–129.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beg ZH, Stonik JA and Brewer HB Jr:
3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of
enzymatic activity by phosphorylation and dephosphorylation. Proc
Natl Acad Sci USA. 75:3678–3682. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zang M, Xu S, Maitland-Toolan KA, et al:
Polyphenols stimulate AMP-activated protein kinase, lower lipids,
and inhibit accelerated atherosclerosis in diabetic LDL
receptor-deficient mice. Diabetes. 55:2180–2191. 2006. View Article : Google Scholar : PubMed/NCBI
|