Introduction
The transcription factor Stat1 is established as an
important antiviral agent, acting via IFN-associated intracellular
signaling, but evidence has indicated that Stat1 also serves an
antioncogenic role. This occurs in part via the upregulation of
caspases (1,2) and cyclin-dependent kinase inhibitor
1A (3), by the IFN-regulatory
factor 1 (IRF1)/p53 pathway (4)
and by the downregulation of Bcl-2 family members (5). By contrast, a number of studies have
indicated that in certain cellular contexts, the IFN/Stat1 pathway
may facilitate tumor cell growth (6,7). One
study reported that resistance to ionizing radiation and IFNs is
associated with constitutive overactivity of the IFN/Stat1 pathway
in radioresistant tumor cells (6).
Previous studies have also demonstrated that constitutive
overexpression of Stat1 is positively correlated with the
protection of tumor cells from genotoxic stress evoked by treatment
with doxorubicin (8) or cisplatin
(9). In addition, since
overactivity of the IFN/Stat1 pathway is associated with poor
prognosis in various types of cancer, IFN-associated genes have
been suggested as predictive markers for patients with breast
cancer that is resistant to adjuvant chemotherapy (7).
Histone deacetylases (HDACs) are essential for the
regulation of the acetylation state of histones, and thus are
required for the maintenance and function of chromatin (10). Previous studies suggested that
HDACs regulate the acetylation state of various non-histone targets
(11–13). In particular, HDAC4 (a class IIa
HDAC) has been recognized as a notable enzyme, due to its
involvement in multiple biological processes (12). One study demonstrated that HDAC4
binds to HIF-1α, in addition to identifying that HDAC4 suppression
with siRNA augments HIF-1α acetylation. This leads to
destabilization of HIF-1α and downregulation of HIF-1α-targeted
gene transcription (11). Another
study indicated that HDAC4 directly interacts with and reduces
levels of acetylation of FOXO1, leading to the upregulation of
FOXO1 transcriptional activity (12). In addition, previous studies
demonstrated the ability of HDAC inhibition or suppression as a
method to activate Stat3 via acetylation (14,15).
However, in ovarian cancer cells resistant to cisplatin, HDAC4
emerges as an activator of Stat1 (13).
Cancer cells commonly acquire anticancer drug
resistance during the administration of chemotherapy. To explore
one mechanism by which cancer cells develop this resistance, the
features of human A549 lung cancer cells resistant to etoposide
were investigated. In the current study, Stat1 and HDAC4 were
demonstrated to be upregulated and involved in etoposide resistance
in A549 cells through P-glycoprotein (P-gp), which is encoded by
the multidrug resistance 1 (MDR1) gene. Based on this
result, Stat1 and HDAC4 were proposed as potential therapeutic
targets for the treatment of chemotherapy-resistant lung cancer
cells that overexpress P-gp.
Materials and methods
Cell cultures
A549 cells and etoposide-resistant A459 cells
(A549RT-eto) were developed and provided by the Laboratory of
Biochemistry at Chulabhorn Research Institute (Bangkok, Thailand),
as previously described (16).
These cells were cultured in RPMI-1640 medium (Gibco Life
Technologies, Grand Island, NY, USA) which was supplemented with
10% fetal bovine serum (FBS), 1% penicillin and 1% streptomycin
(all from Gibco Life Technologies) at 37°C in a humidified
atmosphere with 5% CO2. Cells were observed under a
light microscope (Nikon ELIPSE TS100, Nikon Corporation, Tokyo,
Japan).
Reagents and antibodies
Antibodies against phospho-Stat1 (rabbit anti-human
polyclonal, #9171) Stat1 (rabbit anti-human polyclonal, #9172),
HDAC4 (mouse anti-human monoclonal, #5392), PARP (mouse anti-human
monoclonal, #9549), caspase 9 (mouse anti-human monoclonal, #9508)
and anti-P-gp (mouse anti-human monoclonal, #517310) were obtained
from EMD Millipore, Billerica, MA, USA. Anti-β-actin (mouse
anti-human monoclonal, reactive to human, sc-47778) antibodies were
also obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Trichostatin A (TSA) and etoposide were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Immunoblotting
Lysis buffer (150 mM NaCl; 1% NP-40; 50 mM Tris-HCl,
pH 7.5) containing 0.1 mM Na2VO3, 1 mM NaF
and Protease Inhibitor Cocktail (Sigma-Aldrich) was used to lyse
the cells subsequent to harvest. Proteins from whole cell lysates
were resolved using 10 or 12% SDS-PAGE (Bio-Rad, Hercules, CA, USA)
using a power-supply (PowerPac, Bio-Rad) and then transferred to
nitrocellulose membranes (Whatman, Dassel, Germany) in order to
complete the immunoblotting. Primary antibodies were used at a
dilution of 1:1,000 (phospho-Stat1 or Stat1) or 1:2,000 (HDAC4,
PARP, caspase 9 or P gp), and secondary antibodies (goat anti-mouse
Ig, sc-2031, or goat anti-rabbit Ig sc-2030, Santa Cruz
Biotechnology, Inc.), which were conjugated with horseradish
peroxidase, were used at a dilution of 1:2,000 in 5% nonfat dry
milk. Subsequent to final washing with a washing buffer (137 mM
NaCl; 0.1% Tween-20; 20 mM Tris-HCl, pH 7.6; Cell Signaling
Technology, Inc. Danvers, MA, USA), the nitrocellulose membranes
were exposed using the ImageQuant LAS 4000 Mini (GE Healthcare,
Cleveland, OH, USA) for an enhanced chemiluminescence assay.
