Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
cancers worldwide and the occurrence rate has increased by 2% per
year for the last six decades (1).
Metastatic RCC has a particularly poor prognosis, with an overall
survival rate of 12 months and a five-year survival rate of <10%
(2). Apart from the effects of
chemical and physical irritants in the environment, the host immune
system is also important in renal carcinogenesis.
Since the cancer immunosurveillance hypothesis
(3) was first proposed, the
suggestion that the immune system can recognize and eliminate tumor
cells has been debated. Several epidemiologic investigations have
indicated that immunocompromised patients are more likely to
develop cancer, whether it is of viral or non-viral origin, which
supports the cancer immunosurveillance hypothesis (4). In certain types of cancer, an
increase in the number of regulatory T cells (Treg) advances the
progression of cancer by interfering with immune surveillance
(5) and exhibiting cancer
tolerance (6). However, in
specific types of cancer, including colorectal carcinoma, Treg
cells have been observed to suppress bacteria-driven inflammation,
which promotes carcinogenesis, and thus benefits the host (7).
CD4+ T cells are the most important
component of the adaptive immune system and have an important
effect in host defense against infection and the maintenance of
immune homeostasis (6,8). CD4+ T cells differentiate
into a number of effector subsets, including T helper (Th)1, Th2,
Th17 and Treg cells (9). The
differentiation of Th1, Th2, Th17 and Treg cells is mediated mainly
by T box transcription factor T-bet, GATA binding protein 3
(GATA3), RAR-related orphan receptor (ROR)γt and forkhead box P3
(Foxp3), respectively (9). Th1 and
Th17 are involved in the cytotoxic response, while Th2 and Treg
mainly suppress the Th1 and Th17-mediated immune response (9). There is a precise dynamic balance in
healthy individuals; however, if this balance is lost, the human
body becomes vulnerable to infection, autoimmune diseases and tumor
growth (10). Enhanced Th2
response accompanied by a decease in Th1 response has been observed
in patients with bladder carcinoma (11) and, in oral cancer, the ratio of
Th17/Treg cells has been observed to increase in the early stages
and decrease in later stages (12).
The Th1/Th2/Th17/Treg-balance in patients with RCC
remains to be fully elucidated. Therefore, in the present study,
the percentages of Th1, Th2, Th17 and Treg cells were assessed in
the peripheral blood of patients with RCC, which was categorized by
tumor grade and stage. In addition, infiltrating Treg cells at
tumor sites were examined.
Materials and methods
Patients and healthy controls
The present study included patients undergoing
nephrectomy for non-metastatic RCC between May 2012 and December
2012 at Zhongshan Hospital, Fudan University (Shanghai, China;
Table I). The mean age of the
patients with RCC and the healthy volunteers was 54.69±13.77 and
48.18±1.78 years, respectively (mean ± standard error of the mean
(SEM); P>0.05). Individuals with additional neoplasms,
autoimmune diseases, lymphatic or lympho-proliferative disorders
were excluded from the study, as were those treated with
chemotherapy or immunotherapy prior to surgery. Pre-operative
clinical evaluations included a chest X-ray and an abdominal
computerized tomography scan. Nephrectomy was performed by an open
extraperitoneal/intraperitoneal approach or by laparoscopy, without
pre-operative embolization. Peripheral blood (two 5-ml EDTA vials)
was obtained from each patient 12 h pre-operatively and from 36
healthy volunteers. Following kidney removal, samples were taken
from the neoplasm for immunohistochemical analysis. Approval of the
present study was obtained from Zhongshan Hospital’s Ethics
Committee (Fudan University, Shanghai, China) and all patients and
healthy volunteers provided prior written informed consent.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | RCC patients
(n) | Healthy volunteers
(n) | P-value |
|---|
| Gender |
| Male | 93 | 21 | >0.05 |
| Female | 38 | 15 | >0.05 |
| Fuhrman grade |
| I | 20 | - | - |
| II | 86 | - | - |
| III | 25 | - | - |
| IV | 0 | | |
| TNM stage |
| I | 65 | - | - |
| II | 4 | - | - |
| III | 59 | - | - |
| IVw | 3 | - | - |
| Pathology |
| Clear cell | 114 | - | - |
| Cystic
papillary | 12 | - | - |
| Chromophobe | 5 | - | - |
Pathological assessment
All tumor specimens were analyzed by two independent
pathologists who were experienced in the diagnosis of RCC. The
Heidelberg classification (13)
was used to assign a histological type to each specimen. RCC was
classified according to the 2010 American Joined Committee on
Cancer tumor, nodes and metastasis (TNM) staging systems (14) and the Fuhrman nuclear grading
system (15).
Isolation of peripheral blood mononuclear
cells (PBMCs)
The peripheral blood, in heparinized tubes, was
diluted 1:2 with fresh sterile phosphate-buffered saline (PBS). The
PBMCs were then isolated by density gradient centrifugation (611 ×
g; 25 min) using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO,
USA). The viability of the cells was determined using a trypan blue
dye exclusion test (Sigma-Aldrich).
