Introduction
There is evidence suggesting that myocardial
ischemia (MI) is a major risk factor of myocardial infarction,
which induces myocardial remodeling. The predominant morphological
changes of ventricular remodeling are the exhibition of an
infarction area, ventricular hypertrophy and ventricular expansion
(1,2). The mechanisms underlying ventricular
remodeling remain to be fully elucidated, however, it is generally
accepted that following myocardial injury, the molecules, cells and
mechanisms change due to altered gene expression levels and the
imbalance between cell apoptosis and proliferation, which is
important in the entire disease process (1).
It is important to develop novel treatments to
inhibit or slow the disease process to enable more time for
subsequent treatment. Traditional Chinese medicine has gained
increased attention for the treatment of various diseases. Although
the use of Chinese medicine in the treatment of ventricular
remodeling has been investigated only relatively recently, it has
been demonstrated that using traditional Chinese medicine can
affect the occurrence and development of ventricular remodeling in
a number of aspects (3). This is
also supported by previous reports suggesting that certain agents,
including statins, angiotensin converting enzyme inhibitors and
angiotensin II receptor inhibitors, can improve ventricular
remodeling by increasing cell apoptosis and preventing cell
proliferation in smooth muscle cells in hypertensive animal models
(4,5).
Ginsenoside Rg3 (GSRg3), extracted from Panax
ginseng, is a traditional Chinese herbal medicine used widely
in clinical treatment and may significantly improve basilar artery
hypertrophic remodeling through the prevention of artery smooth
muscle cell proliferation (6). The
present study predominantly investigated how GSRg3 attenuates
MI/reperfusion (MI/R) injury and examined the main pathways
involved.
Materials and methods
Animals and drugs
The present study was performed in accordance with
the National Institutes for Food and Drug Control (http://www.nicpbp.org.cn) for the Use of Laboratory
Animals and was approved by the Tongji Hospital of Tongji
University (Shanghai, China) Committee on Animal Care. A total of
30 male eight-week-old Sprague-Dawley rats (Experimental Animal
Center, Tongji University) weighing between 260 and 280 g were
housed in diurnal lighting conditions (12 h light/12 h dark;
22–24°C) and allowed free access to food and water for 7 days prior
to performing the investigation. GSRg3 (purity>98%; Fig. 1) was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China).
MI/R procedure in the rat hearts
The cardial MI/R surgery was performed, as described
previously (5). Briefly, the rats
were anaesthetized via intraperitoneal injection of pentobarbital
sodium (60 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA)
and were placed on a warm board (25°C) to control the body
temperature at 37°C for surgery. The neck was opened with a ventral
midline incision and the animals were ventilated with room air
using a rodent respirator (tidal volume 8 ml/kg body weight; 60–80
breaths/min; Shanghai Alcott Biotech Co., Ltd., Shanghai, China).
An electrocardiogram in lead II was recorded through needle
electrodes attached to the limbs. Following the adjustment of the
respiratory rate and the tidal volume of gases, the chest was
opened by a middle thoracotomy. Following pericardiotomy, a 4-0
black silk thread (Millar, Inc., Houston, TX, USA) was passed
behind the left anterior descending coronary artery and was
occluded by a knot for 30 min to cause ischemia. Subsequently, the
knot was released and reperfusion was performed for 3 h. For the
sham control group, the black silk was placed under the left
anterior descending coronary artery without occlusion.
Hemodynamic measurements
The right common carotid artery was exposed and
cannulated with a Millar vessel (Millar, Inc.) into the left
ventricular cavity of the rat through the ascending aorta. The
heart function, including the left ventricular systolic pressure
(LVSP), heart rate (HR) and first derivative (±dp/dt) of left
ventricular pressure of the rats in each group (sham, I/R and
I/R+Rg3) were recorded and programmed using a biotic signal
collection and processing system (PowerLab; AD Instruments, New
South Wales, Australia), as described previously (6).