Short interference (si)RNA
transfection
Cells were trypsinized (Gibco Life Technologies) and
incubated overnight in order to achieve 60–70% confluence prior to
transfection with siRNA. Stat1 siRNA [pre-made commercially at
Bioneer Corporation, Daejeon, Korea; 200 nM sense, 5′-CUG ACU UCC
AUG CGG UUG A(dTdT)-3′ and antisense, 5′-UCA ACC GCA UGG AAG UCA
G(dTdT)-3′] or negative control siRNA (Bioneer Corporation) were
mixed with Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). The cells underwent a 6-h incubation with the
transfection mixture and were then rinsed with RPMI-1640 medium
containing 10% FBS. The cells were incubated for 48 h prior to
harvest.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using the RNeasy
Mini Kit (Qiagen, Valencia, CA, USA) in accordance with the
manufacturer’s instructions. Total RNA (3 μg) was converted into
cDNA using SuperScript II Reverse Transcriptase (Invitrogen Life
Technologies) and PCR was performed using the following specific
primers: Human MDR1 sense, 5′-CCC ATC ATT GCA ATA GCA GG-3′
and antisense, 5′-GTT CAA ACT TCT GCT CCT GA-3′; MRP2 sense,
5′-ACA GAG GCT GGT GGC AAC C-3′ and antisense, 5′-ACC ATT ACC TTG
TCA CTG TCC-3′; and breast cancer resistance protein (BCRP)
sense, 5′-GAT CAC AGT CTT CAA GGA GAT C-3′ and antisense, 5′-CAG
TCC CAG TAC GAC TGT GAC A-3′ (all from Bioneer Corporation). The
cDNAs of each sample were diluted and PCR was run at the optimal
cycle number (95°C for 1 min, 58°C for 1 min and 72°C for 1 min; 28
cycles for MDR1, and 30 cycles for MRP2 and BCRP). β-Actin mRNA was
measured as an internal standard. Subsequent to amplification, the
products were subjected to electrophoresis on a 2.0% agarose gel
and detected using ethidium bromide (Sigma-Aldrich) staining.
Stained band intensity was measured using Multi Gauge software
version 2.1 (FujiFilm, Tokyo, Japan).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay (CellTiter 96 Non-Radioactive Cell
Proliferation assay; Promega Corporation, Madison, WI, USA) was
used to measure cell survival, as previously described (17). Dye solutions containing tetrazolium
were added to the cells in the 96-well plate and incubated for 2 h.
The absorbance of the formazan produced by living cells was
measured at a wavelength of 570 nm (Victor3, PerkinElmer, Waltham,
MA, USA). The relative percentage of cell survival was calculated
by the mean absorbance of the treated cells (ODT) and
the mean absorbance of control cells (ODC) with the
following formula: % Cell survival =
(ODT/ODC).
Luciferase reporter assay
A549 and A549RT-eto cells were transfected with
hMDR1-luciferase (18) or pGL3
empty vector (Promega Corporation) as a control luciferase vector.
To normalize transfection efficiency, a pGK-β-gal vector (19) expressing β-galactosidase from a
phosphoglucokinase promoter was included in the transfection
mixture. At 48 h subsequent to transfection, cells were washed with
