Introduction
The protein kinase casein kinase 2 (CK2) is a highly
conserved serine/threonine kinase with a broad spectrum of
substrates (1). CK2 is a
multifunctional protein kinase and is involved in cell
proliferation and survival (2–4). An
increase in the levels and activity of CK2α has been observed in
several types of human cancer, including ovarian cancer (5,6). CK2
also affects several cell signaling pathways, including those of
phosphoinositide 3-kinase, nuclear factor-κB and Wnt (7–9). A
previous study revealed that Hedgehog (Hh)/glioma-associated
oncogene (Gli) signaling is downregulated following inhibition of
CK2, with a subsequent reduction in the number of stem-like side
population cells in human lung cancer cells (10).
Sonic hedgehog (Shh), a member of the Hh family of
proteins, consists of secreted signaling molecules and has several
functions during vertebrate development (11). Activation of the Hh pathway is
initiated at the cell surface, in which the Hh ligand binds to its
receptor Patched. This leads to the removal of repression of the
effector protein Smoothened (Smo), a G-protein-coupled receptor
(12). Ultimately, Smo activates
the Gli family of transcription factors and its target genes and
Gli1 activates the Hh target genes (13,14).
Deregulation of Hh/Gli signaling is considered to be an initiating
or maintaining factor in the progression of various types of
cancer, including ovarian cancer (13,15).
The Gli1 gene is amplified in human glioma and is activated in
basal cell carcinoma (16,17) and in ovarian cancer stem-like cells
(18). These findings suggest that
investigation of the pharmacological inhibitors of the Shh pathway
may be beneficial in the treatment and/or prevention of ovarian
cancer.
Apigenin is chemically known as
4′,5,7,-trihydroxyflavone and has the molecular formula
C15H10O5, with a molecular weight
of 270 g/mol. Apigenin has been observed to have marked effects in
inhibiting cancer cell growth in cell culture systems and in in
vivo tumor models (19,20).
Apigenin has also been demonstrated to inhibit tumor cell invasion
and metastasis (21) and suppress
mitogen-activated protein kinases and downstream oncogene
expression (22). These findings
suggest that apigenin possesses significant preventative effects
against cancer. In addition, a previous study by our group
demonstrated that apigenin affected the number of glioma stem-like
cells derived from U251 cells (23), implying the potential of apigenin
to interfere with cancer stem cells. However, whether apigenin
affects the self-renewal capacity of ovarian cancer stem-like cells
(OCSLCs) and its underlying mechanisms of action remain to be
elucidated.
The present study investigated the self-renewal
capacity, a main feature for identifying OCSLCs, of sphere-forming
cells (SFCs) of the human ovarian cancer cell line SKOV3. The
effect of apigenin on the self-renewal capacity of SKOV3-derived
SFCs and the involvement of CK2α and the Hedgehog signaling pathway
in this process were studied. The results provide important
evidence for the potential benefits of CK2 inhibitors, including
apigenin, in the treatment of ovarian cancer.
Materials and methods
Reagents
Apigenin was obtained from Sigma-Aldrich (St. Louis,
MO, USA) and was dissolved in dimethyl sulfoxide to a final
concentration of 0.1% in media without causing cytotoxicity. The
following reagents were purchased: anti-CK2α (Millipore, Billerica,
MA, USA), anti-Gli1 (Cell Signaling Technology, Inc., Beverly, MA,
USA), anti-β-actin (Sigma-Aldrich), Dulbecco’s modified Eagle’s
medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) and
fetal bovine serum (Invitrogen Life Technologies). All other
chemicals were obtained from Sigma-Aldrich.
Cell culture and tumorsphere formation
assay
The human ovarian cancer SKOV3 cells (American Type
Culture Collection, Manassas, VA, USA) were maintained in DMEM
supplemented with 10% fetal bovine serum, 4 mM glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin and incubated at 37°C in a
humidified atmosphere of 5% CO2.