Surface-intracellular staining and flow
cytometry
The following anti-human monoclonal antibodies were
used: Fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD4
(clone OKT4), FITC-conjugated mouse anti-CD8 (clone HIT8a),
allophycocyanin (APC)-conjugated mouse anti-CD25 (clone BC96),
APC-conjugated rat anti-RORγt (clone AFKJS-9), phycoerythrin
(PE)-conjugated mouse anti-CD45RA (clone HI100), PE-conjugated
mouse anti-T-bet (clone eBio4B10), peridinin chlorophyll protein
(PerCP)-Cy5.5-conjugated mouse anti-CD127 (clone eBioRDR5) and
PerCP-eFluor-conjugated mouse anti-GATA-3 (clone TWAJ). All
antibodies were obtained from eBioscience (San Diego, CA, USA).
Cells without antibody treatment were used as negative controls.
Briefly, the cells were stained with surface markers, fixed and
permeabilized with fixation buffer [Dulbecco’s phosphate-buffered
saline (pH 7.4) with 4% w/v paraformaldehyde (0.22-μm
pore-filtered)]/permeabilization buffer [PBS, 1% fetal calf serum,
0.1% sodium azide, 0.1% saponin (0.2-μm pore-filtered; pH7.4–7.6);
eBioscience] and then stained with anti-T-bet, anti-RORγt and
anti-GATA-3. Cells (~3×105) were loaded into a BD
FACSAria II flow cytometer and data were analyzed using FACSDiva
software (v6.1.3; BD Biosciences, Franklin Lakes, NJ, USA). The
CD4+ T-bet+, CD4+
GATA-3+ and CD4+ RORγt+ cells were
referred to as Th1, Th2 and Th17 cells, respectively. The
CD4+CD25hi CD127lo
CD45RA− cells were referred to as activated Treg and the
CD4+ CD25hi CD127lo
CD45RA+ cells as naïve Treg cells.
Immunohistochemical staining
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded kidney tissue sections. Human
tonsil tissue, provided as formalin/paraformaldehyde-fixed