cold phosphate-buffered saline and lysed in lysis solution [25 mM
Tris (pH 7.8), 2 mM EDTA, 2 mM DTT, 10% glycerol, and 1% Triton
X-100; Sigma-Aldrich]. Luciferase activity was measured with a
luminometer (Lumat LB9507; Berthold Technologies, Oak Ridge, TN,
USA) using a luciferase kit (ONE-Glo Luciferase System; Promega
Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-test was used for statistical analysis (SigmaPlot 9,
Systat Software Inc., San Jose, CA, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
A549RT-eto cells exhibit higher levels of
HDAC4, phospho-Stat1 and P-gp compared with A549 parental
cells
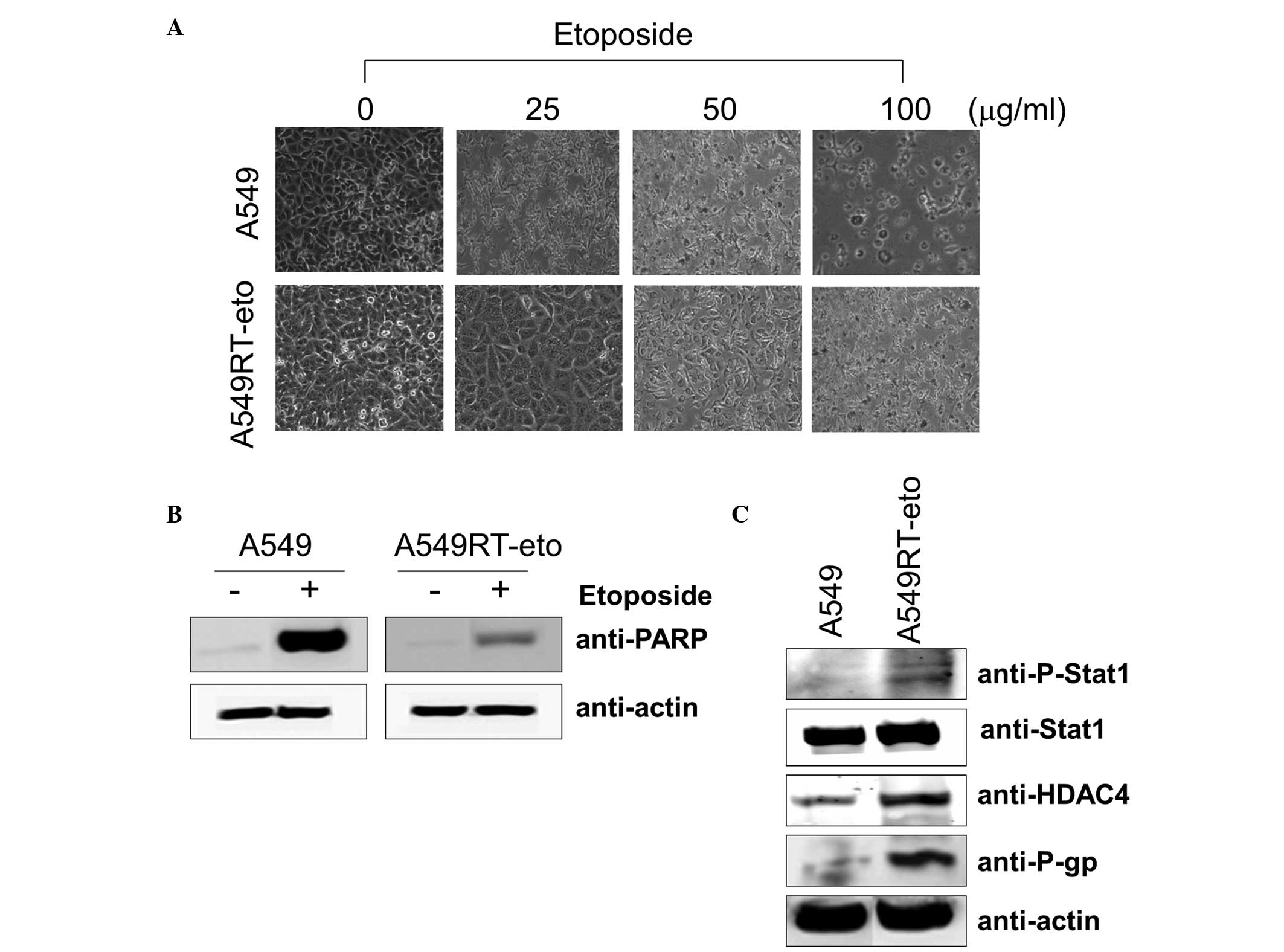
The first experiments aimed to determine the
concentration of etoposide required to affect the cell viability of
the A549 and A549RT-eto cells. The A549 parental cells were
sensitive to growth inhibition at a lower concentration of
etoposide (5 μg/ml) while A549RT-eto cells were relatively
resistant to growth inhibition (data not shown). The majority of
A549 cells died following 24–48 h exposure to 50 μg/ml etoposide,
whereas the majority of A549RT-eto cells under similar conditions
survived (Fig. 1A). A549 cells
were more sensitive to apoptosis during etoposide treatment than
A549RT-eto cells, as illustrated by the detection of cleaved PARP,
a substrate of active caspase-3 and -7 (Fig. 1B).
Subsequent to confirmation of the resistance of
A549RT-eto cells to etoposide, the differences between A549RT-eto
and A549 parental cells, which may explain this resistance, were
investigated. When expression levels of Stat1, HDAC4 and P-gp were
compared, it was noted that the levels of HDAC4 and P-gp proteins
were significantly enhanced in A549RT-eto cells compared with those
in the A549 parental cells (Fig.
1C). Total Stat1 protein levels in A549RT-eto cells were not
identified to be significantly different from those in A549 cells,
but the active form of phospho-Stat1 was more strongly expressed in
the A549RT-eto cells compared with the corresponding control level
(Fig. 1C). Based on these results,
the enhanced levels of phospho-Stat1, HDAC4 and P-gp were predicted
to be involved in A549 cell etoposide resistance.
HDAC inhibition enhances susceptibility
to etoposide in A549RT-eto cells
Enhanced HDAC4 levels in A549RT-eto cells were
demonstrated to be involved in etoposide resistance in A549 cells.
Thus, A549 and A549RT-eto cells were treated with the HDAC
inhibitor TSA (6.25 nM) and cell viability was examined by MTT
assay. TSA treatment alone did not influence cell growth in the two
cell types (Fig. 2A). As expected,
A549 cells exhibited greater sensitivity to etoposide, leading to
reduced cell survival compared with the A549RT-eto cells
(P<0.05). Additionally, the combined treatment of etoposide and
TSA was observed to inhibit cell growth by ~85% and ~65%, in A549
and A549RT-eto cells, respectively.
