Introduction
Ultraviolet (UV) irradiation impairs human skin and
causes premature skin aging (photoaging), which results in deep
wrinkles and pigment formation (1,2).
Type I collagen, the most abundant structural protein in skin
connective tissue, is essential for maintaining the strength and
elasticity of the skin. Disorganization, fragmentation and
dispersion of collagen bundles are three characteristics in human
photoaging skin (1,3). Destroying the structural integrity of
the collagenous extracellular matrix is well-established to be the
major reason for the wrinkled appearance of photoaged skin
(4). UV irradiation decreases type
I collagen through two interdependent pathways: Stimulation of
collagen degradation and inhibition of type I procollagen (COL1)
production (3,5). Thus, UV-induced control of type I
collagen production is one of the critical factors in the mechanism
of photoaging.
Transforming growth factor-β (TGF-β) is the primary
regulator of collagen synthesis in human skin (6–9).
TGF-β functions by binding to specific receptor complexes,
including TGF-β type I (TβRI) and TGF-β type II (TβRII) receptors
on the cell surface (9). Smad7 is
one of the negative factors in the TGF-β/Smad signaling pathway,
which interacts with TβRI to prevent activation of Smad2/3, thereby
inhibiting TGF-β signaling. It has been reported that UV
irradiation impairs TGF-β/Smad signaling through downregulating the
transcription of TβRII. This impairment is a major reason for the
reduced procollagen synthesis in human skin fibroblasts (10). For this reason, the prevention of
UV-induced loss of TβRII may precede the recovery of type I
collagen reduction in photoaging skin.
UV irradiation leads to direct or indirect DNA
damage and the formation of radical oxygen species, which causes
the subsequent activation of complex signaling pathways, followed
by the induction of matrix metalloproteinases (MMPs) in skin cells.
MMPs are a group of extracellular matrix (ECM) enzymes, which can
degrade the protein components of the ECM (11). Upregulation of MMPs, particularly
collagenase-1 (MMP-1), generated by several types of cells,
including fibroblasts, keratinocytes, endothelial cells,
macrophages, hepatocytes, chondrocytes and osteoblasts, is
responsible for the lysis of dermal collagen in skin aging.
Astragaloside IV (AST) is a small molecular saponin
with multiple activities under pathophysiological conditions,
including antihypertensive, positive inotropic action,
anti-inflammatory and anti-infarct properties. However, the effect
of AST in photoaging skin remains to be elucidated. The present
study focused on whether AST prevents collagen degradation in
photoaging skin and the possible underlying mechanisms, in
vivo and in vitro to determine whether AST inhibits
collagen reduction in photoaging skin by improving TGF-β/Smad
signaling suppression and inhibiting MMP-1.
Materials and methods
Chemicals and reagents
Rabbit polyclonal immunoglobulin G (IgG) anti-TβRII
(sc-220) and mouse monoclonal IgG2b anti-tubulin
(sc-23950) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Cell culture
Human skin fibroblasts (HSFs), derived from newborn
skin were acquired from the Chinese Academy of Medical Science
(Beijing, China). The cells were then cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA),
supplemented with 10% fetal calf serum (FCS; Invitrogen Life
Technologies, Victoria, Australia), 100 U/ml penicillin and 100
μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). HSFs were
cultivated in 75-cm2 culture flasks in an incubator at
37°C with a humidified atmosphere containing 5% carbon dioxide.
When the cells reached 80–90% confluency, they were subcultivated
to 60-mm culture dishes.
UVB irradiation
A total of four F36T12 ERE-VHO UV tubes were used in
the present study as the UV source. A Kodacel TA401/407 filter
(Kodak, Tokyo, Japan) was mounted 4 cm in front of the tubes to
block UVC (wavelengths >290 nm). The irradiation intensity was
monitored using a UVR radiometer equipped with a UVA sensor
(Bioblock Scientific, Tournai, Belgium). Subconfluent HSFs were
cultured in DMEM containing 0.1% FCS for 24 h and subsequently
incubated in DMEM with various concentrations of AST (10, 20, 30,
40, 50 μml; Sigma-Aldrich) for 24 h. HSFs were then washed twice
with fresh phosphate-buffered saline (PBS; Sigma-Aldrich) and
exposed to UVA irradiation (10 J/cm2) in a thin layer of
PBS. Following irradiation, the cells were incubated in DMEM for
the indicated time.