Determination of the serum levels of SOD,
LDH and CK
Following 3 h reperfusion, blood samples (5 ml) were
collected through the ventral aorta using a scalp vein set, and the
serum was frozen at −80°C until subsequent analysis. The activities
of LDH, CK and SOD were determined using an ELISA kit, according to
manufacturer’s instructions (BioAssay Systems, Hayward, CA,
USA).
Infarct size measurement
Following reperfusion, the hearts were rapidly
extracted from the rats using surgical scissors and frozen at
−20°C. The left ventricular area was sliced into six 2–3 mm-thick
slices perpendicular to the base-apex and incubated in 2%
triphenyltetrazolium chloride (TTC; pH 7.4; Xiya Reagent, Chengdu,
China) buffer for 15 min at 37°C. The viable tissues were stained
dark red with TTC, while the infarcted portion remained
grayish-white. The area of infraction was measured using an image
analysis system [National Institutes of Health (NIH) image
software, version 1.60; NIH, Bethesda, MA, USA]
Isolation of primary neonatal rat
cardiomyocytes (NRCs) and anoxia-reoxygenation injury
Primary neonatal Sprague-Dawley rats (1–3 days-old)
were purchased from the experimental Animal center of Tongji
University (Shanghai, China). Cardiac myocytes were cultured from
1–3 day-old Sprague-Dawley rats with Dulbecco’s modified Eagle’s
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) at a
density of 5×105 for three days, as described previously
(1,2). The primary neonatal rats were
anaesthetized with sodium pentobartbital and decapitated, and then
immersed in 75% ethanol (20 ml) for 30 sec. The chest was opened
and the heart ventricles were dissected rapidly and immersed in
ice-cold Krebs-Ringer buffer containing 137 mM NaCl, 2 mM
CaCl2, 5.4 mM KCl, 1.1 mM
MgCl2·6H2O, 0.4 mM
NaH2PO4·2H2O, 11.9 mM
NaHCO3 and 5.6 mM glucose (pH 7.4), and the ventricles
were minced into small sections using eye scissors and digested
with trypsin (0.1%; Beyotime Institute of Biotechnology, Guangzhou,
China). The cardiomyocytes were cultured in DMEM containing 10%
newborn calf serum (NCS; Gibco Life Technologies), in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. The neonatal
rat cardiomyocytes (NRCs) were used and the ginsenoside-Rg3 and
solvent were preincubated with cells for 30 min prior to I/R
injury.
Simulated I/R (SI/R) was performed, as described
previously (1,2). Briefly, simulated ischemia buffer,
containing 98.5 mM NaCl, 1.2 mM MgSO4, 10 mM KCl, 1 mM
CaCl2, 40 mM sodium lactate and 20 mM HEPES (pH 6.8),
and simulated reoxygenation buffer, containing 20 mM
HCO3, 0.9 mM NaH2PO4, 1 mM
CaCl2, 1.2 mM MgSO4, 20 mM HEPES, 5 mM KCl,
129.5 mM NaCl and 5.5 mM glucose (pH 7.4), were prepared in
advance. The medium of the NRCs was replaced with 1 ml simulated
ischemia buffer, incubated in a hypoxic chamber (humidified
atmosphere 5% CO2/0% O2 balanced with
N2 at 37°C) for 3 h, and then reoxygenated in a standard
incubator for 2 h with reoxygenation buffer. The cells subjected to
control conditions were cultured with normal Tyrode solution (pH
7.4; Beijing Leagene Biotech Co., Ltd., Beijing, China) in a
humidified atmosphere of 5% CO2/21% O2
balanced with N2 at 37°C for 5 h (4). The cells were divided randomly into
four groups: Control group, incubated with Tyrode solution during
the entire experimental period; SI/R group, incubated with
simulated ischemia buffer for 3 h hypoxia, followed by 2 h
re-oxygenation; Vehicle group, subjected to 0.2% (v/v) dimethyl
sulfoxide administration 30 min prior to SI/R; SI/R+GSRg3 group,
subjected to GSRg3 (10 mM) administration 30 min prior to SI/R
(5).