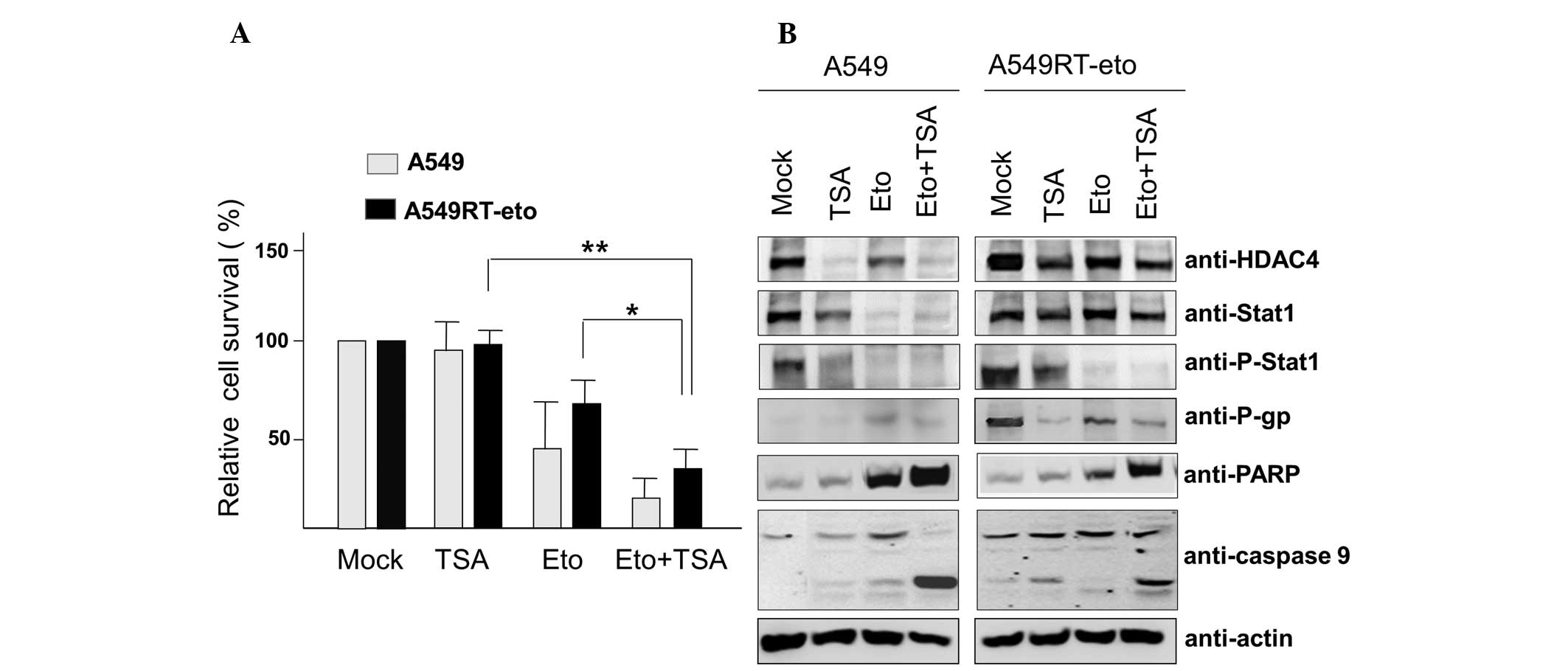 | Figure 2TSA treatment suppresses protein
levels of HDAC4, phospho-Stat1 and P-gp, leading to sensitization
to etoposide-induced apoptosis. (A) A549 and A549RT-eto cells were
treated with etoposide (50 μg/ml) alone, TSA (6.25 nM) alone, or
etoposide + TSA for 48 h, then cell survival was assessed by MTT
assay. Data were calculated as the percentage of relative cell
viability and expressed as the average of three experiments.
*P<0.05 and **P<0.01. (B) Cell lysates
from the treated A549 and A549RT-eto cells were prepared and
separated by SDS-PAGE. The expression levels of HDAC4, Stat1,
phospho-Stat1 and P-gp were detected by immunoblotting with the
corresponding antibodies. Apoptosis was denoted as the level of
cleaved PARP and caspase-9 measured by immunoblotting. TSA,
trichostatin A; HDAC4, histone deacetylase 4; P-gp, P-glycoprotein;
A549RT-eto, A549 cancer cells resistant to etoposide; p-Stat1,
phospho-Stat1; PARP, poly-ADP-ribose polymerase. |
Cleaved PARP and caspase-9 (indicators of intrinsic
apoptotic cell death) were detected during the combined treatments,
suggesting that the TSA and etoposide treatment sensitizes A549 and
A549RT-eto cells to apoptosis, compared with etoposide or TSA alone
(Fig. 2B). In addition, when the
protein levels of HDAC4, P-gp and phospho-Stat1 were observed, TSA
treatment was demonstrated to reduce HDAC4 and P-gp expression
levels in the two types of cell. Protein levels of Stat1 were not
significantly altered in A549RT-eto cells, while there was a clear
reduction in A549 cell Stat1 protein levels during TSA treatment
(Fig. 2B). It was also observed
that TSA treatment alone inhibited the activation of phospho-Stat1
in the two cell types. Etoposide treatment alone diminished protein
levels of HDAC4 in the two cell lines (Fig. 2B). It was observed that etoposide
treatment alone reduced Stat1 protein levels in A549 cells but not
in A549RT-eto cells (Fig. 2B). The
combined treatment reduced protein levels of HDAC4, P-gp and
phospho-Stat1 in A549RT-eto cells. These results suggest that HDAC4
inhibition sensitizes A549RT-eto cells to etoposide-induced
apoptosis through a reduction in P-gp protein levels.