Western blotting
A total of 40 μg of protein from each sample was
separated by 10–12% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Bedford, MA, USA). Following
blocking with 10% instant non-fat dry milk for 1 h, membranes were
incubated with specific antibodies overnight at 4°C followed by
incubation with horseradish-conjugated secondary IgG antibodies
(anti-rabbit, #7074 and anti-mouse, #7076; Cell Signaling
Technology, Inc., Danvers, MA, USA) for 1 h. Antibody binding was
detected with the enhanced chemiluminescence detection system
(Amersham Biosciences, Piscataway, NJ, USA).
Cell viability assay
Cell viability was measured using the
3-(4,5-dimethylthylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
(MTT) method as described previously (12).
Quantification of apoptosis by ELISA
The ELISA Detection kit (Roche, Palo Alto, CA, USA)
was used to detect MMP-1 and Smad7 in HSFs with different
treatments. Briefly, following the indicated treatments, the
cytoplasmic histone/DNA fragments from cells were extracted and
bound to immobilized anti-histone antibody. Subsequently, the
peroxidase-conjugated anti-DNA antibody was used for the detection
of immobilized histone/DNA fragments The antibodies used were from
the ELISA Detection kit (Roche). Following the addition of a
substrate for peroxidase, the spectrophotometric absorbance of the
samples was determined using the Dynatech MR5000 plate reader at
405 nm (Dynatech Laboratories, Chantilly, VA, USA).
RNA isolation, reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from human skin (Chinese
Academy of Medical Science) using TRIzol reagent (Invitrogen Life
Technologies, Shanghai, China) and reverse transcription was
conducted on 2 μg RNA using the PrimeScript RT reagent kit (TaKaRa
Bio Inc. Ohtsu, Japan) and standard RT-PCR primers for human COL1:
Forward: 5′-CGC CAT CAA GGT CTA CTG C-3′ and reverse: 5′-GAA TCC
ATC GGT CAT GCT CT-3′ and tubulin forward,
5′-ATCAGCAATGCCTCCTGCAC-3′ and reverse, 5′-CGTCAAAGGTGGAGGAGTGG-3′.
Data were normalized to tubulin expression and the untreated group
was set as one. The PCR was semi-quantitative and the cycling
conditions were 50°C for 2 min, 95°C for 1 min and 40 cycles of
amplification at 95°C for 15 sec, 60°C for 1 min, followed by 95°C
for 15 sec, 60°C for 30 sec and 95°C for 15 sec.
Statistical analysis
The values in the figures are expressed as the mean
± standard deviation. The figures in the present study were
representative of >3 different experiments. Statistical analysis
of the data between the control and treated groups was performed