Cell viability
The cell viability was assessed using a Cell
Counting Assay kit-8 (CCK-8, Beyotime Institute of Biotechnology)
according to the manufacturer’s instructions. The NRCs (100
μl) were plated into 96-well plates at a density of
1×105 cells/well, followed by 30 min pre-incubation with
different concentrations of GSRg3 (0.1–100 μM). Following
treatment, the cells were exposed to 10 μl CCK-8 solution
for a futher 2 h and the absorbance at 450 nm was measured using a
microplate reader (ELx808; Bio-Tek Instruments, Winooski, VT, USA)
(1).
Determination of apoptosis by flow
cytometry
The apoptotic rate of the NRCs was determined by
flow cytometry using annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining according to the
manufacturer’s instructions. Briefly, the NRCs were pretreated with
10 μM GSRg3 for 30 min followed by SI/R treatment. The cells
were digested with trypsin (0.25%) and were then resuspended in
phosphate-buffered saline (PBS) containing 10% NCS. The cells were
centrifuged at 200 × g for 10 min at 4°C and then washed twice with
cold PBS. The cells were then treated with 5 μl annexin
V-FITC (1:80) and 10 μl PI (1:40) (Bioworld Technology Co.,
Ltd., Nanjing, China), and incubated in the dark at room
temperature for 15 min. Each sample was analyzed using a
Beckton-Dickinson flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
The NRCs were lysed in lysis buffer (Beyotime
Institute of Biotechnology) containing Protease Inhibitor Cocktail
(Merck Millipore, Billerica, MA, USA) for 30 min on ice. The
cellular proteins were collected using a cell scraper. Following
centrifugation for 15 min at 12,000 rpm, a Bicinchoninic acid
Protein Assay kit (Beyotime Institute of Biotechnology) was used to
determine the protein concentrations. Equal quantities of protein
were separated using 10% SDS-PAGE gels and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked using 5% non-fat milk in
Tris-buffered saline (8 g NaCl and 6 g Tris), containing 1%
Tween-20 (TBST), for 1 h at room temperature. The membranes were
then incubated with primary antibodies against eNOS (610297; IgG1;
polyclonal; 1:3,000; BD Biosciences), p-Akt (2920; mouse
monoclonal; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), Akt (4691; rabbit monoclonal; 1:1,000; Cell Signaling
Technology, Inc.) Bcl-2 (2870; mouse poly-clonal; 1:10000; Cell
Signaling Technology, Inc.), Bax (2772; mouse polyclonal; 1:2,000;
Cell Signaling Technology, Inc.) and PARP (5625; rabbit monoclonal;
1:1,000; Cell Signaling Technology, Inc.) overnight at 4°C.
Following washing three times for 5 min with TBST, the membranes
were incubated with secondary antibodies, including alkaline
phosphatase-linked anti-mouse (7056; IgG; 1:5,000; Cell Signaling
Technology, Inc.) or horseradish peroxidase-linked anti-rabbit
antibodies at room temperature for 1 h. β-actin (7074; IgG;
1:5,000; Cell Signaling Technology, Inc.) was used as an internal
control. The protein bands were visualized using a
Chemiluminescence Electophoretic Mobility Shift Assay kit (Beyotime
Institute of Biotechnology) and X-ray film (Kodak BioMax MS Film;
Kodak. Corp., Rochester, NY, USA). The band density was
statistically analyzed using Image J software (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The statistical comparisons between groups were performed
using one-way analysis of variance. SPSS version 19.0 was used to
perform all statistical analyses (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of GSRg3 on cardiac function
GSRg3 had no effect on blood glucose, cardiac
function or blood pressure normality and no significant differences
were observed between the groups at the baseline conditions.
Pretreatment with GSRg3 increased LVSP and +dp/dt max, and
decreased LVEDP and −dp/dt max following 3 h reperfusion, compared
with the MI/R group (P<0.05; Fig.
