Introduction
Critical-sized bone defects often demand the
transplantation of bone tissue or substitutes, to restore bone
integrity. The gold standard method for orthopedic surgical
procedures is the use of autologous bone grafts to stimulate bone
growth and implant fixation. However, limited quantities of bone
are available for autografting and the harvest procedure involves
potential donor site morbidity (1). While allografted bone has been widely
used, it is limited by the associated risks, including
immunogenicity and transmission of infectious diseases (2,3).
Bone tissue engineering is a promising approach to overcome these
limitations.
One strategy utilized to repair bone defects by bone
tissue engineering, involves the combination of osteogenic cells
with the appropriate porous absorbable scaffolds. In this
cell-based therapy, mesenchymal stem cells (MSCs) are regarded as
an excellent cellular source for bone tissue engineering because of
their self-replication and osteogenic differentiation capacities
(4,5). A variety of adult MSCs have been
isolated from a diverse range of tissue types and ontogenies,
including bone marrow, periosteum, synovium, umbilical cord blood,
amniotic fluid, liver and adipose tissue (6–12).
Among these, bone marrow and periosteum are the most commonly used
cellular source for bone regeneration therapy. However, each source
has its disadvantages, including the fact that MSCs isolated from
bone marrow have limited proliferation capacity and high cellular
senescence, and their osteogenic potential decreases with age
(13,14). Furthermore, periosteal-derived stem
cells isolated from different donor sites and species have been
reported to demonstrate wide viability in osteogenic potential
(15,16). Thus, the correct selection of MSCs
as a cell source is of high importance in constructing engineered
bone tissue.
It is well established that fracture healing
requires the mobilization of MSCs, to allow deposition of cartilage
and bone at the injury site. These cells are considered to be
recruited locally and concurrently from the periosteum and bone
marrow during bone repair. Although the periosteum and bone marrow
generate osteoblasts, these cell types have demonstrated distinct
cellular responses in the process of bone healing and it has been
confirmed that the periosteum is critical in new bone tissue
mineralizaion (17,18). Furthermore, it has also been
identified that injured periosteum and bone marrow heal in a
different manner. Periosteum injuries heal by endochondral
ossification, whereas bone marrow injuries heal by intramembranous
ossification (17,19).
However, whether MSCs isolated from the bone marrow
and periosteum have synergistic effects on osteogenic potential
remains unclear. In the present study, hBMSCs with hPCs from the
same donors were co-cultured with the aim of determining whether
this strategy would accelerate the osteogenic potential of MSCs.
For in vitro evaluation, alizarin red S and ALP staining
were used for monolayer cultivation, and osteogenic-specific mRNA
expression was tested in three-dimensional (3-D) cultivation. For
in vivo assessment, the MSCs from each group were seeded
onto porous β-tricalcium phosphate (TCP) scaffolds and transplanted
to critical-sized femoral condylar defects in rabbits, and the bone
formation volume, mature bone percentage and blood vessel ingrowth
were subsequently determined.
Materials and methods
Samples, animals and ethics
Human bone marrow and periosteum samples were
obtained from patients undergoing lower limb amputation surgery
because of severe limb trauma. Samples were obtained from 8 healthy
donors (six males and two females; range, 22–30 years of age) in
accordance with the local ethics committee and after obtaining
informed consent. The bone marrow was harvested from the inferior
segment of the tibia. During the same surgical procedure, the
periosteum was harvested from the distal part of the tibia.
New Zealand rabbits (n=36; weighing, 2.5±3.2 kg)
were provided by the Laboratory Animal Centre of the Sixth People’s
Hospital of Shanghai Jiaotong University (Shanghai, China). All
procedures were approved by the Animal Care and Use Committee of
Shanghai Sixth People’s Hospital, Shanghai Jiaotong University
(Shanghai, China).
Isolation of hBMSCs
The isolation of hBMSCs was performed as previously
described (20). Briefly, a
single-cell suspension was passed through an 80 μm cell strainer
(BD Biosciences, San Diego, CA, USA). The cells were then plated in
25 cm2 culture flasks and cultured in a complete medium
(CM) consisting of Dulbecco’s modified Eagle’s medium (Gibco,
Gaithersburg, MD, USA), supplemented with 10% fetal bovine serum
(Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in
95% humidified air and 5% CO2. Non-adherent cells were
removed by changing the medium twice a week. When the hBMSCs
reached 80–90% confluence, the adherent cells were detached with
0.25% trypsin/EDTA (Gibco) and subcultured at a density of
1×104/cm2 in 25 cm2 culture
flasks.
Isolation of hPCs
The culture of hPCs was performed as described
previously (21). Following
rinsing the periosteum thoroughly with phosphate-buffered saline
(PBS) containing 100 U/ml penicillin and 100 μg/ml streptomycin,
the biopsy specimens were minced into small pieces and digested in
0.2% type II collagenase (Sigma, St. Louis, MO, USA) for 4 h at
37°C. The isolated cells were centrifuged and resuspended in the CM
at 37°C in 95% humidified air and 5% CO2. The hPCs were
subcultured as described above for the hBMSCs. The hBMSCs and hPCs
at passages 3 were used in the experiments.
Co-culture design of hBMSCs and hPCs
The hBMSCs and hPCs from the same donors were used
for co-culture experiments with three different ratios. The ratio
of hBMSCs to hPCs in group 1 was 1:1; in group 2 was 1:2 and in
group 3, was 2:1. For the monolayer culture, three types of
co-cultured MSCs, hBMSCs and hPCs were seeded at a density of 5,000
cells/cm2 into 6-well dishes with CM. Following 24 h in
culture, the medium was replaced by an osteogenic medium consisting
of CM supplemented with 10 nM dexamethasone (Sigma), 0.05 mM
L-ascorbic acid 2-phosphate (Sigma) and 10 mM β-glycerophosphate
(Sigma). The cells were cultured in osteogenic medium for three
weeks and the medium was changed twice a week.