using SPSS software version 6.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of AST on cell viability in
HSFs
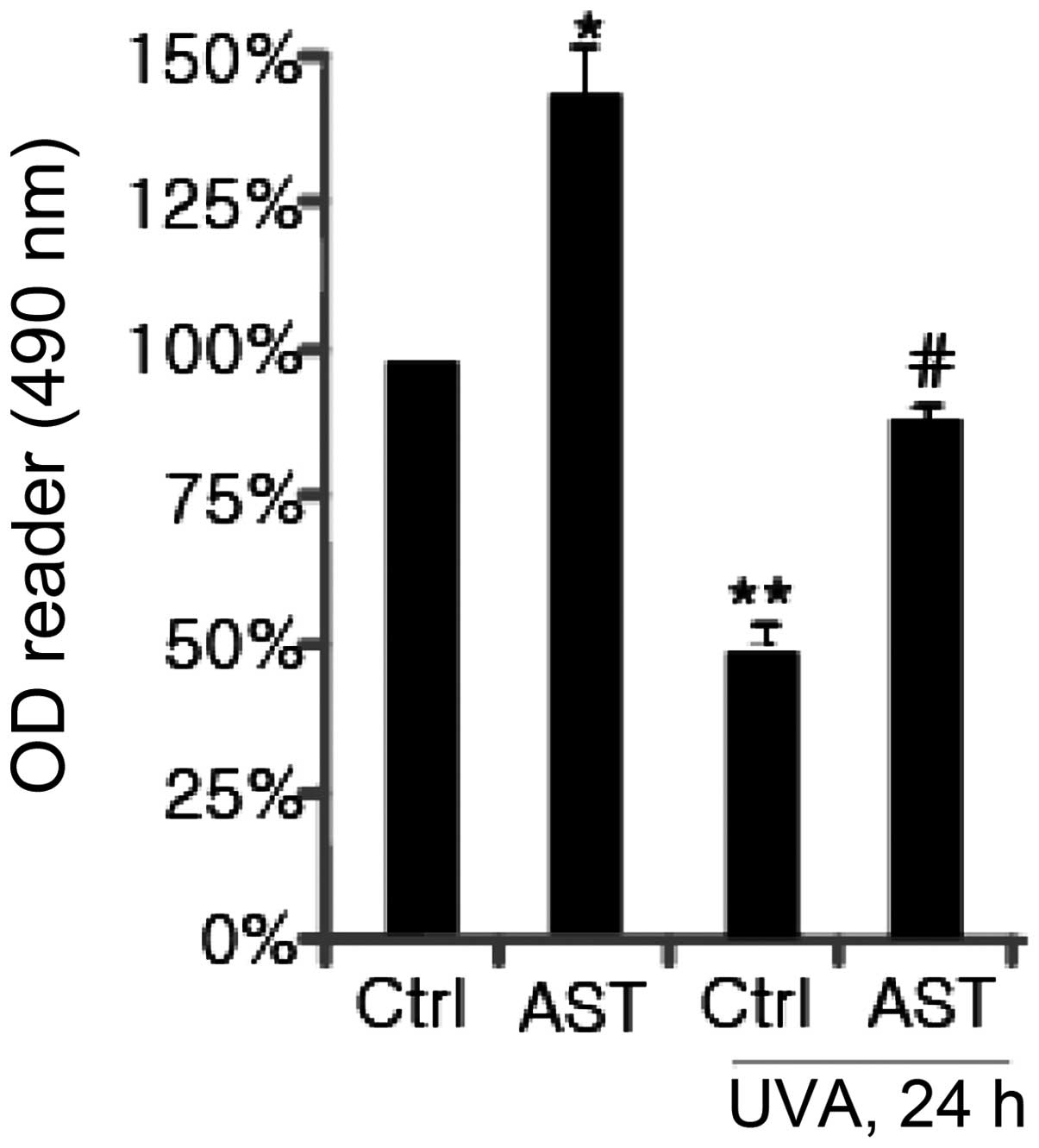
Initially, it was assessed whether AST affects cell
viability in HSFs using an MTT assay. As shown in Fig. 1, at a low concentration (20 μg/ml),
AST did not affect cell viability. AST at 30 μg/ml exhibited almost
a 25% increase in cell viability. When the concentration was 40
μg/ml, AST had the most significant effect (~50%) on HSFs compared
with the control. Subsequently, the effect of AST on cell viability
in UVA-exposed HSFs was examined. The results revealed that UVA
irradiation (10 J/cm2) exhibited marked cytotoxicity.
AST (40 μg/ml) enhanced cell viability in HSFs irradiated with UVA
(Fig. 2).
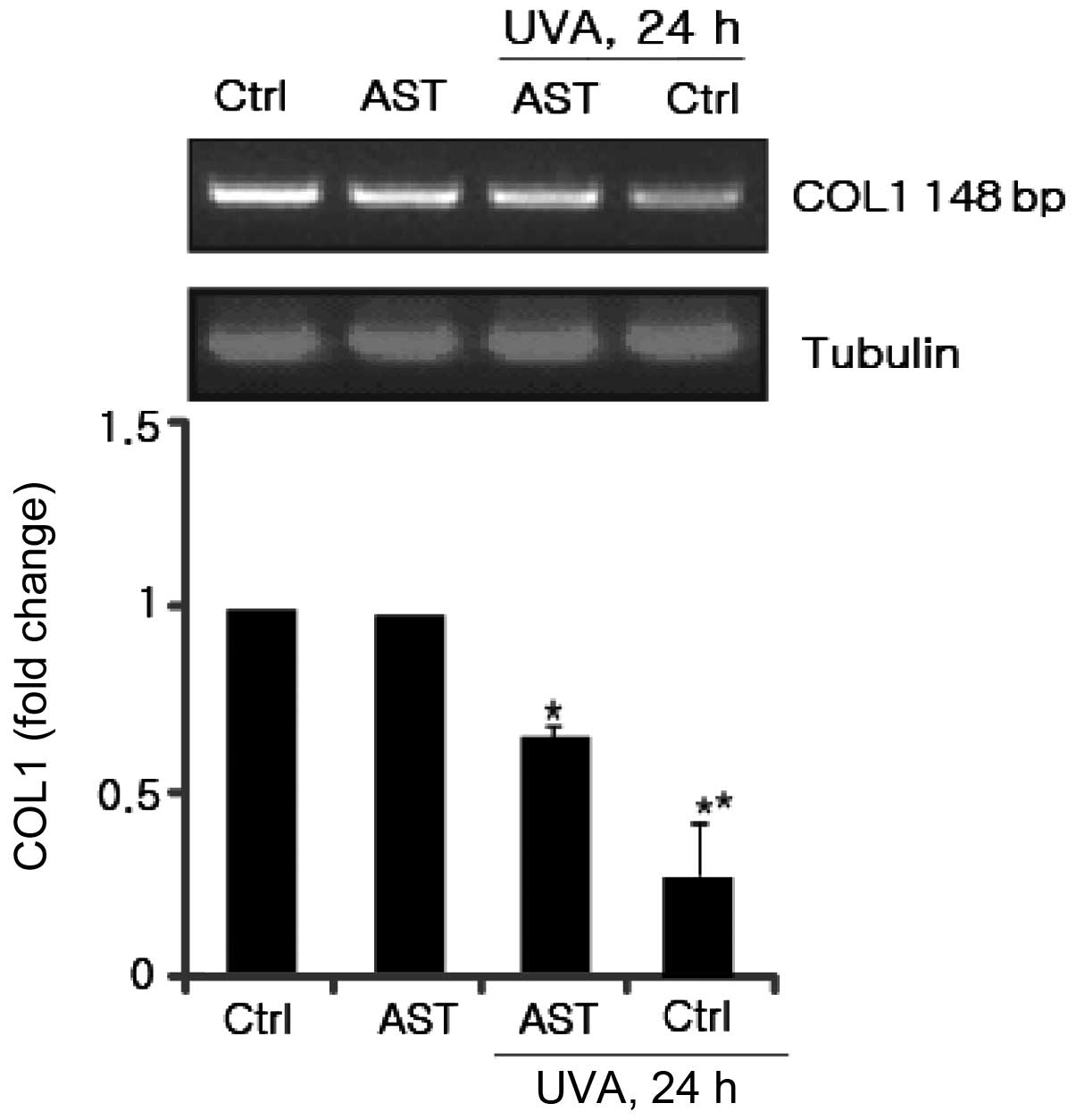
Effect of AST on UVA-induced COL1
downregulation
Type I collagen is the most abundant structural
protein in skin connective tissue. UV irradiation decreases type I
collagen through inhibition of COL1 production. Therefore, the
present study aimed to elucidate the effect of AST on UVA-induced
COL1 downregulation. HSFs were pretreated with AST. Following UVA
irradiation, secreted COL1 in the supernatants were harvested and
identified using RT-PCR. As shown in Fig. 3, COL1 secretion was inhibited by
UVA, while AST reversed this inhibitory effect. These results