paraffin-embedded tonsil tissue sections by eBioscience, was used
as a positive control, while several negative controls were
included using renal tissue and omitting certain steps (use of the
primary and secondary antibodies) of the staining technique. Using
a modified avidin-biotin-peroxidase complex (ABC) method according
to Hsu et al (16), the
samples were stained overnight with monoclonal rat anti-human Foxp3
primary antibodies (1:150; eBioscience) and biotinylated rabbit
anti-rat immunoglobulin G secondary antibodies (Abcam, Cambridge,
UK). Detection was achieved using ABC-peroxidase solution following
peroxidase inhibition and the applied chromogen was
diaminobenzidine. The cell nuclei were counterstained using
hematoxylin. Non-specific background staining was reduced using
DAKO blocking solution (DAKO, Carpinteria, CA, USA) according to
the manufacturer’s instructions. The Foxp3+ cells were
examined (magnification, ×400; TH4-200; Olympus, Tokyo, Japan) in
20 fields of tumor areas and scored semi-quantitatively.
Statistical analysis
The results obtained from the T-cell subsets are
expressed as the mean ± SEM. At the baseline, the proportion gender
ratio was compared using a χ2 test of independence and
the mean age and serum creatinine levels were compared using
two-tailed Student’s t-tests. All other variables are presented
descriptively. Statistical analysis of the T-cell subsets was
performed using a two-tailed independent t-test between two groups
and one-way analysis of variance among more than three groups using
SPSS 18.0 software (International Business Machines, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
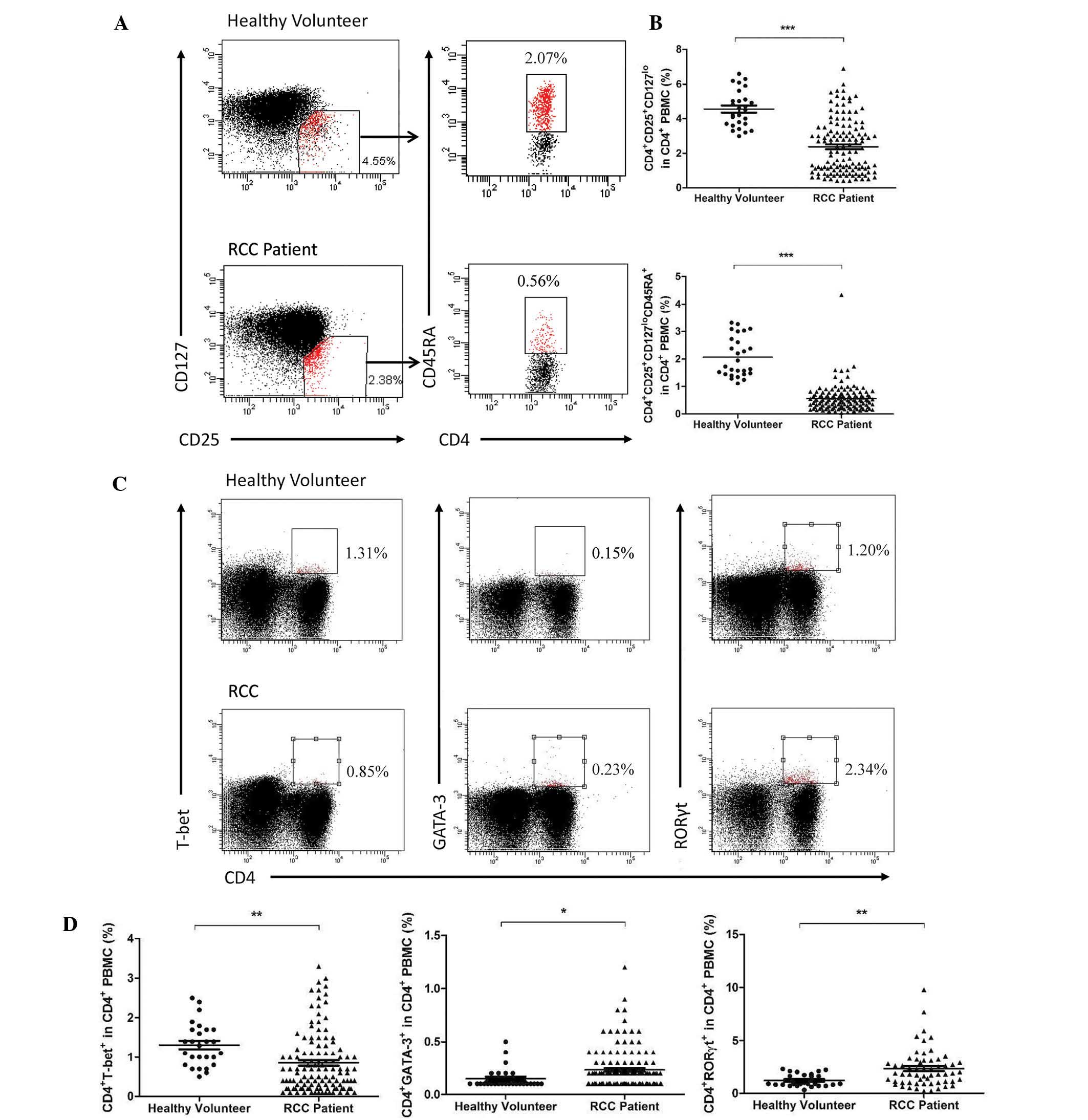
Proportion of Th1, Th2, Th17 and Treg