Suppression of Stat1 with siRNA enhances
susceptibility to etoposide in A549RT-eto cells
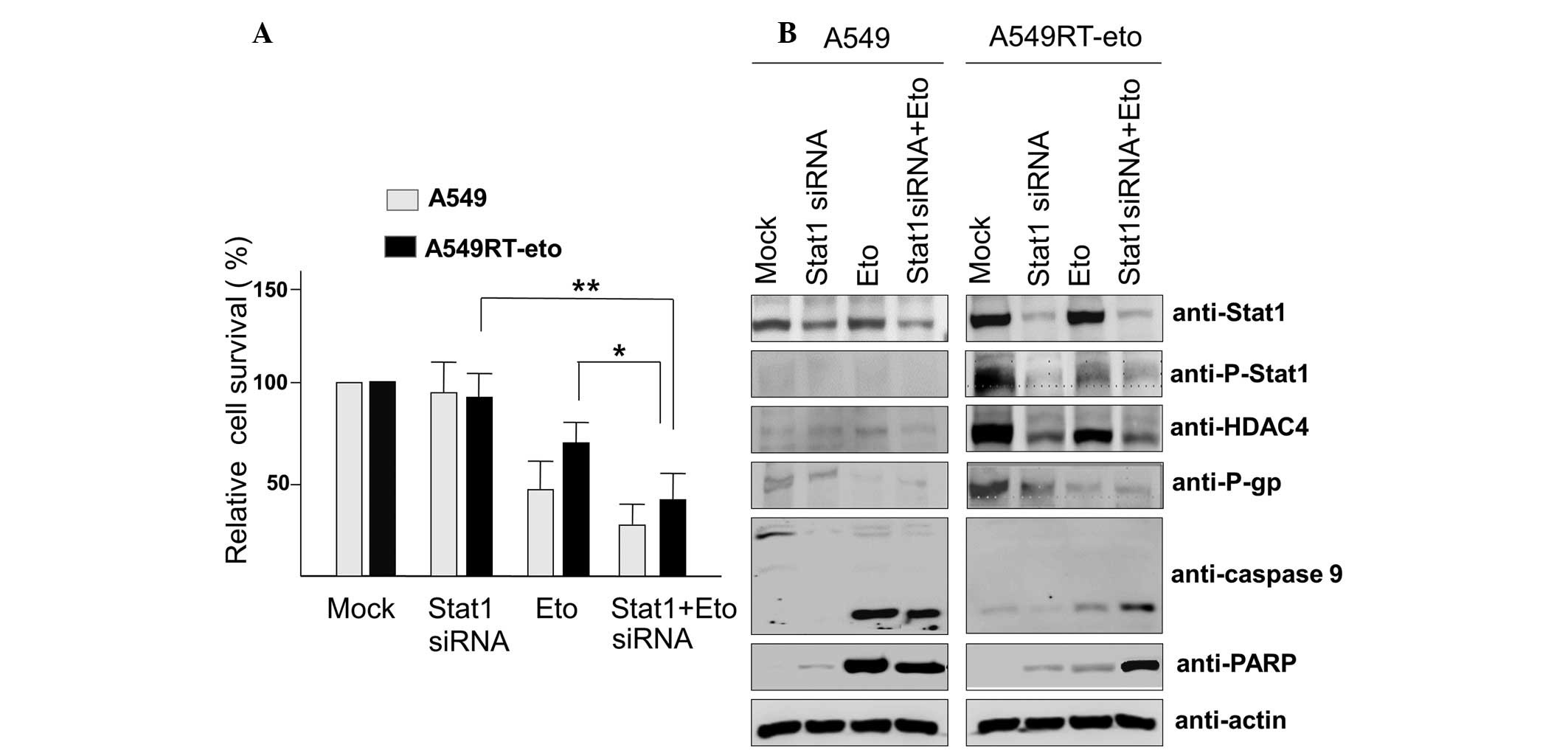
Since enhanced levels of phospho-Stat1 were observed
in A549RT-eto cells, it was hypothesized that elevated Stat1 may be
involved in resistance to etoposide. To assess this, Stat1 siRNA
was introduced to suppress Stat1 levels in A549RT-eto cells. The
optimal Stat1 siRNA concentration for the suppression of Stat1
expression was identified according to the results obtained from
transfection using Lipofectamine 2000, and according to the
results, 100 nM Stat1 siRNA was used (data not shown). Suppression
of Stat1 was observed to induce a reduction in HDAC4 and P-gp
expression levels, indicating that HDAC4 and Stat1 cross-talk with
each other (Fig. 3A). Etoposide
treatment alone reduced HDAC4, P-gp and phospho-Stat1 protein
levels, but was not sufficient to induce significant apoptosis in
A549RT-eto cells (Fig. 3B). The
combined treatment with Stat1 siRNA and etoposide induced a
reduction of HDAC4, P-gp and phospho-Stat1 protein levels (Fig. 3A), resulting in the acceleration of
apoptosis by the activation of caspase-3, -7 and -9 in A549RT-eto
cells. Together, these results suggest that Stat1 is involved in
P-gp expression, resulting in resistance to etoposide.