Osteogenic differentiation in monolayer
cultures
ALP staining
Following rinsing of the monolayer cells with PBS,
they were fixed in an ice-cold, 90% ethanol solution for 10 min and
washed in PBS for 5 min. Then, the cells were stained with fast
5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium
(BCIP/NBT) ALP substrate (Beyotime Biotechnology, Jiangsu, China)
for 30 min at room temperature. The reaction was terminated by
removing the substrate solution and washing with distilled water.
The results were expressed as the percentage of positive staining
area per field of view (magnification, ×100).
Alizarin red S staining
For the mineralized nodule formation assay, the
mineralized matrix was analyzed using alizarin red S staining. The
cell cultures were rinsed with PBS and fixed in ice-cold, 90%
ethanol solution for 10 min. The cells were washed with distilled
water, treated with a 2% alizarin red S solution (Amresco, Solon,
OH, USA) for 5 min and washed with distilled water to remove the
remaining staining. The results were expressed as the percentage of
positive staining area in per field of view (magnification,
×100).
Scaffold preparation and MSCs in 3-D
cultures
The β-TCP scaffolds (Bio-lu Biomaterials, Shanghai,
China) were molded into a circular cylinder (6 mm diameter and 10
mm length) with a porosity of 70% and pore diameter of 450±50 μm.
The scaffolds were sterilized by 60Co irradiation prior to use. The
MSCs were suspended in a fibrin gel (Sigma) and statically loaded
into porous β-TCP scaffolds (1.2×106 cells/scaffold), as
described previously (21). The
cellular scaffolds were cultured in 6-well plates with CM overnight
prior to being transferred to the osteogenic medium, which was
changed twice a week. For the in vitro osteogenic gene
expression assay, the cellular scaffolds were harvested on days 3,
7, 14 and 21 for quantitative polymerase chain reaction (qPCR)
analysis. For in vivo evaluation, the cellular scaffolds
were pre-differentiated in an osteogenic differentiation medium for
21 days prior to implantation.
qPCR
The total cellular RNA on the scaffolds was
extracted using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The concentration of RNA was determined from the optical absorbance
at 260 nm of the extract. Complementary DNA (cDNA) was synthesized
using the PrimeScript First Strand cDNA Synthesis kit (TaKaRa
Biotechnology, Inc., Dalian, China). Reactions were performed and
monitored in a PTC 200 Thermal Cycler PCR machine (Bio-Rad,
Waltham, MA, USA). qPCR was performed using a quantitative
real-time amplification system (Light Cycler 480; Roche Diagnostics
(Schweiz) AG, Risch, Switzerland). SybrGreen Premix Ex TaqII
(TaKaRa Biotechnology, Inc.) was used in each reaction. Reactions
were performed with 40 cycles (95°C for 5 sec, 55°C for 30 sec and
72°C for 30 sec). The primers used for qPCR were as follows: BMP-2,
5′-TGGAAGTGGCCCATTTAGAG-3′, 5′-TGACGCTTTTCTCGTTTGTG-3′; Collagen
typeIalpha1 (COL1α1), 5′-CCTGCGTGTACCCCACTCA-3′,
5′-ACCAGACATGCCTCTTGTCCTT-3′; Osteopontin (OPN), 5′-AC
ACATATGATGGCCGAGGTGA-3′, 5′-TGTGAGGTGATGTCCTCGTCTGTA G-3′;
Osteocalcin (OC), 5′-CAAAGGTGCAGCCTTTGTGTC-3′,
5′-TCACAGTCCGGATTGAGCTCA-3′; GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ and
5′-ATGGTGGTGAAGACGCCAGT-3′. Results were normalized against the
housekeeping gene GAPDH and relative gene expression was analyzed
with the 2-ΔΔCt method. The human osteoblasts were obtained as the
control cell types. Each measurement was assessed in
triplicate.
Surgical procedure
The animal model was adapted from Giavaresi et
al, as described previously (22). Briefly, following induction of
general anesthesia, transversal, critical-sized bone defects were
created (6 mm diameter, 10 mm length) in the femoral distal
epiphysis of the posterior limbs by a standardized surgical
procedure. A 2 cm skin incision was established on the lateral
aspect of the distal femoral condyle. Bilateral confined cancellous
defects were stepwise drilled in both limbs with a 3.2 mm drill and
these defects were subsequently expanded with a 6.0 mm drill. The
depth of the defects was 10±0.5 mm as measured by a digital
caliper. The soft tissues were sutured with Dexon 3-0 and the skin
was closed with silk 3-0. Analgesics (carprofen, 4 mg/kg) were
prescribed in the immediate post-operative period. Antibiotic
therapy (cefazolin, 25 mg/kg) was administered pre-operatively and
for five days following surgery. Six experimental conditions were
used: (i) hBMSCs, (ii) hPCs, (iii) co-culturing hBMSCs and hPCs
with 1:1 ratios, (iv) co-culturing hBMSCs and hPCs with 1:2 ratios,
(v) co-culturing hBMSCs and hPCs with 2:1 ratios and (vi) blank
β-TCP scaffolds. A sample size of n=6 defect sites per group per
time point were examined. Following four and 12 weeks,
respectively, the animals were euthanized and the implants were
retrieved for histological analysis.
Quantification of newly formed bone
To evaluate the total new bone formation and the
mature bone volume, the harvested femoral condyles were fixed in 4%
formaldehyde, decalcified and embedded in paraffin wax. Three
middle sections (5 μm thickness) of each implant were stained with
hematoxylin and eosin (H&E) for total new bone tissue area and
Van Geison’s for mature bone volume. The results were observed
under a light microscope (magnification, ×100) and at least ten
images were randomly obtained in one section. Using image
analytical software Image-Pro Plus (Media Cybernetics, Bethesda,
MD, USA), the total new bone volume was expressed as a percentage
of newly formed bone area in the total cross sectional area and the
mature bone volume was calculated as a percentage of mature bone
area of the total new bone tissue area.