cells in the CD4+ T cells in PBMCs
To determine the overall changes in CD4+
T cells and their subsets in the PBMCs, peripheral blood
lymphocytes from patients with RCC and healthy volunteers were
stained using the above-mentioned antibodies. A significant
decrease was observed in the activated Treg and naïve Treg
(P<0.0001) as well as the Th1 (P=0.004) cells (Fig. 1A–C), while the Th17 (P=0.0022) and
Th2 (P=0.0317) cells increased compared with those in the healthy
volunteers (Fig. 1C and D).
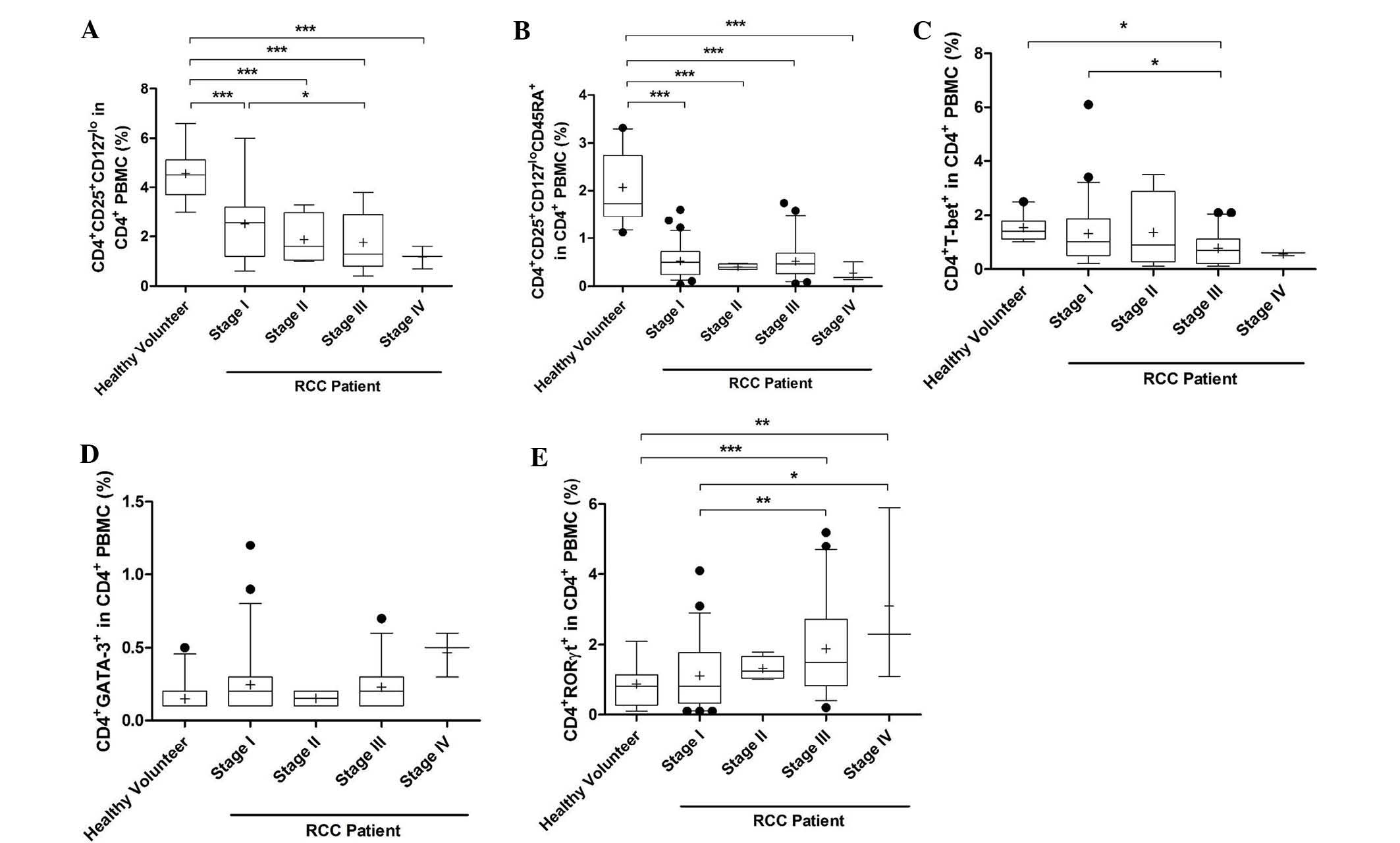
Proportion of Th1, Th2, Th17 and Treg
cells in patients with different stages of RCC
The percentage of activated Treg cells was
significantly decreased in PMBCs of stage I-IV RCC patients
compared with that in healthy volunteers (P<0.0001). In
addition, the percentage of activated Treg cells in PMBCs of stage
III RCC patients was lower than that in stage I patients
(P<0.05; Fig. 2A). The
percentage of naïve Treg cells in PMBCs of patients with stage I-IV
RCC was also decreased (P<0.0001, Fig. 2B). The percentage of Th1 cells in
PMBCs of stage III patients was significantly decreased compared
with that in stage I patients and healthy volunteers (P<0.05;
Fig. 2C). However, the percentage
of Th2 cells was marginally increased in stage I-IV RCC patients
compared with that in healthy volunteers, but no significant
differences were observed (Fig.
2D). The percentage of Th17 cells in stage III and IV patients
was markedly increased compared with that in healthy volunteers
(stage III, P<0.0001; stage IV, P=0.0001; Fig. 2E). In addition, the proportion of
Th17 cells in stage III and IV patients was higher than that in
stage I patients (stage III, P=0.008; stage IV, P=0.002).