2). Treatment with GSRd markedly increased the mean heart rate
compared with the MI/R group (P<0.05; Fig. 3). The hemodynamic data demonstrated
that GSRg3 improved rat cardiac systolic and diastolic function
following MI/R.
 | Figure 2GSRg3 improves rat cardiac function
following 30 min ischemia and 3 h reperfusion. Improvements were
observed in (A) LVSP, (B) LVEDP, (C) +dP/dt max and (D) −dP/dt max.
Data are expressed as the mean ± standard error of the mean (n=8;
*P<0.05, vs. sham, #P<0.05 and
##P<0.01, vs. I/R). LVSP, left ventricular systolic
pressure; LVEDP, left ventricular end diastolic pressure; ±dP/dt
max, instantaneous first derivation of left ventricle pressure;
sham, untreated; I/R, ischemia/reperfusion; I/R+Rg3, I/R+Rg3
administration 3 days prior to surgery; baseline, immediately
following stabilization; I0, pre-ischemic treatment; R0, start of
reperfusion; R30, 30 min after reperfusion; R60, 60 min after
reperfusion; R180, 180 min after reperfusion. GSRg3, ginsenoside
Rg3. |
 | Figure 3Changes in heart rate. Data are
expressed as the mean ± standard error of the mean (n=8;
*P<0.05, vs. sham, #P<0.05, vs. I/R).
Sham, no treatment, I/R, ischemia/reperfusion; I/R+Rg3, I/R+Rg3
treatment 3 days prior to experimental surgery; baseline,
immediately after stabilization; I0, pre-ischemic treatment; R0,
start of reperfusion; R30, 30 min after reperfusion; R60, 60 min
after reperfusion; R180, 180 min after reperfusion; GSRg3,
ginsenoside Rg3. |
GSRg3 reduced rat myocardial injury
(infarct size, necrosis, and apoptosis) post MI/R
The infacted areas and areas at risk are shown in
Fig. 4. No MI was observed in the
hearts from the sham group. Pretreatment with GSRg3 significantly
decreased the infarct size compared with the MI/R group
(P<0.05). To determine whether GSRg3 attenuated MI/R induced
cardio-myocyte necrosis, the plasma levels of CK, LDH and SOD were
measured following reperfusion. GSRg3 treatment markedly decreased
the levels of CK and LDH, and increased the levels of SOD compared
with the MI/R group (P<0.05). These data demonstrated that GSRg3
reduced myocardial necrosis following MI/R.
 | Figure 4GSRg3 reduces rat myocardial injury
(infarct size, necrosis, and apoptosis) post I/R. (A) INF in rats
subjected to 30 min I, followed by 3 h R. Red-staining represents
the AAR and pale areas indicate infracted regions. INF is expressed
as a percentage of the AAR. (B) Plasma CK levels. (C) Plasma LDH
levels. (D) Plasma SOD levels. Data are expressed as the mean ±
standard error of the mean (n=6; *P<0.05, vs. I/R,
#P<0.05, vs. sham). I/R, ischemia/reperfusion; INF,
myocardial infract size; AAR, area at risk; CK, creatine kinase;
LDH, lactate dehydrogenase; SOD, superoxide dismutase; GSRg3,
ginsenoside Rg3. |
GSRg3 improves SI/R-induced in vitro cell
injury, increasing viability and decreasing apoptosis
The NRCs were treated with different concentrations
of GSRg3 (0.1–100 mM) to determine the effects of GSRg3 alone.
Treatment with these concentrations of GSRg3 for 24 h were not
cytotoxic, as demonstrated by the CCK-8 assay (Fig. 5A). As shown in Fig. 5B, the concentration response curves
determined cellular viability, and this was observed at dosage of
10 mM GSRg3.