Neovasculogenesis analysis
To determine the extent of blood vessel ingrowth,
the middle sections of the implants were immunostained for vWF
(Biosynthesis Biotechnology, Beijing, China), a protein present in
large quantities in subendothelial matrices, including blood vessel
basement membranes (20). Circular
vWF staining was obtained to indicate a blood vessel
subendothelium. Blood vessels were counted manually with Adobe
Photoshop 8.0 software (Adobe Systems, Mountain View, CA, USA) and
were assessed by the mean blood vessel numbers per pore and
percentage of blood vessels in the pore center region.
Statistical analyses
Parametric data are represented as the mean ± SD,
analyzed using one-way ANOVA. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Osteogenic differentiation capacity in
monolayer cultures
As demonstrated by alizarin red S staining, when
exposed to osteogenic medium, the mineralized nodule was generated
abundantly in the co-culture condition, compared with the hBMSC and
hPC groups (Fig. 1A and C).
Furthermore, co-cultured MSCs formed alizarin red S positive
mineralization nodules earlier than hBMSCs and hPCs, which was
first observed at day 8 in all three co-cultured MSCs and at day 10
in both hBMSCs and hPCs. The more robust osteogenic differentiation
was also confirmed by ALP staining. ALP-positive staining in the
early stage was confirmed in all five types of MSCs, although the
staining was slightly decreased on day 21 in hBMSCs and hPCs.
Co-culturing hBMSCs and hPCs did however, demonstrate a relatively
strong positive staining in the early stage (day 3) and in the late
stage (day 21), compared with hBMSCs and hPCs (Fig. 1B and D).
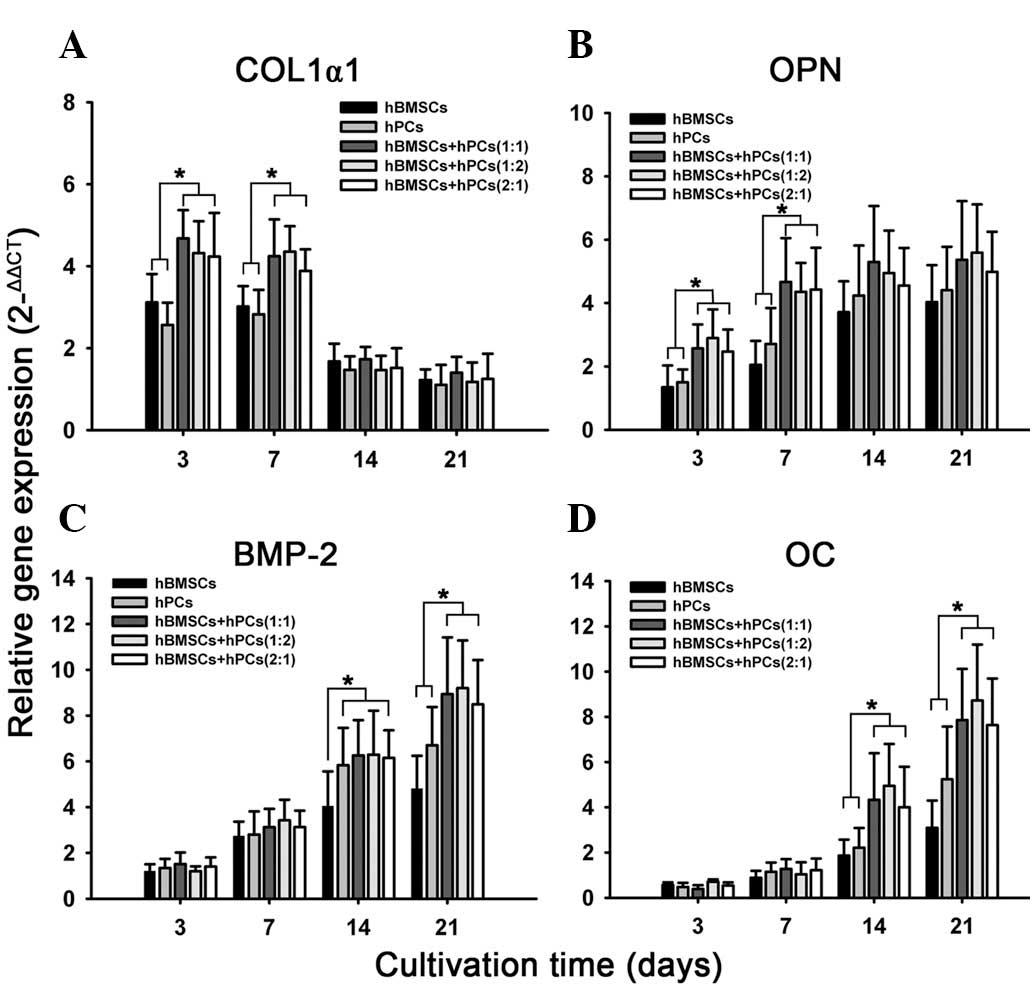
Osteogenic gene expression in 3-D
cultures
During culturing in the osteogenic medium, hBMSCs,
hPCs and co-cultured MSCs of the three different ratios, were
assessed for mRNA expression of gene encoding. qPCR demonstrated
that co-culturing MSCs in the 3-D model upgraded mRNA expression of
COL1α1, BMP2, OPN and OC at different time points (Fig. 2). High expression of COL1α1 mRNA
was recorded in the early differentiation stage, but a decrease was
observed in the late stage. Co-culturing hBMSCs and hPCs
significantly increased COL1α1 expression at days 3 and 7,
respectively (Fig. 2B). OPN was
expressed in the early differentiation stage and this effect
progressively increased with time. The co-cultured MSCs upgraded
the OPN mRNA transcript on days 3 and 7 (Fig. 2B). BMP-2 and OC mRNA transcript was
highly expressed at day 14, which steadily increased at 21 days.
Co-culturing significantly enhanced the expression of BMP-2 and OC
in the late stage, which were confirmed in all three co-culturing
MSCs (Fig. 2C and D).