Etoposide resistance is attributed to the
enhancement of MDR1 transcript levels and its transcriptional
activity in A549RT-eto cells
Other genes, such as those encoding MRP2 and
BCRP, are established to be involved in drug resistance
(20,21). Therefore, the levels of these
transcripts (MDR1, MRP2 and BCRP genes) were
examined in A549 and A549RT-eto cells in the present study.
A549RT-eto cells were observed to exhibit increased levels of
MDR1 mRNA, whereas low levels were observed in A549 cells
(Fig. 4A). However, levels of
MRP2 and BCRP transcript were similar between
A549RT-eto and A549 parental cells. This result indicates that the
MDR1 gene is specifically activated to create etoposide
resistance in A549 cells. Furthermore, MDR1 promoter
activity was examined in A549RT-eto and A549 cells. An increase in
MDR1 promoter activity was observed in A549RT-eto cells
compared with that of the A549 parental cells (Fig. 4B), in concordance with the higher
levels of MDR1 mRNA observed in A549RT-eto cells.
TSA treatment inhibits P-gp expression at
the transcriptional level in A549RT-eto cells but Stat1 siRNA
treatment does not
Next, the effect of TSA or Stat1 siRNA treatment on
MDR1, MRP2 and BCRP mRNA levels in A549RT-eto
cells was investigated. TSA and etoposide treatments alone reduced
transcriptional levels of MDR1, while neither treatment
reduced the levels of MRP2 or BCRP (Fig. 5A). The combined treatment of TSA
and etoposide also reduced transcriptional levels of MDR1.
When Stat1 siRNA was administered to the A549RT-eto cells, there
was no reduction in MDR1 mRNA levels, yielding results
similar to those treated with the control siRNA (Fig. 5B). The combined treatment of Stat1
siRNA and etoposide significantly reduced MDR1 mRNA levels
due to the action of etoposide. Based on these results, it can be
theorized that HDAC4 affects the expression of P-gp protein at the
transcriptional level, while Stat1 influences its expression at the
post-transcriptional level.
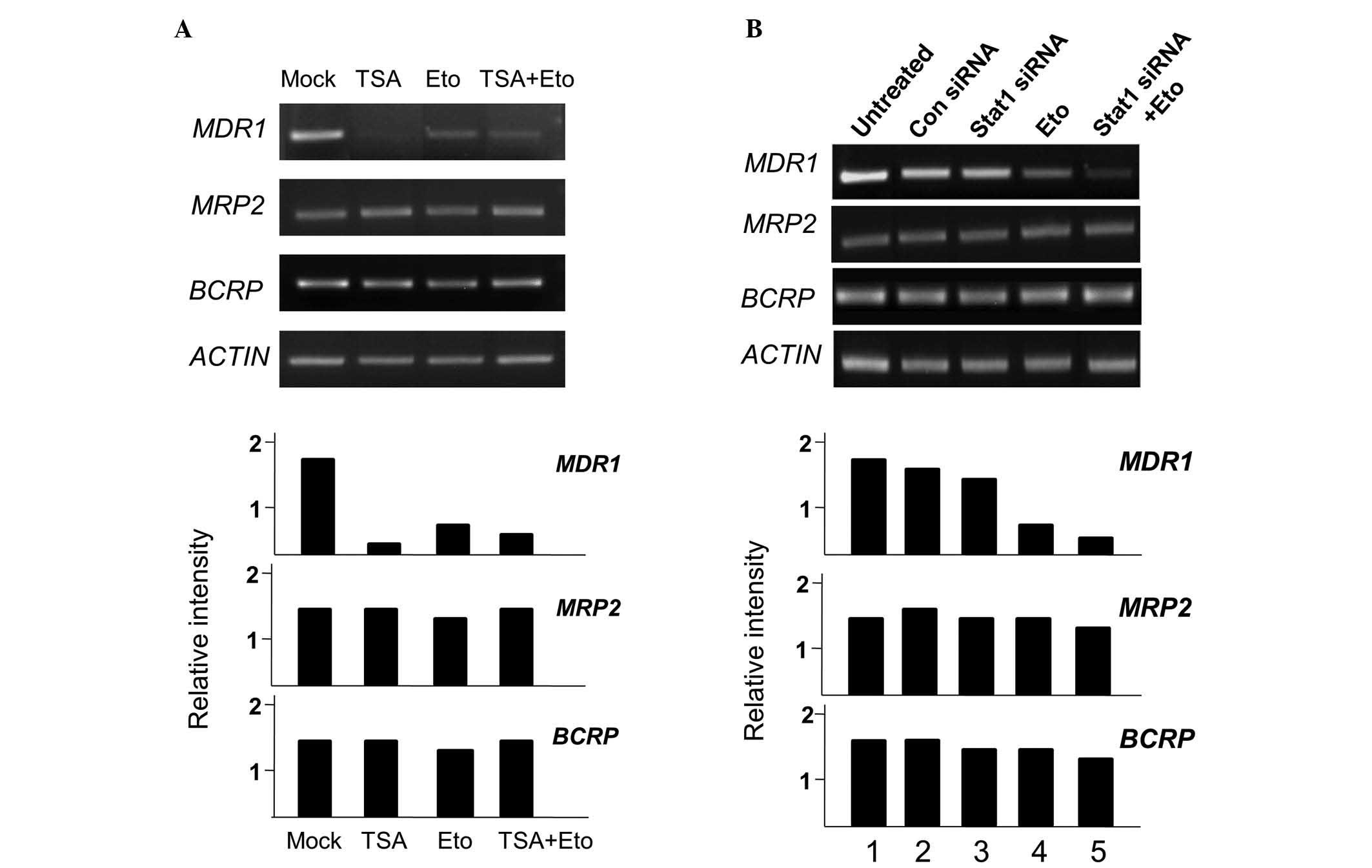 | Figure 5MDR1 gene transcript levels are
reduced by treatment with TSA, but not by treatment with Stat1
siRNA. A549RT-eto cells; (A) untreated, treated with TSA alone,
etoposide alone, or TSA + etoposide; (B) untreated, treated with
control siRNA, Stat1 siRNA alone, etoposide alone, or Stat1 siRNA +
etoposide. Total RNA from each group was isolated and subjected to
RT-PCR. RNA levels of MDR1, MRP2 and BCRP were
examined. Relative mRNA ratios of each MDR-associated gene were
described in comparison with mRNA levels of β-actin subsequent to
measurement of band intensities using Multi Gauge version 2.1.