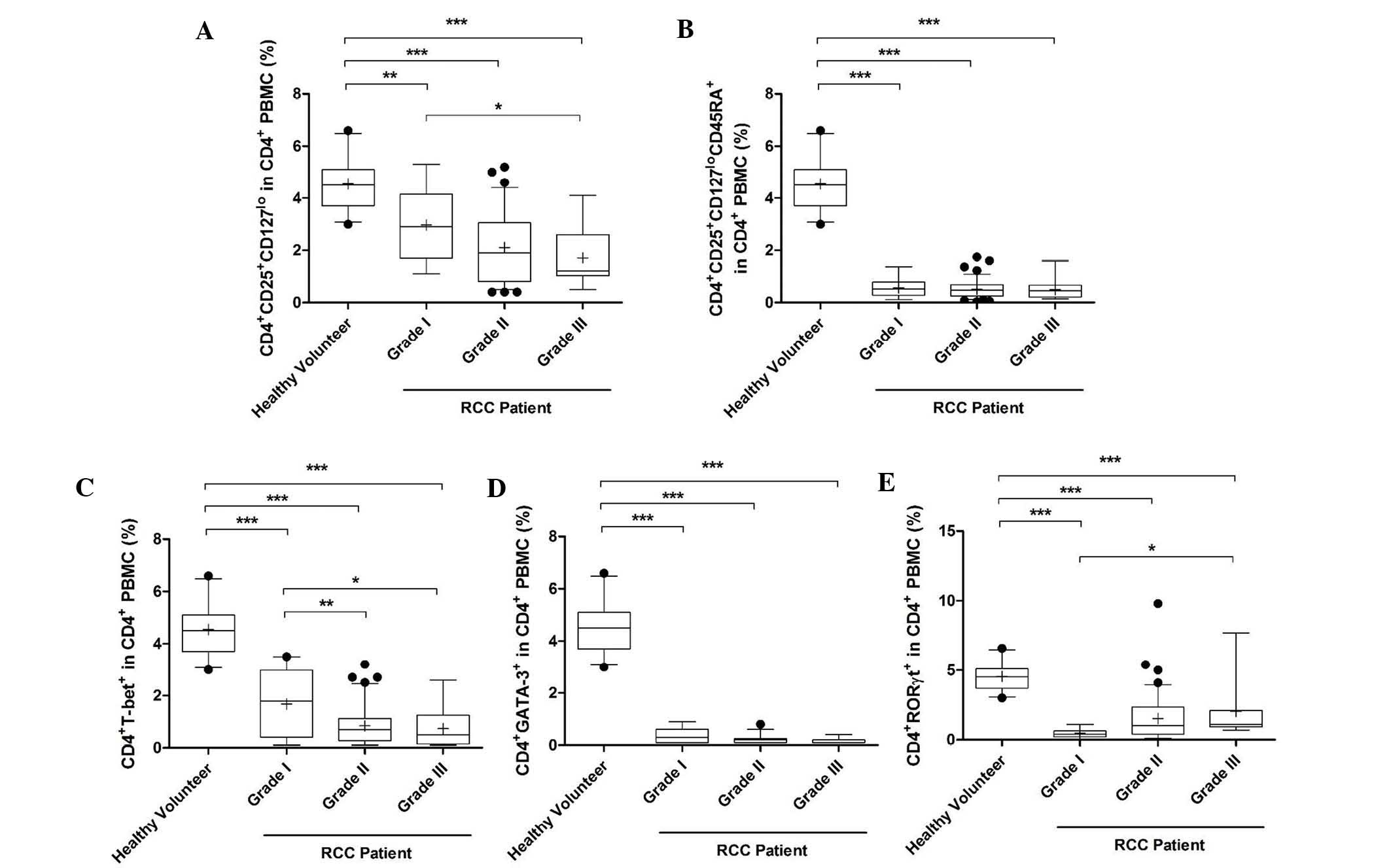
Proportion of Th1, Th2, Th17 and Treg
cells in different grades of RCC
The proportion of activated Treg cells was reduced
significantly in PMBCs of grade I-III RCC patients compared with
that in healthy volunteers (grade II and III, P<0.0001; grade I,
P<0.05). Furthermore, this proportion was reduced as the grade
increased and the proportion in grade III patients was
significantly lower than that in grade I patients (Fig. 3A). Similarly, the percentage of
naïve Treg and Th1 cells in grades I-III was also decreased
(P<0.0001; Fig. 3B and C). In
addition, the percentage of Th1 cells in grades II and III was
significantly lower than that in grade I (P<0.01 and P<0.05,
respectively; Fig. 3C). However,
the percentage of Th2 and Th17 cells in grades I-III was increased
significantly compared with that in healthy volunteers
(P<0.0001; Fig. 3D and E). The
percentage of Th17 cells was also increased in grade III patients
compared with that in grade I patients (Fig. 3E).