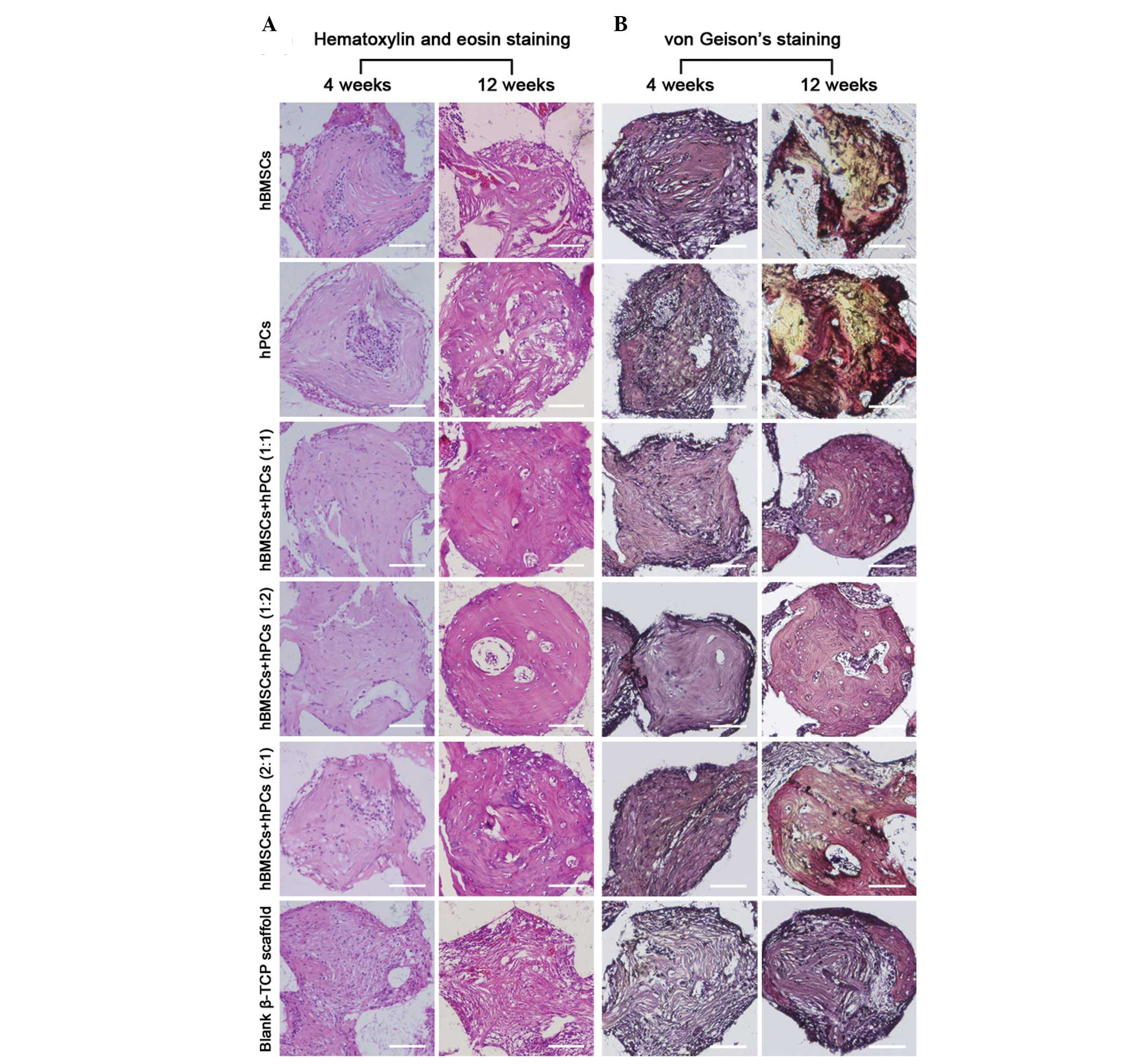
Histological analysis
The cellular-scaffold constructs in the femoral
condyle were retrieved for histological evaluation following 4 and
12 weeks’ implantation. The total new bone formation in the 3-D
β-TCP scaffolds was indicated by H&E staining (Fig. 3A). Mature bone volume in the six
groups of implants was revealed by Van Geison’s staining (Fig. 3B). As summarized in Table 1, the β-TCP scaffolds seeded with
all three different ratios of co-culturing MSCs, significantly
increased new bone formation, compared with the scaffolds loaded
with hBMSCs and hPCs. The most evident difference in newly formed
bone tissue was observed at four weeks. This indicated that this
synergetic effect in bone formation was initiated from the early
stages of osteogenic differentiation. Furthermore, mature bone
volume analysis confirmed that, compared with the hBMSCs and hPCs,
co-culturing MSCs exhibited a significantly higher percentage of
mature bone formation in the critical-sized femoral condyle
defects.
 | Table IBone formation area and mature bone
percentage of engineering bone in critical-sized femoral condyle
defects. |
Table I
Bone formation area and mature bone
percentage of engineering bone in critical-sized femoral condyle
defects.