suggested that AST significantly prevented UV-induced reduction of
COL1 mRNA expression.
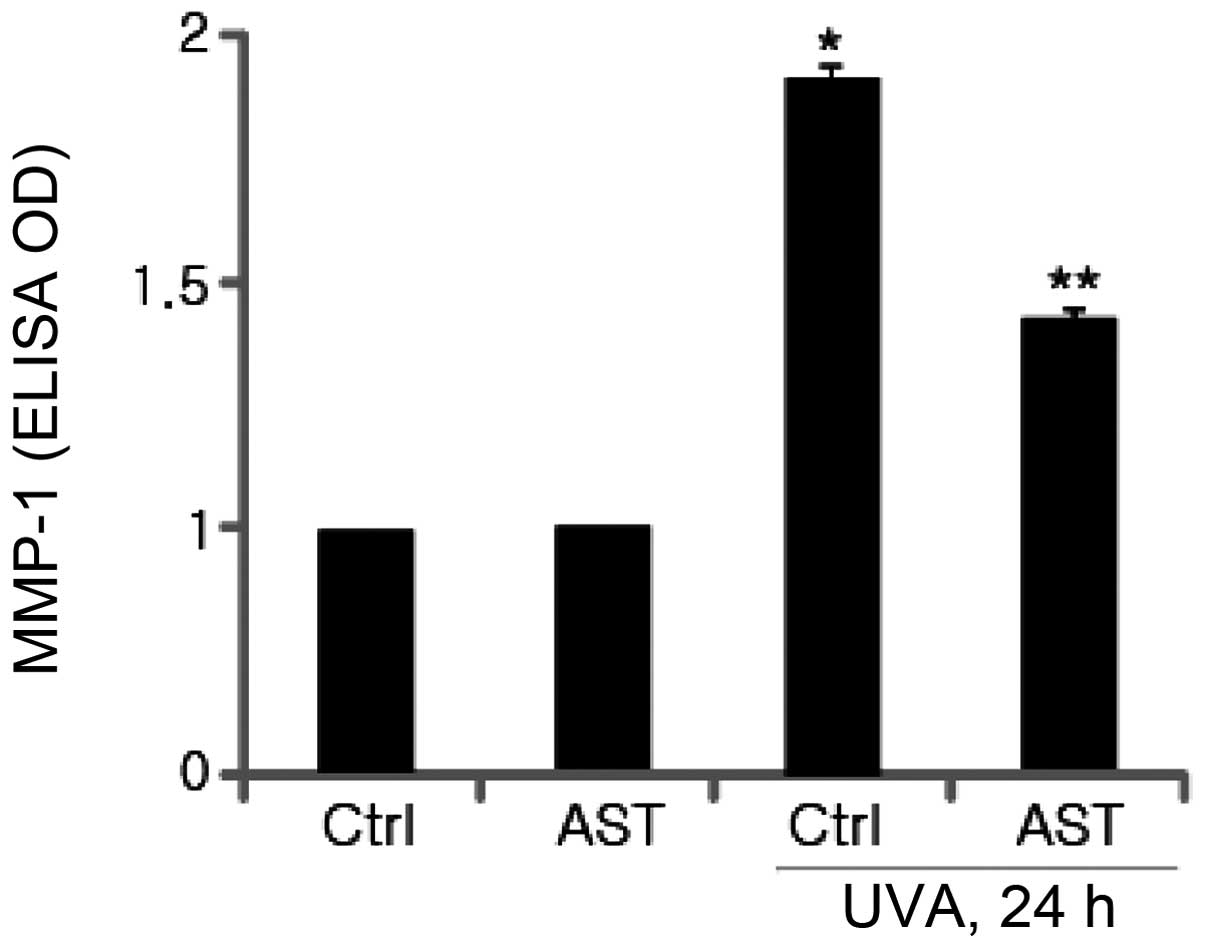
Effect of AST on UVA-induced MMP-1
expression
It is well-established that UV irradiation damages
human skin cells and causes photoaging. UVA and UVB irradiation of
dermal fibroblasts induced MMP-1 expression, which is implicated in
the degradation of human skin matrix proteins, including collagen
and other components of the ECM. In the present study, to
investigate whether AST affects the expression of MMP-1 in
UVA-irradiated HSFs, cultured fibroblasts were pretreated with AST
followed by UVA irradiation. MMP-1 protein levels were determined
using ELISA. As expected, the results revealed that UVA irradiation
significantly enhanced MMP-1 expression. The ELISA results
demonstrated that pretreatment with AST markedly inhibited
UVB-induced MMP-1 expression compared with the UVA-irradiated group
(Fig. 4).
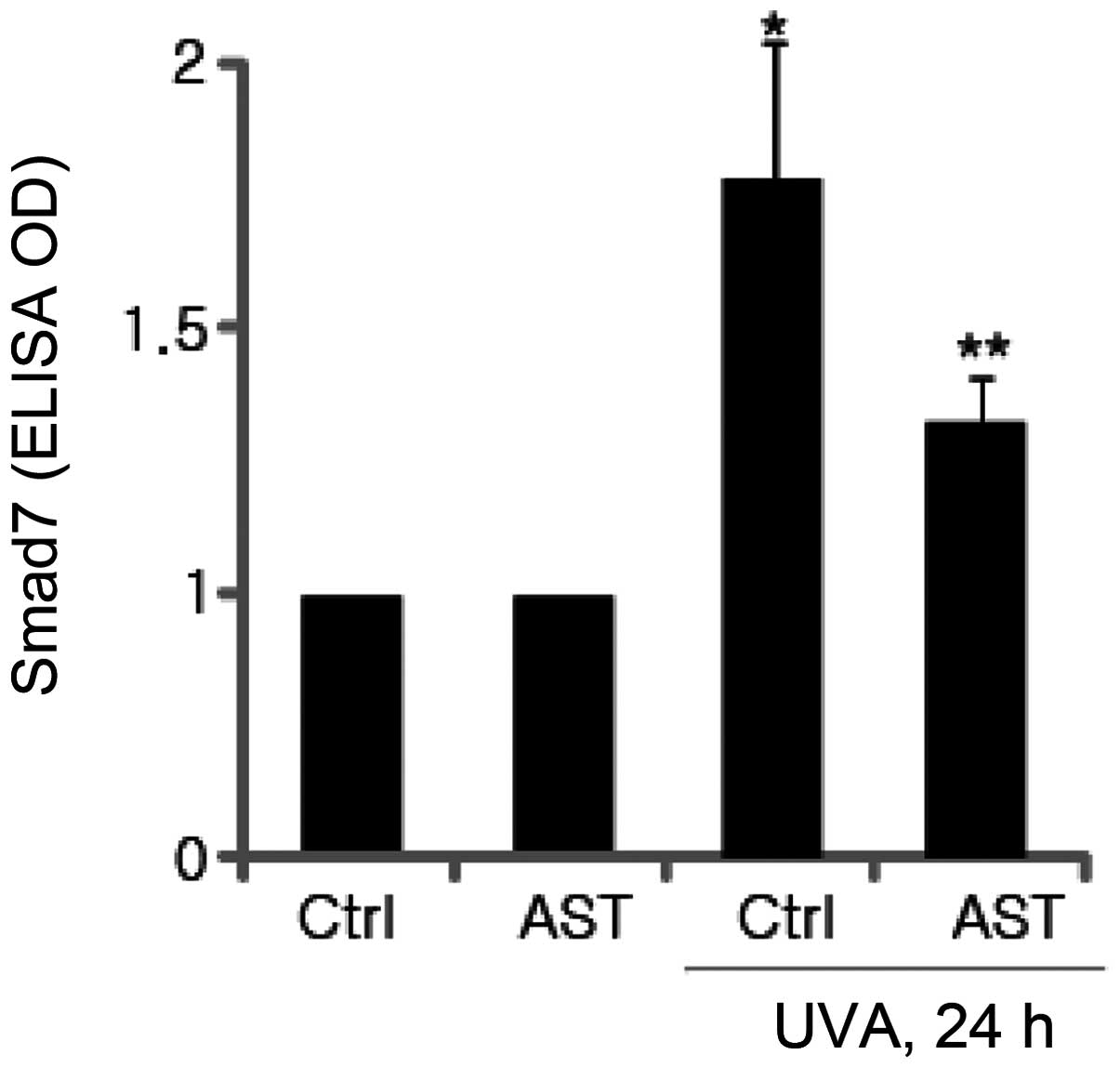
Inhibitory effect of AST on UVA-induced
Smad7 expression
Smad7 is one of the negative factors in the
TGF-β/Smad signaling pathway, which interacts with TβRI to prevent
activation of Smad2/3, thereby inhibiting TGF-β signaling. It was
subsequently investigated whether AST affects the expression of