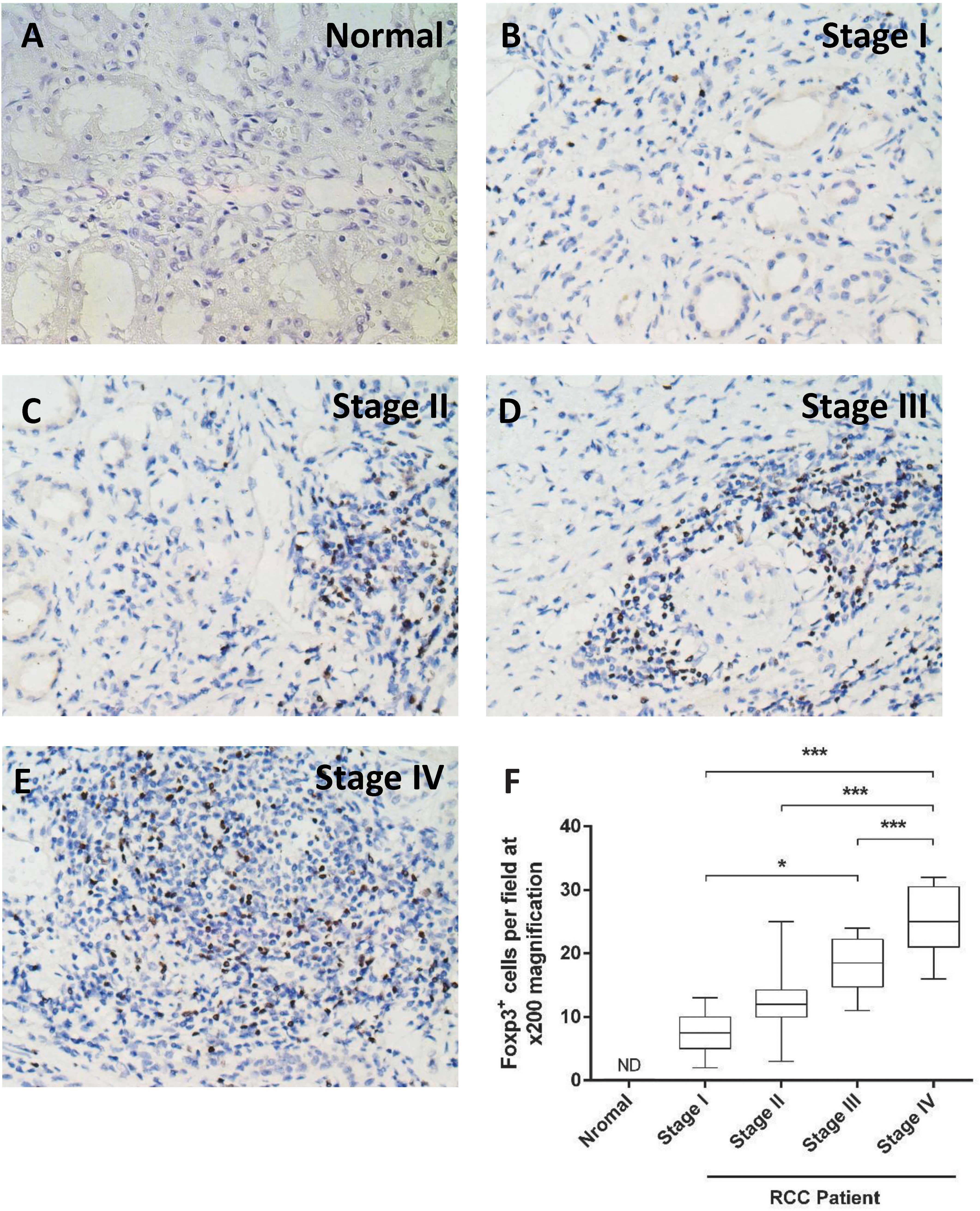
Foxp3 immunostaining in tumors at
different stages
In order to determine tumor infiltration by Treg,
Foxp3 immunostaining was performed (Fig. 4A–E). The number of
Foxp3+ cells in the tumor samples increased from stage I
to stage IV. Furthermore, the number of Foxp3+ cells was
significantly higher in stage IV compared with that in stages I-III
(P<0.0001). The number was also significantly higher in stage
III compared with that in stage I (P=0.002; Fig. 4F).
Discussion
The change in CD4+ T cells is
representative of the immune status and is important for
tumorigenesis and in maintaining homeostasis (12). In the present study, a shift from
Th1 toward Th2 cells was observed in patients with RCC, which
reflected a skewed balance between the Th1 and Th2 profiles. The
cytokines produced by Th1 and Th2 cell subsets are important for
the anti-tumor immune function (11). Th1 cells produce type 1 cytokines,
including interleukin (IL)-2 and interferon-γ, which exert potent
anti-tumor effects and activate cytotoxic lymphocytes (CTL) and
natural killer (NK) cell-mediated cytolytic function, associated
with effective anti-tumor defense mechanisms (17). Type 2 cytokines, including IL-4 and
IL-10, downregulate the tumor-specific immune response by directly
suppressing the production of Th1 cytokines, which prevents CTL and
NK cell activation and by inhibiting tumor antigen presentation by
antigen-presenting cells (18,19).
Immune dysfunction in tumor patients includes a skew from a Th1 to
a Th2 response, which impairs T-cell immunity towards the tumor
(18,20).
In the present study, the skewed proportion of
Treg/Th17 cells was associated with the severity of RCC, which was
categorized by its stage and grade. This observation was similar to
that reported by certain previous studies. In breast cancer, the
complete response of breast carcinoma to neoadjuvant chemotherapy
was associated with a disappearance of tumor-infiltrating
Foxp3+ Treg cells (21). However, controversy remains in
other studies. Wang et al (22) demonstrated an increase in the
percentage of Th17 and Treg cells in the circulation of patients
with colorectal adenoma and carcinoma. In addition, the percentage
of Th17 cells in the circulation increased during early stages;
however, Treg cells increased only in advanced stages (23). A possible reason for these
differences in results in the circulation may be due to a small
sample size (<40 patients in each tumor group). However, rather
than the absolute Treg number, its localization pattern has been
associated with cancer prognosis (23). Liotta et al (24) examined 30 patients with RCC and
found that the frequency of Treg cells was significantly higher in
tumor-infiltrating lymphocytes than in the peripheral blood,
whereas no significant difference was observed in the peripheral
blood between healthy volunteers and patients.