| Bone formation area
(%) | Mature bone volume
(%) |
|---|
|
|
|
|---|
| Group | 4 weeks | 12 weeks | 4 weeks | 12 weeks |
|---|
| hBMSCs | 25.09±3.56 | 36.82±5.38 | 36.48±4.89 | 48.47±4.72 |
| hPCs | 26.58±4.89 | 38.73±7.14 | 39.31±5.12 | 63.43±4.77a,b |
| hBMSCs+hPCs
(1:1) | 40.30±6.83a,b | 46.83±7.36a,b | 48.46±4.33a,b | 76.42±5.82a,b |
| hBMSCs+hPCs
(1:2) | 38.53±7.15a,b | 47.39±6.88a,b | 51.54±3.79a,b | 76.85±6.43a,b |
| hBMSCs+hPCs
(2:1) | 37.58±7.87a,b | 45.65±8.21a,b | 48.48±5.17a,b | 74.15±5.38a,b |
| blank β-TCP
scaffold | 15.22±3.15a,b | 26.32±5.41a,b | 33.70±4.82 | 44.18±4.79b |
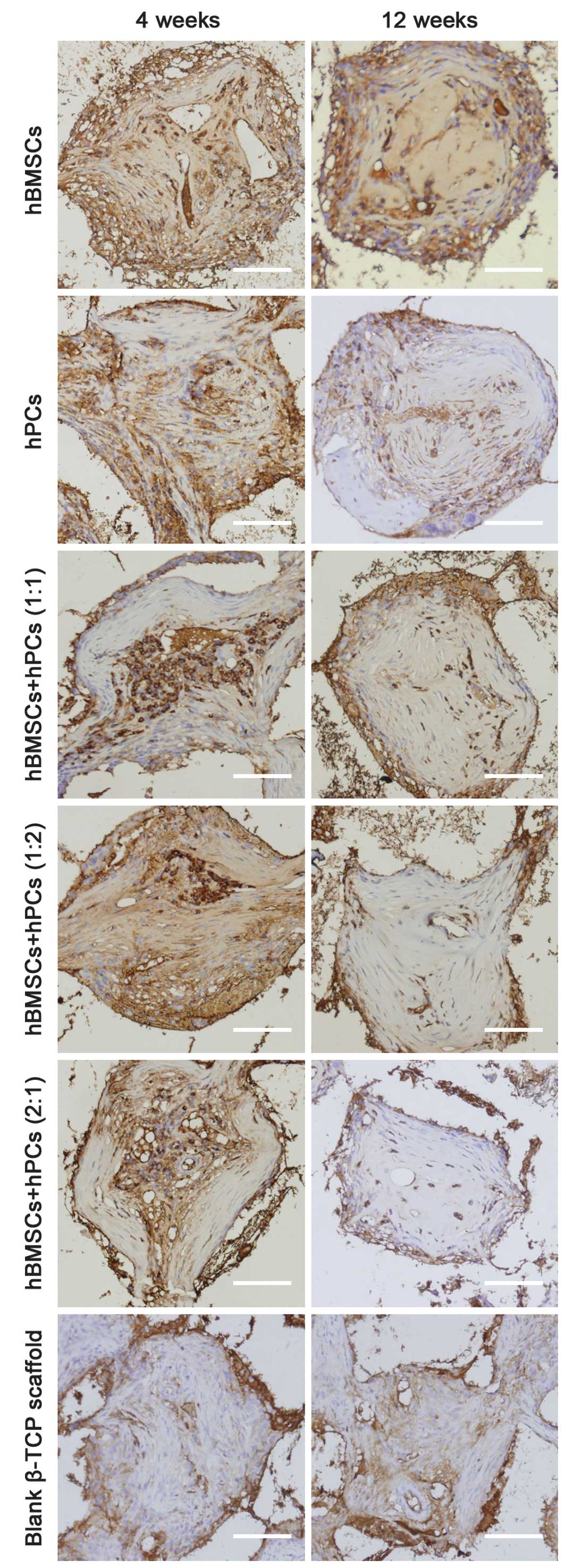
Neovasculogenesis analysis
The effects of blood vessel ingrowth in the 3-D
β-TCP scaffolds were determined by immunostaining tissue sections
for vWF (Fig. 4), a component of
blood vessel extracellular matrix (ECM). There was a marked
increase in immunostained vessels in the TCP scaffolds seeded with
MSC vs. the controls (acellular β-TCP scaffolds). Co-culturing MSCs
did not significantly enhance vascularization within the 3-D β-TCP
scaffolds, compared with the hBMSCs and hPCs. Of note, however, all
co-culturing MSCs demonstrated a remarkably higher percentage of
blood vessels in the pore center region than that exhibited in the
hBMSCs or hPCs (Table II).
 | Table IINeovascularization of engineering
bone in critical-sized condyle defects. |
Table II
Neovascularization of engineering
bone in critical-sized condyle defects.
| 4 weeks | 12 weeks |
|---|
|
|
|
|---|
| Group | Blood
number/pore | Center region
(%) | Blood
number/pore | Center region
(%) |
|---|
| hBMSCs | 87.48±12.87 | 26.75±4.75 | 72.81±9.32 | 25.82±3.64 |
| hPCs | 78.33±10.45 | 28.82±4.97 | 69.77±7.45 | 23.76±5.79 |
| hBMSCs+hPCs
(1:1) | 82.59±7.85 | 51.23±5.39a,b | 72.36±5.14 | 37.32±4.85a,b |
| hBMSCs+hPCs
(1:2) | 86.75±9.77 | 49.85±5.88a,b | 70.42±6.12 | 33.29±5.37a,b |
| hBMSCs+hPCs
(2:1) | 79.56±11.46 | 44.75±6.58a,b | 68.39±8.19 | 32.84±6.29a,b |
| Blank β-TCP
scaffold | 37.57±7.35a,b | 29.23±3.97 | 30.84±4.97a,b | 24.72±4.86 |
Discussion
Engineering bone based on combined multipotent MSCs
and a 3-D scaffold, presents a promising new strategy in bone
regeneration that restores bony tissue following extensive loss as
a result of trauma or disease (23–25).
The identification of various types of MSCs from different
ontological and anatomical sites has raised the question as to the
optimal cellular source for such allogeneic applications. In the
present study, hBMSCs and hPCs from the same donors were
co-cultured. Their osteogenic potential was investigated using
three different models (monolayer culture, 3-D culture and an in
vivo model), in an attempt to determine whether this
co-culturing strategy may be an alternative cellular source for
hBMSCs and hPCs in cell-based approaches to bone repair. The
results confirmed that, co-culturing hBMSCs with hPCs exhibits an
overall enhanced capacity to differentiate towards the osteogenic
lineage in vitro, which was reflected by robust mineralized
node formation, steadily ALP positive staining and upgraded
osteogenic-specific mRNA expression. Furthermore, for the repair of
critical-sized femoral condylar defects, engineering bone
constructed by co-culturing MSCs and porous β-TCP scaffolds
exhibited noteably abundant, newly formed bone tissue, enhanced
mature bone formation and increased center neovascularization,
compared with the constructs seeded with hBMSCs or hPCs.