For the tumorsphere assay, single-cell suspensions
were prepared at a density of 5,000 cells/ml in condition-medium
comprising serum-free DMEM/F12 supplemented with 100 IU/ml
penicillin, 100 μg/ml streptomycin, 20 ng/ml human recombinant
epidermal growth factor, 10 ng/ml human recombinant basic
fibroblast growth factor, 2% B27 supplement without vitamin A and
1% N2 supplement (Invitrogen Life Technologies), which were then
seeded into ultra low attachment six-well plates (Corning Inc.,
Corning, NY, USA) at a density of 3,000 cells/ml. The suspension
cultures were continued for six days until the formation of tumor
spheres. To propagate the spheres in vitro, the sphere cells
were collected by centrifugation (300xg, 6 min, 4°C), dissociated
into single-cell suspensions and cultured to enable the
regeneration of spheres. Third generation spheres were used for all
subsequent experiments.
To investigate the percentage of single cells able
to regenerate new spheres, the cells were seeded at a density of
1,000 cells/ml in a six-well plate to obtain new spheres. The total
number of tumor spheres was counted after six days of culture. The
efficiency of sphere formation was calculated using the following
formula: Total number of spheres formed/total number of live cells
seeded × 100.
Small interfering (si)RNA and plasmid DNA
transfection
The CK2α-specific siRNA and control RNA were
purchased from Thermo Fisher Scientific (Waltham, MA, USA). In
brief, the cells were seeded into six-well plates at 105
cells/well one day prior to transfection, with a target of 30–50%
confluency at the time of transfection. The cells were transfected
with 50 nmol/l siRNA using Lipofectamine RNAiMAX (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
Adequate inhibition of the siRNA-mediated-knockdown was confirmed
using western blot analysis. The pcDNA3.1-CK2α or control
pcDNA3.1-LacZ plasmid vectors were then transfected into the SKOV3
cells or SKOV3-derived SFCs (0.5 μg/ml in a 24-well plate) using
Lipofectamine 2000 transfection reagent (Invitrogen Life
Technologies), according to the manufacturer’s instructions. The
cells were harvested for western blot analysis and tumorsphere
formation assay.
Western blot analysis
The cells were lysed in buffer containing 50 mM
Tris-HCl (pH 7.5), 137 mM NaCl, 1% (w/v) SDS, 0.5 mM
phenylmethanesulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml
aprotinin and 1 mM dithiothreitol. The cell lysate, containing 50
μg protein, was separated by 12.5% SDS-PAGE and then blotted onto
polyvinylidene difluoride membranes (Millipore). The murine