Smad7 in UVA-irradiated HSFs. Smad7 protein levels were determined
using ELISA. The results demonstrated that UVA irradiation
significantly enhanced Smad7 expression and the expression of Smad7
induced by UVB irradiation was significantly attenuated by AST
(Fig. 5).
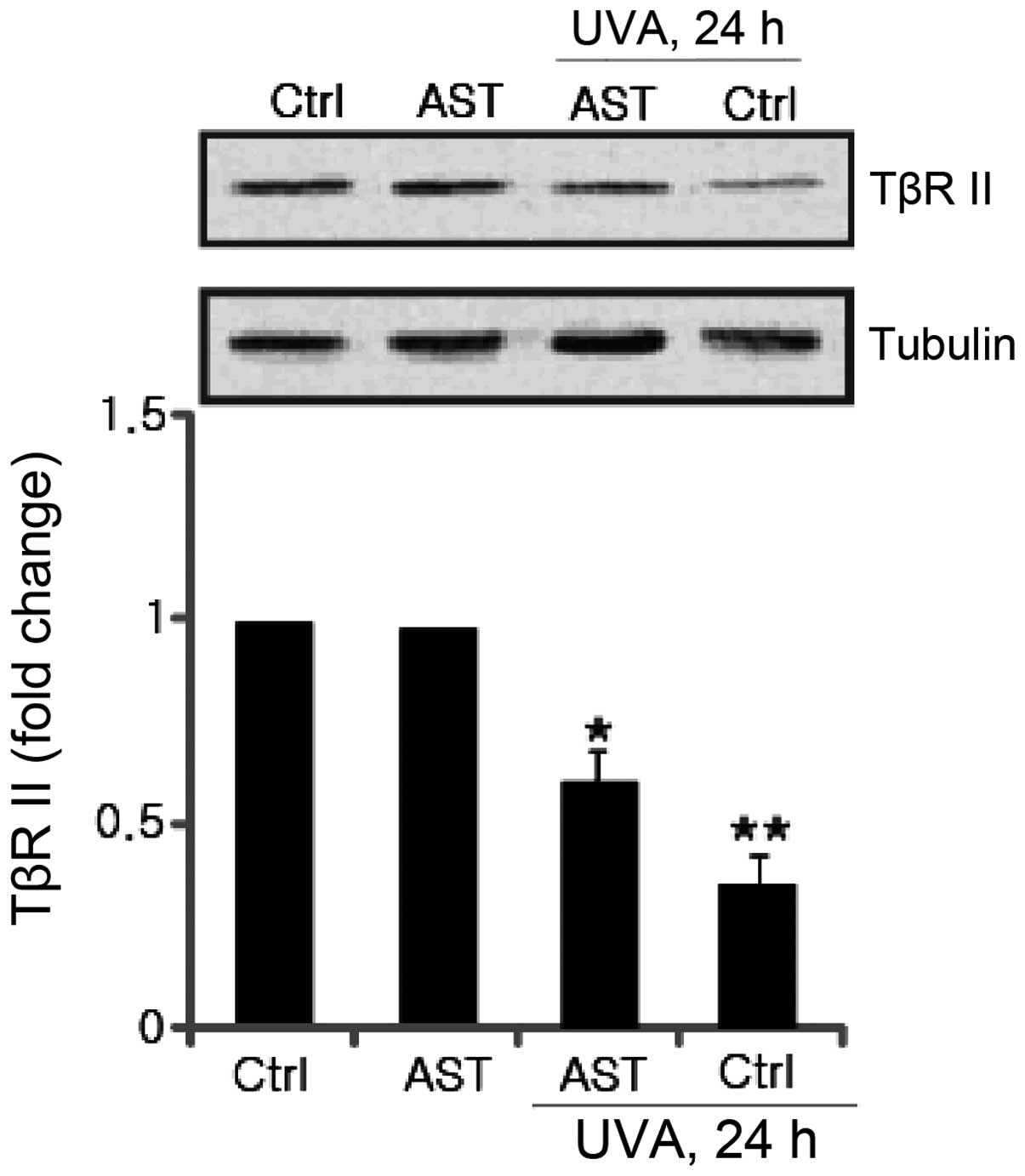
Effect of AST on UVA-induced TβRII
downregulation
Significant progress has been made towards
understanding the molecular mechanisms underlying the UV-induced
TGF-β/Smad signaling pathway. It is reported that UV irradiation
impairs TGF-β/Smad signaling through transcriptional downregulation
of TβRII (7). The effect of AST on
UVA-induced TβRII downregulation was further investigated. The
results revealed that attenuated TβRII expression induced by UVA
irradiation was significantly inhibited by AST pretreatment
(Fig. 6).
Discussion
UV radiation causes premature skin aging, which is
termed photoaging. It has been well-established that this
complicated procedure is due to UV-induced collagen degradation
through its effects on various signaling factors, including MMP-1
in the TGF-β/Smad signaling pathway.
TGF-β is a major regulator of procollagen production
in human skin. TGF-β acts through its cell surface receptors to
activate transcription factors Smad 2/3, which regulate TGF-β
target gene expression (6–9). Considering that regulation of COL1
expression occurs via a complicated mechanism, which remains to be
elucidated, multiple studies have indicated that transcriptional
regulation has a major role in controlling its production (13,14).
Transcription of the COL1 gene is directly regulated by TGF-β via a
Smad3 binding element in its promoter (15). It was reported that UV irradiation
impairs the TGF-β/Smad pathway by downregulating its type-II
receptor and inducing Smad7 (11,16),
and this impairment reduces procollagen synthesis in UV-irradiated
human skin. Therefore, the UV-induced reduction of TβRII and
UV-induced increase of Smad7 may provide novel insights for the
molecular mechanisms of photoaging and suppression of UV-induced
downregulation of TβRII and upregulation of Smad7, which may lead
to the identification of novel approaches for the prevention of
photoaging. The present data indicated that AST inhibits the
downregulation of TβRII and the upregulation of Smad7, followed by
suppression of the reduction of COL1 synthesis in the AST group,
which revealed that the pathway and signaling factors regulated by
AST were involved in its functions against photoaging.
UV irradiation is known to induce expression of
MMP-1, −3 and −9 in human skin in vivo, and cultured human
skin cells in vitro (17).