MDR1, multidrug resistance 1; TSA, trichostatin A; RT-PCR,
reverse transcription-polymerase chain reaction; MRP2,
multidrug resistance-associated protein 2; BCRP, breast
cancer resistance protein; A549RT-eto, A549 cancer cells resistant
to etoposide. |
Discussion
The majority of patients with non-small cell lung
cancer display intrinsic chemoresistance, which limits the
possibility of successful treatment with chemotherapy (22). The most prevalent form observed in
these patients is multidrug resistance, which has been associated
with the overexpression of the ATP-binding cassette superfamily of
transporters, including P-gp and MRP (23,24).
In the current study, A549 cells with acquired etoposide resistance
were observed to specifically exhibit the upregulation of P-gp but
not other MDR-associated genes, such as MRP2 and
BCRP, which are frequently overexpressed in patients with
cancer that exhibits chemotherapeutic resistance. In addition,
human H460 lung cancer cells resistant to etoposide have exhibited
overexpression of lung resistance protein (25). It is thus concluded that the
upregulation of various specific MDR gene family members is
associated with the context of the cell origin and chemotherapeutic
drug exposure.
In the present study, it has been reported that the
expression of P-gp is modulated by HDAC4 and Stat1. Suppression of
HDAC4 activity and Stat1 levels induced a reduction of P-gp
expression levels, leading to sensitization to apoptosis,
suggesting that HDAC4 and Stat1 are responsible for etoposide
resistance. Notably, it was also observed that inhibition of HDAC4
activity by TSA reduced Stat1 activity, indicating that HDAC4 may
regulate Stat1 activity. The results of the current study are
supported by another that suggested that HDAC4 interacts with
Stat1, resulting in a reduction of Stat1 acetylation, which
eventually enhances Stat1 phosphorylation in cisplatin-resistant
cancer cells (13). Furthermore,
when expression of Stat1 was suppressed by its siRNA, HDAC4 protein
levels were also diminished in etoposide-resistant A549 cells,
leading to sensitization of etoposide-induced apoptosis through the
downregulation of P-gp. Based on these results, it is proposed that
Stat1 and HDAC4 coregulate each other.
However, it was observed that the transcript levels
of P-gp were reduced during Stat1 siRNA treatment. Thus, it is
hypothesized that the suppression of Stat1 may influence protein
levels of P-gp at a post-transcriptional level, or indirectly
through other proteins. Supporting this hypothesis, a previous
study demonstrated that FBXO15/Fbx15, an F-box protein in the
ubiquitin E3 ligase complex, regulates P-gp expression levels
through the ubiquitin-proteosome pathway (26). However, it remains unclear how
Stat1 suppression is associated with the upregulation of
FBXO15/Fbx15. Thus, future studies should focus upon the
investigation of the detailed mechanisms by which the suppression
of Stat1 induces a reduction in protein levels of P-gp.
Acknowledgements
The current study was supported by a grant from the
World Class University Program (R31-2008-000-20004-0) through the
National Research Foundation funded by the Korean government; the
Office of the Higher Education Commission, Thailand, under the
Strategic Scholarships Fellowships Frontier Research Networks
(specifically for the southern region) for the Joint PhD Thai
Doctoral Degree Program, a CHE-SSR-PhD SW Scholarship to Ms.
Chutima Kaewpiboon.
References
|
1
|
Chin YE, Kitagawa M, Kuida K, Flavell RA
and Fu XY: Activation of the STAT signaling pathway can cause
expression of caspase 1 and apoptosis. Mol Cell Biol. 17:5328–5337.
1997.PubMed/NCBI
|
|
2
|
Kumar A, Commane M, Flickinger TW, Horvath
CM and Stark GR: Defective TNF-alpha-induced apoptosis in
STAT1-null cells due to low constitutive levels of caspases.