In the monolayer culture, in vitro osteogenic
differentiation of co-culturing MSCs was determined by mineralized
nodule formation and ALP staining. ALP is an early marker of
osteoblast differentiation, whereas mineralization of the matrix is
associated with the late phase of osteoblast differentiation
(26,27). ALP is an ectoenzyme, produced by
osteoblasts, that is involved in the degradation of inorganic
pyrophosphate, providing sufficient local concentrations of
phosphate or inorganic pyrophosphate for mineralization (28,29).
Therefore, ALP was used as a biochemical marker to determine
osteoblast phenotype and it is considered as an important factor in
determining bone differentiation (30–32).
In addition, alizarin red S staining is the most common method of
examining a mineralized matrix (26,33).
In the present study, during the early and late osteogenic
differentiation stages, the number of ALP positive cells in the
three co-culturing MSCs was higher than those in the hBMSCs or
hPCs. Furthermore, it was confirmed that co-culturing hBMSCs and
hPCs not only demonstrated abundant mineralized nodule formation,
but also that alizarin red S positive staining appeared earlier
than in the hBMCs and hPCs. The results suggest that co-culturing
hBMSCs with hPCs strongly promotes MSC differentiation into their
osteogenic phenotype and greatly enhances mineralized nodule
formation in vitro.
For constructing engineered bone, biodegradable
scaffolds are critical in providing 3-D space for the growth of
osteogenic cells at an early stage and enough space for new bone
formation when they are later degraded (34). Furthermore, the scaffolds offer a
3-D framework on which a temporary matrix, for cellular
proliferation, differentiation and deposition of the ECM,
facilitating the development of the neovasculature (25,35).
In the present study, the porous β-TCP scaffold was employed for
the 3-D cultivation of MSCs. Its biocompatibility and its
osteoconductive and osteoinductive features have been reported in
several studies (36,37). To further confirm the osteogenic
capacity of co-culturing MSCs in 3-D cultivation, the
osteogenic-specific mRNA expression of COL1α1, BMP-2, OPN and OC
were evaluated using qPCR. In skeletal tissues, the cells are
distributed within a dense ECM composed of collagens,
proteoglycans, a complex mixture of phosphoproteins and other
inorganic materials (38). COL1α1
is an essential element in bone formation and it contributes to
matrix production (39). During
osteogenic differentiation, markers of the osteoblast phenotype
appear, including the accumulation of extracellular bone matrix
proteins, of which COL I is the most prevalent (32). OC is the most abundant
non-collagenous protein in bone and is a useful tool as a
bone-specific marker for terminal osteoblast differentiation. OC is
released by calcified tissue and expressed at the late stage of
differentiation (40,41). OPN is a phosphorylated
glycoprotein, recognized as a early marker of osteogenic
differentiation and involved in the regulation of bone development
and calcification (42). BMP-2 is
secreted in an autocrine and paracrine fashion, and acts to
directly induce differentiation of the MSCs into osteoblasts and to
initiate the differentiation of osteoprogenitors from the host
tissue into bone-forming cells (20). BMP-2 also serves as an
osteoinductive signal, to increase infiltration and recruitment of
surrounding repair cells, to further enhance bone regeneration and
induce blood vessel ingrowth (43–45).
In 3-D cultivation, the results demonstrated that
all three ratios of co-culturing MSCs significantly enhanced the
mRNA expression of COL1α1, OPN, OC and BMP-2 at different times.
OPN and COL1α1 were expressed at the early osteogenic inductive
stage, however there was a discrepancy in that OPN mRNA transcripts
were increased in a time-dependent manner, whereas COL1α1 mRNA was
steadily decreased following seven days in culture. The results
suggest that the OPN mRNA was expressed earlier than the
independent calcification. This may indicate that COL1α1
participates in the initiation of osteogenic differentiation but
does not directly participate in late-stage osteoblastic
mineralization. Although gene expression of OC and BMP-2 in this
3-D cultivation were detected in the early osteogenic
differentiation stage, their noteable expressions were recorded
following 14 days in culture and their levels increased as
calcification progressed from days 14 to 21. These results indicate
that the expression of OC and BMP-2 are closely correlated to
biomineralization or hydroxyapatite crystallization. Based on these
findings in 3-D cultivation, it may be confirmed that co-culturing
hBMSCs with hPCs not only enhances the synthesis of the ECM and
accelerates osteoblastic mineralization, but also stimulates
osteoinductive signals to increase osteogenic differentiation.
The results from in vitro osteogenic
differentiation and the capacity of new bone regeneration in
vivo followed the same trends. For repairing critical-sized
femoral condyle defects, co-culturing hBMSCs and hPCs demonstrated
not only synergetic effects in promoting new bone formation, but
greatly enhanced mature bone formation in vivo. This
phenomenon is consistent with in vitro studies where
co-culturing MSCs facilitated osteoblastic mineralization and
upgraded osteogenic-specific mRNA expression. Furthermore,
co-culturing MSCs revealed the formation of large quantities of
bone tissue in the central (scaffolded) portion of the pore,
whereas in the hBMSCs and hPCs alone, the newly formed bone was
confined mainly to the margin of the pore.