monoclonal anti-CK2α immunoglobulin (Ig)G at 1:2,000 dilution
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA), murine
monoclonal anti-Gli1 IgG at 1:2,000 dilution (Santa Cruz
Biotechnology, Inc.) and monoclonal anti-β-actin at 1:1,000
dilution (Sigma-Aldrich) antibodies were used as primary
antibodies. Signals were detected using the enhanced
chemiluminescence (ECL plus) detection kit (Amersham Pharmacia
Biotech, Piscataway, NJ, USA). Images were scanned by transmission
scanner (E85 Plus; Unisplendour Corp., Ltd, Beijing, China)
followed by densitometric analysis using Alphazmager 2200 software
(Silk Scientific, Orem, UT, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
for triplicate experiments and were analyzed using Student’s t-test
(SPSS software, version 15.0 for Windows; SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
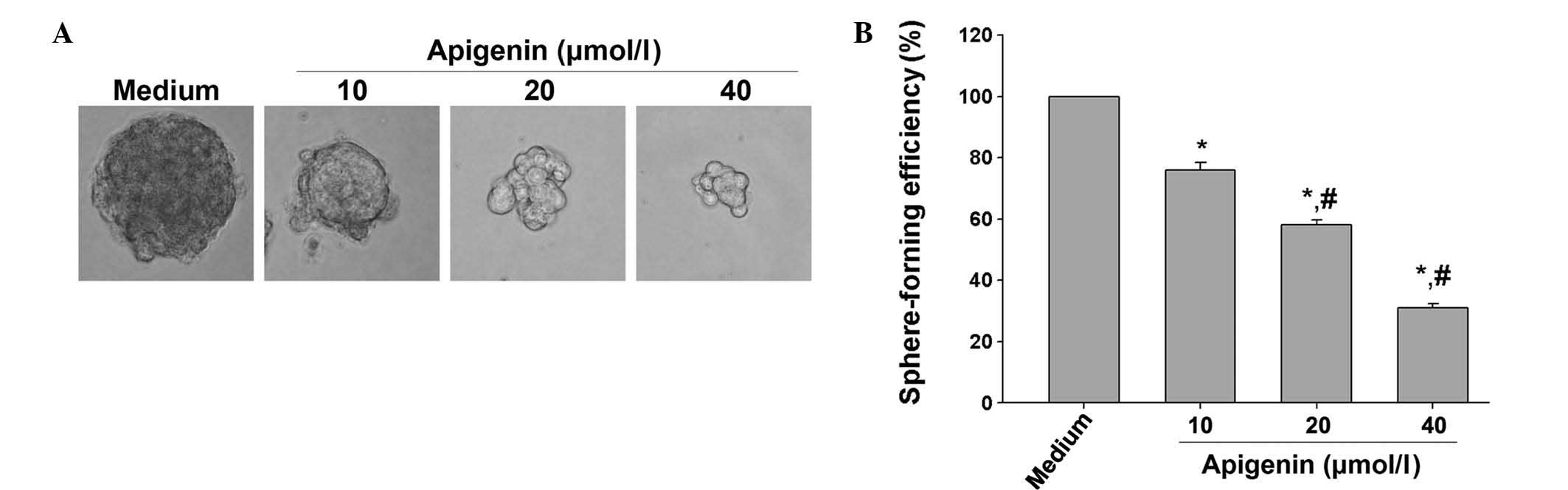
Apigenin inhibits the self-renewal of
SKOV3-derived SFCs
Self-renewal capacity is one of the main
characteristics of cancer stem cells (CSCs) (24). Therefore, the present study
examined whether apigenin inhibited the self-renewal capability of
OCSLCs isolated from the human SKOV3 cell line by measuring sphere
formation. OCSLCs were grown in cancer stem cell condition-medium
in suspension and treated with apigenin. At the end of the
incubation period, images of the spheroids in each well were
captured. The results revealed that apigenin decreased the size of
the spheroids in suspension in a dose-dependent manner (Fig. 1A). The spheroids from each
treatment group were then collected and resuspended to evaluate the
sphere formation efficiency. The results demonstrated that apigenin
inhibited the sphere formation efficiency of the SKOV3-derived SFCs
in a dose-dependent manner (Fig.
1B). These results demonstrated that apigenin effectively
inhibited the self renewal of SKOV3-derived SFCs, suggesting that
apigenin was able to suppress the growth of OCSLCs.
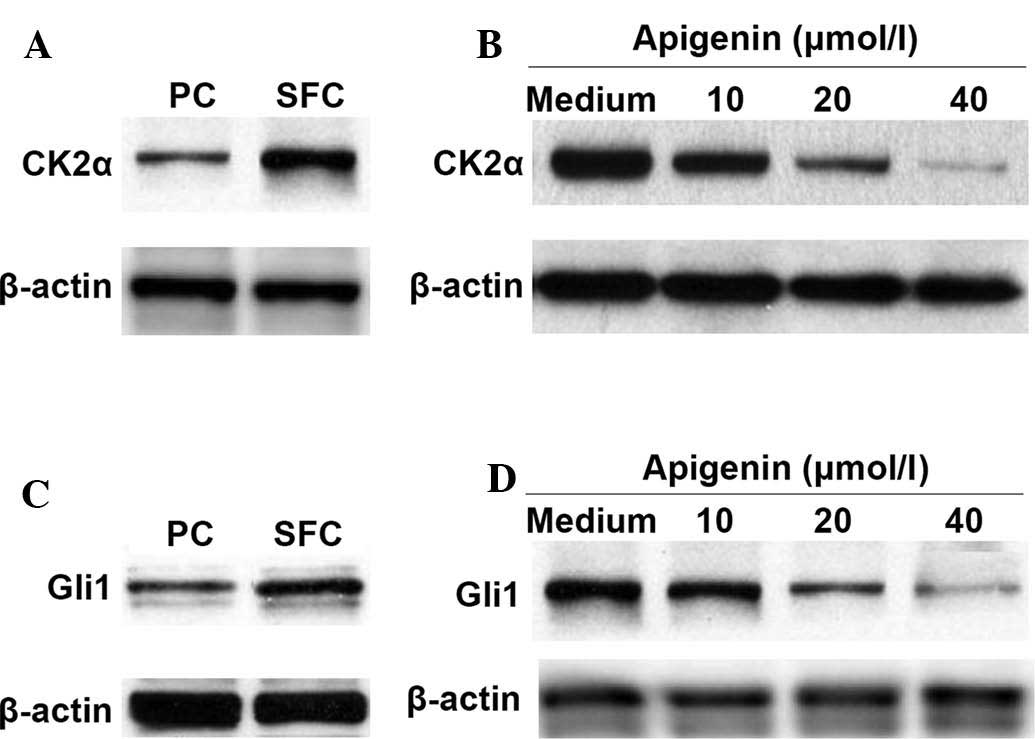
Apigenin downregulates the expression of
CK2α and Gli1 proteins in SKOV3-derived SFCs
Protein kinase CK2 is frequently activated in
various types of human cancer. The activation of CK2α is involved
in the activation of the Hh/Gli1 pathway and is associated with the
stemness maintenance of CSCs (10). The protein expression of CK2α was
then compared between the parental cells and the SKOV3-derived
SFCs. The results demonstrated that the expression of CK2α was
higher in the SKOV3-derived SFCs compared with that in the parental
cells (Fig. 2A). In addition, the
expression of CK2α in the SKOV3-derived SFCs was downregulated by
apigenin (Fig. 2B).