Science. 278:1630–1632. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chin YE, Kitagawa M, Su WC, You ZH,
Iwamoto Y and Fu XY: Cell growth arrest and induction of
cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1.
Science. 272:719–722. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Townsend PA, Scarabelli TM, Davidson SM,
Knight RA, Latchman DS and Stephanou A: STAT-1 interacts with p53
to enhance DNA damage-induced apoptosis. J Biol Chem.
279:5811–5820. 2004. View Article : Google Scholar
|
|
5
|
Stephanou A, Brar BK, Knight RA and
Latchman DS: Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and
Bcl-x promoters. Cell Death Differ. 7:329–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khodarev NN, Beckett M, Labay E, Darga T,
Roizman B and Weichselbaum RR: STAT1 is overexpressed in tumors
selected for radioresistance and confers protection from radiation
in transduced sensitive cells. Proc Natl Acad Sci USA.
101:1714–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weichselbaum RR, Ishwaran H, Yoon T, et
al: An interferon-related gene signature for DNA damage resistance
is a predictive marker for chemotherapy and radiation for breast
cancer. Proc Natl Acad Sci USA. 105:18490–18495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fryknäs M, Dhar S, Oberg F, et al: STAT1
signaling is associated with acquired crossresistance to
doxorubicin and radiation in myeloma cell lines. Int J Cancer.
120:189–195. 2007. View Article : Google Scholar
|
|
9
|
Roberts D, Schick J, Conway S, et al:
Identification of genes associated with platinum drug sensitivity
and resistance in human ovarian cancer cells. Br J Cancer.
92:1149–1158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang XJ and Seto E: HATs and HDACs: from
structure, function and regulation to novel strategies for therapy
and prevention. Oncogene. 26:5310–5318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng H, Harvey CT, Pittsenbarger J, et al:
HDAC4 protein regulates HIF1α protein lysine acetylation and cancer
cell response to hypoxia. J Biol Chem. 286:38095–38102. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mihaylova MM, Vasquez DS, Ravnskjaer K, et
al: Class IIa histone deacetylases are hormone-activated regulators
of FOXO and mammalian glucose homeostasis. Cell. 145:607–621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stronach EA, Alfraidi A, Rama N, et al:
HDAC4-regulated STAT1 activation mediates platinum resistance in
ovarian cancer. Cancer Res. 71:4412–4422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Chin YE, Weisiger E, et al: Cutting
edge: Negative regulation of dendritic cells through acetylation of
the nonhistone protein STAT-3. J Immunol. 182:5899–5903. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan ZL, Guan YJ, Chatterjee D and Chin
YE: Stat3 dimerization regulated by reversible acetylation of a
single lysine residue. Science. 307:269–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanintronkul Y, Worayuthakarn R, Thasana
N, et al: Overcoming multidrug resistance in human lung cancer with
novel benzo[a]quinolizin-4-ones. Anticancer Res. 31:921–927.
2011.PubMed/NCBI
|
|
17
|
Kaewpiboon C, Lirdprapamongkol K,
Srisomsap C, et al: Studies of the in vitro cytotoxic, antioxidant,
lipase inhibitory and antimicrobial activities of selected Thai
medicinal plants. BMC Complement Altern Med. 12:2172012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HG, Hien TT, Han EH, et al: Metformin
inhibits P-glycoprotein expression via the NF-κB pathway and CRE
transcription activity through AMPK activation. Br J Pharmacol.
162:1096–1108. 2011. View Article : Google Scholar :
|
|
19
|
Cho IR, Jeong S, Jhun BH, et al:
Activation of non-canonical NF-kappaB pathway mediated by STP-A11,
an oncoprotein of Herpesvirus saimiri. Virology. 359:37–45. 2007.
View Article : Google Scholar
|
|
20
|
Sugimoto Y, Tsukahara S, Ishikawa E and
Mitsuhashi J: Breast cancer resistance protein: molecular target
for anticancer drug resistance and
pharmacokinetics/pharmacodynamics. Cancer Sci. 96:457–465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Young LC, Campling BG, Cole SP, Deeley RG
and Gerlach JH: Multidrug resistance proteins MRP3, MRP1, and MRP2
in lung cancer: correlation of protein levels with drug response
and messenger RNA levels. Clin Cancer Res. 7:1798–1804.
2001.PubMed/NCBI
|
|
22
|
Ihde DC and Minna JD: Non-small cell lung
cancer. Part II: Treatment. Curr Probl Cancer. 15:105–154.
1991.PubMed/NCBI
|
|
23
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: the multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan HS, Lu Y, Grogan TM, et al: Multidrug
resistance protein (MRP) expression in retinoblastoma correlates
with the rare failure of chemotherapy despite cyclosporine for
reversal of P-glycoprotein. Cancer Res. 57:2325–2330.
1997.PubMed/NCBI
|
|
25
|
Lee E and Lim SJ: The association of
increased lung resistance protein expression with acquired
etoposide resistance in human H460 lung cancer cell lines. Arch
Pharm Res. 29:1018–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katayama K, Noguchi K and Sugimoto Y:
FBXO15 regulates P-glycoprotein/ABCB1 expression through the
ubiquitin-proteasome pathway in cancer cells. Cancer Sci.
104:694–702. 2013. View Article : Google Scholar : PubMed/NCBI
|