The present study revealed a reduction in activated
and naïve Treg cells in the peripheral blood, which may be due to
the majority of Treg cells being recruited into the tumors.
Similarly, the infiltration of Th17 cells which possess an
anti-tumor function may be inhibited by tumors; therefore, the
proportion of these cells in the PBMCs was increased. It has been
demonstrated that tumor cells secrete certain chemokines to recruit
Treg cells in order to suppress the attack of cytotoxic T cells in
the tumor microenvironment. A study of 31 patients with colon
adenocarcinoma revealed that CCR4+ CTLA4hi
Treg cells accumulated in the tumors. However, there were decreases
in the frequencies of activated conventional Th1 cells due to a
decrease in the Th1-associated chemokine receptor CXCR3 in the
tumors (25). In addition, Chen
et al (26) demonstrated
that the CCL20-CCR6 axis mediated the migration of circulating Treg
cells into the tumor microenvironment, which resulted in tumor
progression and poor prognosis patients with hepatocellular
carcinoma. In the present study, infiltrating Treg cells were also
detected using Foxp3 immunostaining. The number of infiltrating
Treg cells increased as the tumor stage increased, which suggested
that the circulating Tregs were recruited into the tumor and
impaired host immunity.
The role of Th17 cells in tumor immunology can be
dichotomous. Th17 cells appear to be important in tumorigenesis and
in the eradication of an established tumor. A potential protective
effect of Th17 has been demonstrated in types of cancer affecting
the mucosal tissues, including gut, lung and skin cancer (27,28).
In addition, an increase in the number of Th17 cells was observed
in the peripheral blood, tumor microenvironment and tumor-draining
lymph nodes of several different types of human and mouse tumor
(29), including ovarian cancer
(30). A previous study on
prostate cancer demonstrated that the infiltration of Th17 cells
into the tumor was inversely correlated with the Gleason score
(31). This implies that Th17
cells mediate an anti-tumor effect in the development of prostate
cancer. In the present study, a decrease in the proportion of Treg
cells in the peripheral blood was associated with increased RCC
grade and a reverse correlation was observed in Th17 cells. These
results suggested that increases in Treg cell recruitment with
reduced Th17 infiltration may affect the prognosis.
Of note, the plasticity of CD4+ T-cell
subsets has been demonstrated. These T helper cells, which were
previously considered to be the terminal differentiation state,
often have the capacity to redirect their functional programs,
including the ability of Treg and Th17 cells to convert into each
other in certain specific microenvironments (9). Therefore, whether the changes in
CD4+ T-cell subsets in RCC patients are the cause of
tumorigenesis or the consequence of tumor development requires
further investigation.
In conclusion, the present study demonstrated that
the skewed immunological balance among Th1, Th2, Th17 and Treg
cells was distinct in healthy volunteers and an impaired balance
was associated with RCC tumor grade and stage. These findings
provide novel data to aid in the understanding of the pathological
immune status in patients with RCC and assist in the modulation of
strategies for potential anti-tumor immunity therapies in these
patients.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (no. 81270832). The authors
would like to thank Dr Yuan Ji at the Department of Pathology,
Zhongshan Hospital, Fudan University for providing assistance with
the immunohistochemical study.
References
|
1
|
Figlin R, Sternberg C and Wood CG: Novel
agents and approaches for advanced renal cell carcinoma. J Urol.
188:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia JA and Rini BI: Recent progress in
the management of advanced renal cell carcinoma. CA Cancer J Clin.
57:112–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn OJ: Human tumor antigens,
immunosurveillance, and cancer vaccines. Immunol Res. 36:73–82.
2006. View Article : Google Scholar
|
|
4
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banerjee A, Vasanthakumar A and
Grigoriadis G: Modulating T regulatory cells in cancer: how close
are we? Immunol Cell Biol. 91:340–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mougiakakos D, Choudhury A, Lladser A,
Kiessling R and Johansson CC: Regulatory T cells in cancer. Adv
Cancer Res. 107:57–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao X: Regulatory T cells and immune
tolerance to tumors. Immunol Res. 46:79–93. 2010. View Article : Google Scholar
|
|
9
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation.
Immunity. 30:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vojdani A: A potential link between
environmental triggers and autoimmunity. Autoimmune Dis.