The Hh/Gli signaling pathway is important in the
maintenance of stemness and tumorigenesis (11) and the inhibition of CK2α causes
downregulation in Hh/Gli signaling, reducing human lung cancer cell
stem-like side populations (10).
Therefore, the present study compared the status of Gli1 protein
expression between parental cells and SKOV3-derived SFCs. The
effects of apigenin on the protein expression of Glil in
SKOV3-derived SFCs was also examined. As shown in Fig. 2C and D, elevated Gli1 protein
expression was observed in the SKOV3-derived SFCs and apigenin
reduced this expression in a dose-dependent manner.
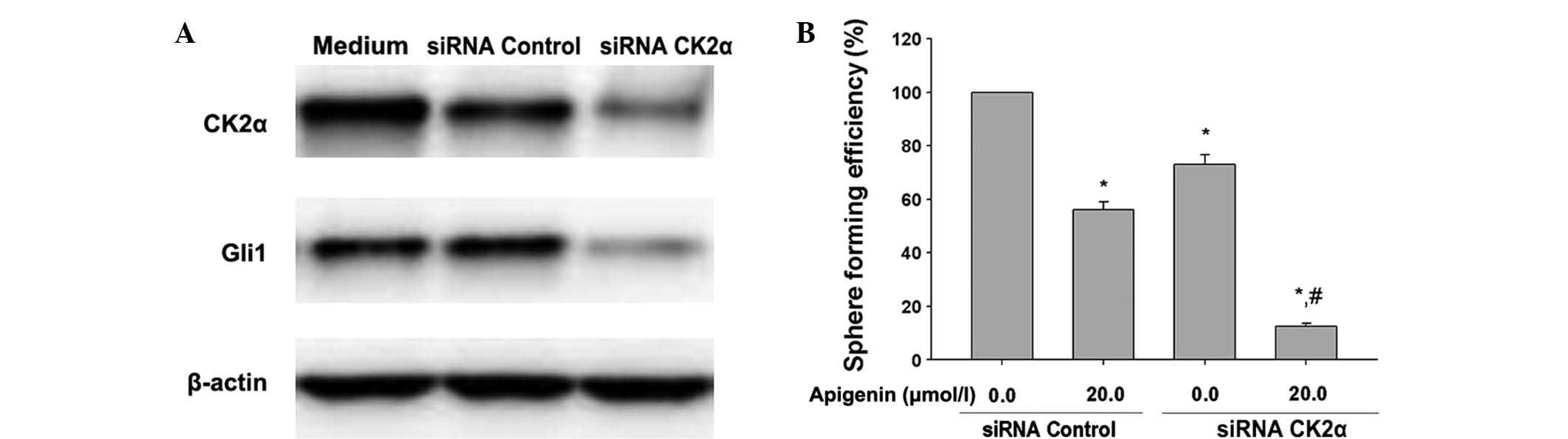
Inhibition of CK2α downregulates Gli1 and
enhances apigenin-inhibited self-renewal of SKOV3-derived SFCs
Since CK2α is highly expressed in CSCs compared with
normal cells (25), the present
study examined whether inhibition of CK2α by siRNA enhanced the
inhibitory effects of apigenin on sphere formation efficiency in
SKOV3-derived SFCs. CK2α siRNA inhibited the protein expression of
CK2α (Fig. 3A), apigenin (20.0
μmol/l) inhibited the sphere formation efficiency in the
SKOV3-derived SFCs transfected with the scrambled siRNA (Fig. 3B) and the inhibition of CK2α by
siRNA further enhanced the inhibitory effects of 20.0 μmol/l
apigenin on the sphere formation efficiency of SKOV3-derived SFCs
(Fig. 3B). These results provided
mechanistic evidence that apigenin-inhibited self-renewal was, in
part, due to the inactivation of CK2α in the SKOV3-derived
SFCs.