Bone tissue is a complex and highly vascularized
tissue. Angiogenesis is an essential component of normal bone
development and fracture healing. Therefore, the evaluation of
angiogenic activity in bone tissue engineering is important. MSCs
are reportedly able to contribute directly to the formation of new
blood vessels (46). The high
degree of neovascularization facilitated the delivery of oxygen and
nutrients for the construct and eventually contributed to the
volume of tissue-engineered bone. It is also considered that
adequate oxygen tension and a supply of other nutrients result from
neovascularization, which allows direct formation of the
mineralized matrix inside the scaffold (47). In addition, signals of newly formed
vessels may positively affect the osteogenic potential of MSCs,
which eventually affects the maturation of tissue-engineered bone
(48). The results of the present
study indicate that β-TCP scaffolds loaded with MSCs increase blood
vessel ingrowth more than acellular scaffolds at the femoral
condyle defect site. Furthermore, it was confirmed that
co-culturing hBMSCs and hPCs notably facilitates central
vascularization in the scaffold pores. These results may explain
why co-culturing MSCs demonstrated a significantly higher
percentage of new bone formation in the central pore region of the
scaffold.
In conclusion, the present study first confirmed
that MSCs, isolated from bone marrow and periosteum, have
synergetic effects on osteogenic differentiation at the level of
monolayer and 3-D cultivation, as well as on the critical-sized
femoral condyle defects model. Co-culturing hBMSCs and hPCs not
only increased osteoblastic mineralization and upgraded osteogenic
specific mRNA expression, but it accelerated bone regeneration and
enhanced mature bone formation. In addition, co-culturing hBMSCs
and hPCs greatly facilitated central vascularization in the
scaffold pores. Based on these findings, we recommend co-culturing
hBMSCs and hPCs as a promising cellular source for bone-tissue
engineering applications. Further study is necessary to clarify the
exact mechanism of this synergetic effect on osteogenesis and
vascularization.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81271998, 81271961, 81071452). The authors
are grateful to Bio-lu Biomaterials Corp for supplying the β-TCP
ceramic blocks.
References
|
1
|
Goulet JA, Senunas LE, DeSilva GL and
Greenfield ML: Autogenous iliac crest bone graft. Complications and
functional assessment. Clin Orthop Relat Res. 76–81. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrara JL and Yanik G: Acute graft versus
host disease: pathophysiology, risk factors, and prevention
strategies. Clin Adv Hematol Oncol. 3:415–419. 4282005.PubMed/NCBI
|
|
3
|
Lietman SA, Tomford WW, Gebhardt MC,
Springfield DS and Mankin HJ: Complications of irradiated
allografts in orthopaedic tumor surgery. Clin Orthop Relat Res.
214–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruder SP, Jaiswal N, Ricalton NS, Mosca
JD, Kraus KH and Kadiyala S: Mesenchymal stem cells in osteobiology
and applied bone regeneration. Clin Orthop Relat Res. (Suppl):
S247–S256. 1998. View Article : Google Scholar
|
|
5
|
Srouji S, Maurice S and Livne E:
Microscopy analysis of bone marrow-derived osteoprogenitor cells
cultured on hydrogel 3-D scaffold. Microsc Res Tech. 66:132–138.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arnsdorf EJ, Jones LM, Carter DR and
Jacobs CR: The periosteum as a cellular source for functional
tissue engineering. Tissue Eng Part A. 15:2637–2642. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barachini S, Trombi L, Danti S, et al:
Morpho-functional characterization of human mesenchymal stem cells
from umbilical cord blood for potential uses in regenerative
medicine. Stem Cells Dev. 18:293–305. 2009. View Article : Google Scholar
|
|
8
|
Campagnoli C, Roberts IA, Kumar S, Bennett
PR, Bellantuono I and Fisk NM: Identification of mesenchymal
stem/progenitor cells in human first-trimester fetal blood, liver,
and bone marrow. Blood. 98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Coppi P, Bartsch G Jr, Siddiqui MM, et
al: Isolation of amniotic stem cell lines with potential for
therapy. Nat Biotechnol. 25:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: limitations and recent
advances. Ann Biomed Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fickert S, Fiedler J and Brenner RE:
Identification, quantification and isolation of mesenchymal
progenitor cells from osteoarthritic synovium by fluorescence
automated cell sorting. Osteoarthritis Cartilage. 11:790–800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mueller SM and Glowacki J: Age-related
decline in the osteogenic potential of human bone marrow cells
cultured in three-dimensional collagen sponges. J Cell Biochem.
82:583–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phinney DG, Kopen G, Righter W, Webster S,
Tremain N and Prockop DJ: Donor variation in the growth properties
and osteogenic potential of human marrow stromal cells. J Cell
Biochem. 75:424–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eyckmans J and Luyten FP: Species
specificity of ectopic bone formation using periosteum-derived
mesenchymal progenitor cells. Tissue Eng. 12:2203–2213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McDuffee LA and Anderson GI: In vitro
comparison of equine cancellous bone graft donor sites and tibial
periosteum as sources of viable osteoprogenitors. Vet Surg.
32:455–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colnot C: Skeletal cell fate decisions
within periosteum and bone marrow during bone regeneration. J Bone
Miner Res. 24:274–282. 2009. View Article : Google Scholar
|
|
18
|
Solchaga LA, Cassiède P and Caplan AI:
Different response to osteo-inductive agents in bone marrow- and
periosteum-derived cell preparations. Acta Orthop Scand.
69:426–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guichet JM, Braillon P, Bodenreider O and
Lascombes P: Periosteum and bone marrow in bone lengthening: a DEXA
quantitative evaluation in rabbits. Acta Orthop Scand. 69:527–531.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang YC, Kaigler D, Rice KG, Krebsbach PH
and Mooney DJ: Combined angiogenic and osteogenic factor delivery
enhances bone marrow stromal cell-driven bone regeneration. J Bone
Miner Res. 20:848–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaquiéry C, Schaeren S, Farhadi J, et al:
In vitro osteogenic differentiation and in vivo bone-forming
capacity of human isogenic jaw periosteal cells and bone marrow
stromal cells. Ann Surg. 242:859–867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giavaresi G, Fini M, Salvage J, et al:
Bone regeneration potential of a soybean-based filler: experimental
study in a rabbit cancellous bone defects. J Mater Sci Mater Med.