2014:4372312014.PubMed/NCBI
|
|
11
|
Lang F, Linlin M, Ye T and Yuhai Z:
Alterations of dendritic cell subsets and TH1/TH2 cytokines in the
peripheral circulation of patients with superficial transitional
cell carcinoma of the bladder. J Clin Lab Anal. 26:365–371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaur P, Qadir GA, Upadhyay S, Singh AK,
Shukla NK and Das SN: Skewed immunological balance between Th17
(CD4(+)IL17A (+)) and Treg (CD4
(+)CD25 (+)FOXP3 (+)) cells in
human oral squamous cell carcinoma. Cell Oncol (Dordr). 35:335–343.
2012. View Article : Google Scholar
|
|
13
|
Bedke J1, Pritsch M, Buse S, et al:
Prognostic stratification of localized renal cell carcinoma by
tumor size. J Urol. 180:62–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB, Byrd DR, Compton CC, et al:
Kidney. AJCC Cancer Staging Manual. 7th edition. Springer; New
York, NY: pp. 479–89. 2010
|
|
15
|
Al-Aynati M, Chen V, Salama S, et al:
Interobserver and intraobserver variability using the Fuhrman
grading system for renal cell carcinoma. Arch Pathol Lab Med.
127:593–596. 2003.PubMed/NCBI
|
|
16
|
Hsu SM, Raine L and Fanger H: Use of
avidin-biotin-peroxidase complex (ABC) in immunoperoxidase
techniques: a comparison between ABC and unlabeled antibody (PAP)
procedures. Journal Histochem Cytochem. 29:577–580. 1981.
View Article : Google Scholar
|
|
17
|
Ruffell B, DeNardo DG, Affara NI and
Coussens LM: Lymphocytes in cancer development: polarization
towards pro-tumor immunity. Cytokine Growth Factor Rev. 21:3–10.
2010. View Article : Google Scholar :
|
|
18
|
Agarwal A, Agrawal U, Verma S, Mohanty NK
and Saxena S: Serum Th1 and Th2 cytokine balance in patients of
superficial transitional cell carcinoma of bladder pre- and
post-intravesical combination immunotherapy. Immunopharmacol
Immunotoxicol. 32:348–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herber DL, Nagaraj S, Djeu JY and
Gabrilovich DI: Mechanism and therapeutic reversal of immune
suppression in cancer. Cancer Res. 67:5067–5069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishimura T, Iwakabe K, Sekimoto M, et al:
Distinct role of antigen-specific T helper type 1 (Th1) and Th2
cells in tumor eradication in vivo. J Exp Med. 190:617–627. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ladoire S, Arnould L, Apetoh L, et al:
Pathologic complete response to neoadjuvant chemotherapy of breast
carcinoma is associated with the disappearance of
tumor-infiltrating foxp3+ regulatory T cells. Clin
Cancer Res. 14:2413–2420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Xu K, Wu J, et al: The changes of
Th17 cells and the related cytokines in the progression of human
colorectal cancers. BMC Cancer. 12:4182012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizukami Y, Kono K, Kawaguchi Y, et al:
Localisation pattern of Foxp3+ regulatory T cells is
associated with clinical behaviour in gastric cancer. Br J Cancer.
98:148–153. 2008. View Article : Google Scholar
|
|
24
|
Liotta F, Gacci M, Frosali F, et al:
Frequency of regulatory T cells in peripheral blood and in
tumour-infiltrating lymphocytes correlates with poor prognosis in
renal cell carcinoma. BJU Int. 107:1500–1506. 2011. View Article : Google Scholar
|
|
25
|
Svensson H, Olofsson V, Lundin S, et al:
Accumulation of CCR4(+)CTLA-4
FOXP3(+)CD25(hi) regulatory T cells in colon
adenocarcinomas correlate to reduced activation of conventional T
cells. PloS One. 7:e306952012. View Article : Google Scholar
|
|
26
|
Chen KJ, Lin SZ, Zhou L, et al: Selective
recruitment of regulatory T cell through CCR6-CCL20 in
hepatocellular carcinoma fosters tumor progression and predicts
poor prognosis. PloS One. 6:e246712011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilke CM, Bishop K, Fox D and Zou W:
Deciphering the role of Th17 cells in human disease. Trends
Immunol. 2:603–611. 2011. View Article : Google Scholar
|
|
28
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kryczek I, Wei S, Zou L, et al: Cutting
edge: Th17 and regulatory T cell dynamics and the regulation by
IL-2 in the tumor microenvironment. J Immunol. 178:6730–6733. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human
CD4+ IL-17-producing T cells in ovarian cancer. Proc
Natl Acad Sci USA. 105:15505–15510. 2008. View Article : Google Scholar
|
|
31
|
Sfanos KS, Bruno TC, Maris CH, et al:
Phenotypic analysis of prostate-infiltrating lymphocytes reveals
TH17 and Treg skewing. Clin Cancer Res. 14:3254–3261. 2008.
View Article : Google Scholar : PubMed/NCBI
|