To investigate whether CK2 suppression had an effect
on the Hh pathway, the expression of CK2 was silenced using
CK2α-specific siRNAs. The results of western blot analysis
confirmed that silencing of CK2α significantly inhibited the
expression of Gli1 in the SKOV3-derived SFCs (Fig. 3A).
Overexpression of CK2α leads to Gli1
upregulation and attenuates apigenin-inhibited self-renewal in
SKOV3-derived SFCs
To confirm whether CK2α activity affected the
expression of Gli1 and sphere-forming capability of the SKOV3 cells
and its sphere forming capability, SKOV3 cells were transfected
with either a pcDNA3.1-CK2α or a control pcDNA3.1-LacZ plasmid.
Western blot analysis revealed that upregulation of CK2α by
pcDNA3.1-CK2α transfection resulted in overexpression of the CK2α
protein in the SKOV3 cells (Fig.
4A). Elevated Gli1 protein levels were also observed in the
SKOV3 cells that exhibited ectopic overexpression of CK2α (Fig. 4A). In addition, the tumor sphere
formation assay revealed that overexpression of the CK2α protein
increased the sphere formation of SKOV3 cells (Fig. 4B). These findings suggested that
the overexpression of the CK2α gene leads to an upregulation
in the expression of Gli1 and a potentiation in the self-renewal
capacity of SKOV3-derived SFCs.
In addition, the overexpression of CK2α attenuated
the apigenin-induced downregulation of CK2α and Gli1 protein
expression (Fig. 4C) and reduced
its inhibition of sphere formation in the SKOV3-derived SFCs
(Fig. 4D). These results
corroborated that apigenin-inhibited self-renewal is, in part, due
to the inactivation of CK2α and the sequential downregulated
expression levels of Gli1 in SKOV3-derived SFCs.
Discussion
The results of the present study suggested that
apigenin inhibits the self-renewal capacity of SKOV3-derived SFCs
via downregulation of Gli1 expression by the inhibition of CK2α.
This was supported by several lines of evidence. Firstly, apigenin
inhibited the sphere formation efficiency of SKOV3-derived SFCs,
accompanied by a downregulation of the expression of CK2α and Gli1.
Secondly, the inhibition of CK2α by siRNA acted synergistically
with apigenin in downregulating the expression of CK2α and Gli1 as
well as inhibiting the self-renewal capability of SKOV3-derived
SFCs. Finally, the forced overexpression of CK2α resulted in an
increase in the expression levels of CK2α and Gli1 and enhanced the
percentage of sphere formation in the parental SKOV3 cells. This
forced overexpression of CK2α also attenuated the
apigenin-inhibited self-renewal capability of the SKOV3-derived
SFCs.
There is no previous evidence for a correlation
between CK2 and Hh/Gli signaling in human ovarian cancer cells,
although CK2 has been suggested as a positive regulator of the Hh
signal transduction pathway and led to the phosphorylation of two
serine residues in Smo in Drosophila (26). However, several studies have
suggested that Smo in mammals and Drosophila are regulated
by distinct mechanisms (27,28).
Chen et al (29)
demonstrated that mammalian Smo are activated by multi-site
phosphorylation by CK1α and GRK2 and the mechanism for Smo
phosphorylation in mammalian cells was considered to proceed via
two steps. The results of the present study indicated that CK2α
silencing reduced the expression of Gli1 protein in SKOV3-derived
SFCs and the forced overexpression of CK2α resulted in an increase
in the expression of Gli1 protein in parental SKOV3 cells. These
data suggested that CK2α may regulate Gli1 in human ovarian cancer
cells in a similar manner to that present in Drosophila. Jia
et al (26) demonstrated
that Gli1 is directly phosphorylated by CK2 in Drosophila,
preventing the ubiquitination and degradation of Gli1. Although the
mechanism underlying CK2 regulation in types of human cancer
remains to be elucidated, there is evidence suggesting that CK2 is
essential in Wnt/β-catenin signaling (30,31).