21:615–626. 2010. View Article : Google Scholar
|
|
23
|
Mistry AS and Mikos AG: Tissue engineering
strategies for bone regeneration. Adv Biochem Eng Biotechnol.
94:1–22. 2005.PubMed/NCBI
|
|
24
|
Otto WR and Rao J: Tomorrow’s skeleton
staff: mesenchymal stem cells and the repair of bone and cartilage.
Cell Prolif. 37:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salgado AJ, Coutinho OP and Reis RL: Bone
tissue engineering: state of the art and future trends. Macromol
Biosci. 4:743–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Igarashi M, Kamiya N, Hasegawa M, Kasuya
T, Takahashi T and Takag M: Inductive effects of dexamethasone on
the gene expression of Cbfa1, Osterix and bone matrix proteins
during differentiation of cultured primary rat osteoblasts. J Mol
Histol. 35:3–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park BW, Hah YS, Kim DR, Kim JR and Byun
JH: Osteogenic phenotypes and mineralization of cultured human
periosteal-derived cells. Arch Oral Biol. 52:983–989. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Asou Y, Sekiya I, Sotome S, Orii H
and Shinomiya K: Enhancement of tissue engineered bone formation by
a low pressure system improving cell seeding and medium perfusion
into a porous scaffold. Biomaterials. 27:2738–2746. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weinreb M, Shinar D and Rodan GA:
Different pattern of alkaline phosphatase, osteopontin, and
osteocalcin expression in developing rat bone visualized by in situ
hybridization. J Bone Miner Res. 5:831–842. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marom R, Shur I, Solomon R and Benayahu D:
Characterization of adhesion and differentiation markers of
osteogenic marrow stromal cells. J Cell Physiol. 202:41–48. 2005.
View Article : Google Scholar
|
|
31
|
Stucki U, Schmid J, Hämmerle CF and Lang
NP: Temporal and local appearance of alkaline phosphatase activity
in early stages of guided bone regeneration. A descriptive
histochemical study in humans. Clin Oral Implants Res. 12:121–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Li Y, Zuo Y, Li J, Ma S and Cheng
L: Biocompatibility and osteogenesis of biomimetic
nano-hydroxyapatite/polyamide composite scaffolds for bone tissue
engineering. Biomaterials. 28:3338–3348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park BW, Hah YS, Kim DR, Kim JR and Byun
JH: Vascular endothelial growth factor expression in cultured
periosteal-derived cells. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 105:554–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan J, Cui L, Zhang WJ, Liu W and Cao Y:
Repair of canine mandibular bone defects with bone marrow stromal
cells and porous beta-tricalcium phosphate. Biomaterials.
28:1005–1013. 2007. View Article : Google Scholar
|
|
35
|
Rai B, Oest ME, Dupont KM, Ho KH, Teoh SH
and Guldberg RE: Combination of platelet-rich plasma with
polycaprolactone-tricalcium phosphate scaffolds for segmental bone
defect repair. J Biomed Mater Res A. 81:888–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marino G, Rosso F, Cafiero G, Tortora C,
Moraci M, Barbarisi M and Barbarisi A: Beta-tricalcium phosphate
3-D scaffold promote alone osteogenic differentiation of human
adipose stem cells: in vitro study. J Mater Sci Mater Med.
21:353–363. 2010. View Article : Google Scholar
|
|
37
|
Neamat A, Gawish A and Gamal-Eldeen AM:
beta-Tricalcium phosphate promotes cell proliferation, osteogenesis
and bone regeneration in intrabony defects in dogs. Arch Oral Biol.
54:1083–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng YX, Ringe J, Liang Z, Loch A, Chen L
and Sittinger M: Osteogenic potential of human periosteum-derived
progenitor cells in PLGA scaffold using allogeneic serum. J
Zhejiang Univ Sci B. 7:817–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ignatius A, Blessing H, Liedert A, et al:
Tissue engineering of bone: effects of mechanical strain on
osteoblastic cells in type I collagen matrices. Biomaterials.
26:311–318. 2005. View Article : Google Scholar
|
|
40
|
Bilkay U, Tokat C, Helvaci E, Ozek C,
Zekioglu O, Onat T and Songur E: Osteogenic capacities of tibial
and cranial periosteum: a biochemical and histologic study. J
Craniofac Surg. 19:453–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stein GS, Lian JB, Gerstenfeld LG,
Shalhoub V, Aronow M, Owen T and Markose E: The onset and
progression of osteoblast differentiation is functionally related
to cellular proliferation. Connect Tissue Res. 20:3–13. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Giachelli CM and Steitz S: Osteopontin: a
versatile regulator of inflammation and biomineralization. Matrix
Biol. 19:615–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bouletreau PJ, Warren SM, Spector JA,
Peled ZM, Gerrets RP, Greenwald JA and Longaker MT: Hypoxia and
VEGF up-regulate BMP-2 mRNA and protein expression in microvascular
endothelial cells: implications for fracture healing. Plast
Reconstr Surg. 109:2384–2397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liang G, Yang Y, Oh S, et al: Ectopic
osteoinduction and early degradation of recombinant human bone
morphogenetic protein-2-loaded porous beta-tricalcium phosphate in
mice. Biomaterials. 26:4265–4271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wozney JM: The bone morphogenetic protein
family and osteogenesis. Mol Reprod Dev. 32:160–167. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dufourcq P, Descamps B, Tojais NF, et al:
Secreted frizzled-related protein-1 enhances mesenchymal stem cell
function in angiogenesis and contributes to neovessel maturation.
Stem Cells. 26:2991–3001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Karageorgiou V and Kaplan D: Porosity of
3-D biomaterial scaffolds and osteogenesis. Biomaterials.
26:5474–5491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou J, Lin H, Fang T, Li X, Dai W, Uemura
T and Dong J: The repair of large segmental bone defects in the
rabbit with vascularized tissue engineered bone. Biomaterials.
31:1171–1179. 2010. View Article : Google Scholar
|