UV-induced MMP-1 expression induces the cleavage of collagen
fibers. Once collagen is cleaved by MMP-1, collagen degradation is
further promoted by MMP-3 and −9. MMP-1, termed fibroblast-type or
interstitial collagenase, is secreted by fibroblasts, keratinocytes
and macrophages. MMP-1 degrades collagens type I, II and III and is
hypothesized to have a pivotal role in the process of photoaging
(18). These properties render
MMP-1 an attractive target for the pharmacological development of
anti-photoaging agents. Therefore, in the present study, the effect
of AST on UV-induced MMP-1 expression was examined. It was
identified that MMP-1 expression was significantly lower in the AST
group. The results suggest that AST is a potent inhibitor of
UV-induced MMP-1 expression.
In conclusion, the present findings demonstrated
that AST inhibits UV-induced COL1 decrease by stimulating the
TGF-β/Smad signaling pathway through upregulating TβRII and
downregulating Smad7 as well as suppressing MMP-1 expression.
Therefore, it is hypothesized that AST may be a potentially
effective agent for the prevention of photoaging.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81101188,
810701297 and 30671894) and the Technology Project Grant from the
Traditional Chinese Medicine Administration of Jiangsu Province
(no. LZ 11082).
References
|
1
|
Fisher GJ, Wang ZQ, Datta SC, et al:
Pathophysiology of premature skin aging induced by ultraviolet
light. N Engl J Med. 337:1419–1428. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warren R, Gartstein V, Kligman AM, et al:
Age, sunlight, and facial skin: a histologic and quantitative
study. J Am Acad Dermatol. 25:751–760. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher GJ, Datta SC, Talwar HS, et al:
Molecular basis of sun-induced premature skin ageing and retinoid
antagonism. Nature. 379:335–339. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talwar HS, Griffiths CE, Fisher GJ, et al:
Reduced type I and type III procollagens in photodamaged adult
human skin. J Invest Dermatol. 105:285–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher GJ, Datta S, Wang Z, et al:
c-Jun-dependent inhibition of cutaneous procollagen transcription
following ultraviolet irradiation is reversed by all-trans retinoic
acid. J Clin Invest. 106:663–670. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massagué J and Chen YG: Controlling
TGF-beta signaling. Genes Dev. 14:627–644. 2000.PubMed/NCBI
|
|
8
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piek E, Heldin CH and Ten Dijke P:
Specificity, diversity, and regulation in TGF-beta superfamily
signaling. FASEB J. 13:2105–2124. 1999.PubMed/NCBI
|
|
10
|
Quan T, He T, Kang S, Voorhees JJ and
Fisher GJ: Solar ultraviolet irradiation reduces collagen in
photoaged human skin by blocking transforming growth factor-beta
type II receptor/Smad signaling. Am J Pathol. 165:741–751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SJ, Yuan W, Lo S, et al: Interaction
of smad3 with a proximal smad-binding element of the human
alpha2(I) procollagen gene promoter required for transcriptional
activation by TGF-beta. J Cell Physiol. 183:381–392. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji C, Yang B, Yang YL, et al: Exogenous
cell-permeable C6 ceramide sensitizes multiple cancer cell lines to
Doxorubicin-induced apoptosis by promoting AMPK activation and
mTORC1 inhibition. Oncogene. 29:6557–6568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inagaki Y, Truter S, Tanaka S, et al:
Overlapping pathways mediate the opposing actions of tumor necrosis
factor-alpha and transforming growth factor-beta on alpha 2(I)
collagen gene transcription. J Biol Chem. 270:3353–3358. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jimenez SA, Varga J, Olsen A, et al:
Functional analysis of human alpha 1(I) procollagen gene promoter.
Differential activity in collagen-producing and -nonproducing cells
and response to transforming growth factor beta 1. J Biol Chem.
269:12684–12691. 1994.PubMed/NCBI
|
|
15
|
Chen SJ, Yuan W, Mori Y, et al:
Stimulation of type I collagen transcription in human skin
fibroblasts by TGF-beta: involvement of Smad 3. J Invest Dermatol.
112:49–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh AK, Yuan W, Mori Y, et al:
Smad-dependent stimulation of type I collagen gene expression in
human skin fibroblasts by TGF-beta involves functional cooperation
with p300/CBP transcriptional coactivators. Oncogene. 19:3546–3555.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji C, Yang Y, Yang B, et al: Trans-Zeatin
attenuates ultraviolet induced down-regulation of aquaporin-3 in
cultured human skin keratinocytes. Int J Mol Med. 26:257–263.
2010.PubMed/NCBI
|
|
18
|
Yang B, Ji C, Kang J, et al: Trans-Zeatin
inhibits UVB-induced matrix metalloproteinase-1 expression via MAP
kinase signaling in human skin fibroblasts. Int J Mol Med.
23:555–560. 2009.PubMed/NCBI
|