Further studies are required to elucidate the precise
mechanisms.
The Hh pathway may be important in the maintenance
of CSCs; however, drug targeting of the Hh pathway is limited. CK2
provides an additional target for inhibition of Hh/Gli signaling.
There has been little development of CK2 inhibitors as therapeutic
agents, partially due to the adenosine triphosphate binding pocket
of CK2 differing from other protein kinase agents (32,33).
Only one small molecule CK2 inhibitor, CX-4945, has been used in
clinical trials as a potential anticancer drug. CX-4945 selectively
inhibits CK2 and is a promising oral therapeutic agent for multiple
types of human cancer, which has demonstrated a favorable safety
profile in the initial phase of clinical trials (34). In addition, a synthetic
peptide-based drug, which targets the CK2 phosphoacceptor domain,
termed CIGB-300, has been identified as safe and of clinical
benefit in the initial phase of cervical cancer trials (35).
As a selective CK2 kinase inhibitor, apigenin has
been demonstrated to induce cell death in ovarian cancer cells and
reduce the risk of ovarian cancer. The present study provided the
first evidence, to the best of our knowledge, that apigenin
inhibits the self-renewal capacity of SKOV3-derived SFCs through
downregulation of the expression of Gli1 by inhibiting CK2α. Due to
the emerging importance of Hh/Gli signaling in tumor initiation and
progression, these results provide important evidence for the
potential benefits of CK2 inhibitors, including apigenin.
Acknowledgements
The authors would like to thank Professor Jian-Guo
Cao and Dr Xi-Yun Deng (Medical College, Hunan Normal University,
Changsha, Hunan, China) for their critical input into the
scientific content. The present study was supported by the
Construct Program of the Key Discipline of Basic Medicine in Hunan
Province, the Youth Fund of Hunan Normal University (grant no.
110637) and the Project of Hunan Provincal Natural Science
Foundation (grant no. 13JJ3061).
References
|
1
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2? FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raaf J, Bischoff N, Klopffleisch K, et al:
Interaction between CK2alpha and CK2beta, the subunits of protein
kinase CK2: thermodynamic contributions of key residues on the
CK2alpha surface. Biochemistry. 50:512–522. 2011. View Article : Google Scholar
|
|
3
|
Buchou T, Vernet M, Blond O, et al:
Disruption of the regulatory beta subunit of protein kinase CK2 in
mice leads to a cell-autonomous defect and early embryonic
lethality. Mol Cell Biol. 23:908–915. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad KA, Wang G, Unger G, Slaton J and
Ahmed K: Protein kinase CK2 - a key suppressor of apoptosis. Adv
Enzyme Regul. 48:179–187. 2008. View Article : Google Scholar
|
|
5
|
Piazza FA, Ruzzene M, Gurrieri C, et al:
Multiple myeloma cell survival relies on high activity of protein
kinase CK2. Blood. 108:1698–1707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prudent R, Moucadel V, Nguyen CH, et al:
Antitumor activity of pyridocarbazole and benzopyridoindole
derivatives that inhibit protein kinase CK2. Cancer Res.
70:9865–9874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dominguez I, Sonenshein GE and Seldin DC:
Protein kinase CK2 in health and disease: CK2 and its role in Wnt
and NF-kappaB signaling: linking development and cancer. Cell Mol
Life Sci. 66:1850–1857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duncan JS and Litchfield DW: Too much of a
good thing: the role of protein kinase CK2 in tumorigenesis and
prospects for therapeutic inhibition of CK2. Biochim Biophys Acta.
1784:33–47. 2008. View Article : Google Scholar
|
|
9
|
Guerra B: Protein kinase CK2 subunits are
positive regulators of AKT kinase. Int J Oncol. 28:685–693.
2006.PubMed/NCBI
|
|
10
|
Zhang S, Wang Y, Mao JH, et al: Inhibition
of CK2α down-regulates Hedgehog/Gli signaling leading to a
reduction of a stem-like side population in human lung cancer
cells. PLoS One. 7:e389962012. View Article : Google Scholar
|
|
11
|
Varjosalo M and Taipale J: Hedgehog:
functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ingham PW, Nakano Y and Seger C:
Mechanisms and functions of Hedgehog signalling across the metazoa.
Nat Rev Genet. 12:393–406. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ng JM and Curran T: The Hedgehog’s tale:
developing strategies for targeting cancer. Nat Rev Cancer.
11:493–501. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nature Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
15
|
Ruiz i Altaba A: Therapeutic inhibition of
Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell
targets? Cancer Cell. 14:281–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinzler KW, Bigner SH, Bigner DD, et al:
Identification of an amplified, highly expressed gene in a human
glioma. Science. 236:70–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Epstein EH: Basal cell carcinomas: attack
of the hedgehog. Nature Rev Cancer. 8:743–754. 2008. View Article : Google Scholar
|
|
18
|
Ciucci A, De Stefano I, Vellone VG, et al:
Expression of the glioma-associated oncogene homolog 1 (gli1) in
advanced serous ovarian cancer is associated with unfavorable
overall survival. PloS One. 8:e601452013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shukla S and Gupta S: Suppression of
constitutive and tumor necrosis factor alpha-induced nuclear factor
(NF)-kappaB activation and induction of apoptosis by apigenin in
human prostate carcinoma PC-3 cells: correlation with
down-regulation of NF-kappaB-responsive genes. Clin Cancer Res.
10:3169–3178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen D, Landis-Piwowar KR, Chen MS and Dou
QP: Inhibition of proteasome activity by the dietary flavonoid
apigenin is associated with growth inhibition in cultured breast
cancer cells and xenografts. Breast Cancer Res. 9:R802007.
View Article : Google Scholar
|
|
22
|
Way TD, Kao MC and Lin JK: Degradation of
HER2/neu by apigenin induces apoptosis through cytochrome c release
and caspase-3 activation in HER2/neu-overexpressing breast cancer
cells. FEBS Lett. 579:145–152. 2005. View Article : Google Scholar
|
|
23
|
Feng X, Zhou Q, Liu C and Tao ML: Drug
screening study using glioma stem-like cells. Mol Med Rep.
6:1117–1120. 2012.PubMed/NCBI
|
|
24
|
Castellanos JA, Merchant NB and
Nagathihalli NS: Emerging targets in pancreatic cancer:
epithelial-mesenchymal transition and cancer stem cells. Onco
Targets Ther. 6:1261–1267. 2013.PubMed/NCBI
|
|
25
|
Bae KM, Su Z, Frye C, et al: Expression of
pluripotent stem cell reprogramming factors by prostate tumor
initiating cells. J Urol. 183:2045–2053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia H, Liu Y, Xia R, et al: Casein kinase
2 promotes Hedgehog signaling by regulating both smoothened and
Cubitus interruptus. J Biol Chem. 285:37218–37226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varjosalo M, Li SP and Taipale J:
Divergence of hedgehog signal transduction mechanism between
Drosophila and mammals. Dev Cell. 10:177–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huangfu D and Anderson KV: Signaling from
Smo to Ci/Gli: conservation and divergence of Hedgehog pathways
from Drosophila to vertebrates. Development. 133:3–14. 2006.
View Article : Google Scholar
|
|
29
|
Chen Y, Sasai N, Ma G, et al: Sonic
Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required
for ciliary accumulation and activation of smoothened. PLoS Biol.
9:e10010832011. View Article : Google Scholar
|
|
30
|
Sarno S and Pinna LA: Protein kinase CK2
as a druggable target. Mol Biosyst. 4:889–894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y and Wang HY: Casein kinase 2 is
activated and essential for Wnt/beta-catenin signaling. J Biol
Chem. 281:18394–18400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cozza G, Meggio F and Moro S: The dark
side of protein kinase CK2 inhibition. Curr Med Chem. 18:2867–2884.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cozza G, Bortolato A and Moro S: How
druggable is protein kinase CK2? Med Res Rev. 30:419–462. 2010.
View Article : Google Scholar
|
|
34
|
Pierre F, Chua PC, O’Brien SE, et al:
Pre-clinical characterization of CX-4945, a potent and selective
small molecule inhibitor of CK2 for the treatment of cancer. Mol
Cell Biochem. 356:37–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perea SE, Baladron I, Garcia Y, et al:
CIGB-300, a synthetic peptide-based drug that targets the CK2
phosphoaceptor domain. Translational and clinical research. Mol
Cell Biochem. 356:45–50. 2011. View Article : Google Scholar : PubMed/NCBI
|