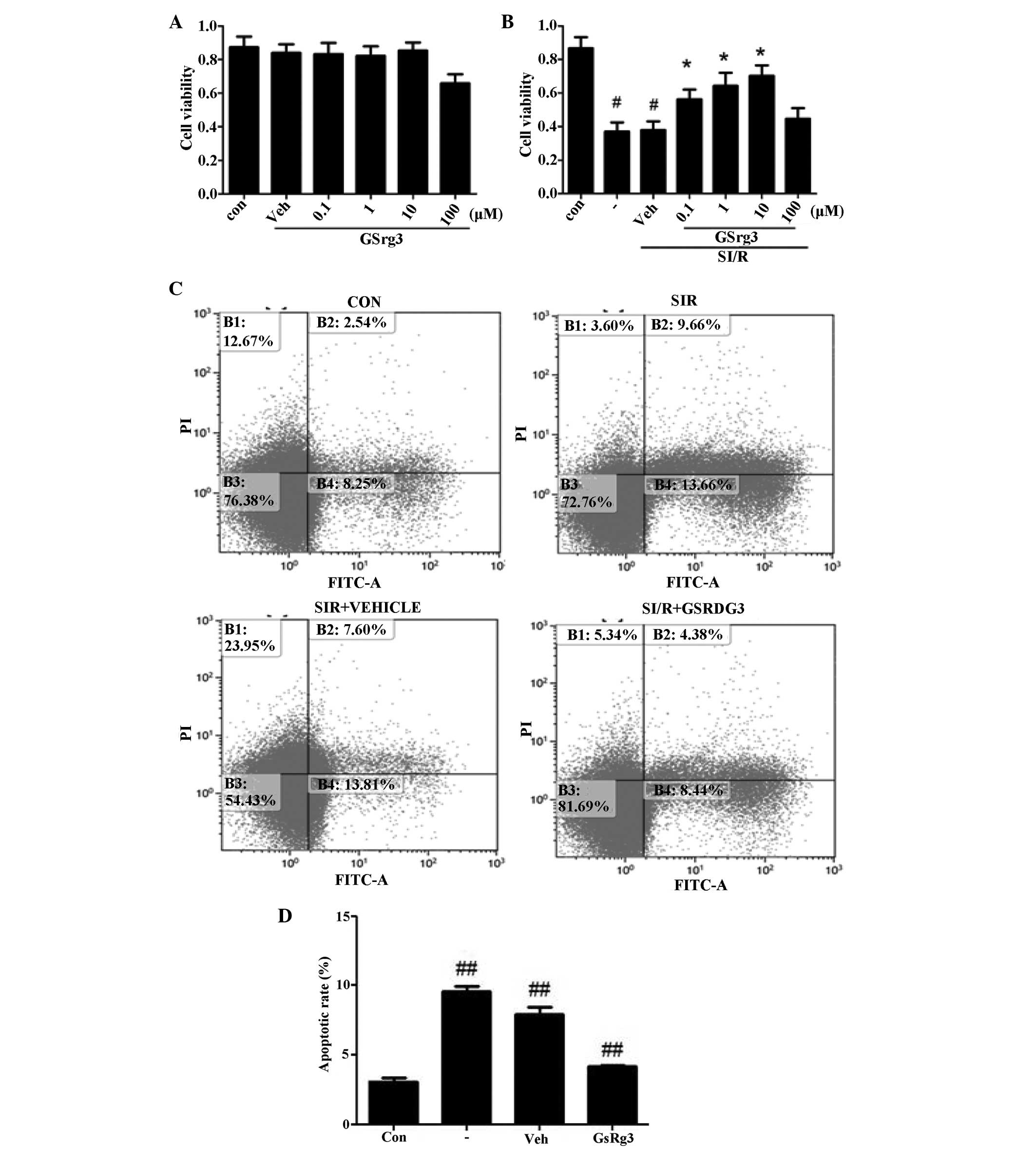 | Figure 5GSRg3 improves SI/R-induced in
vitro cell injury (viability and apoptosis). (A) GSRg3
treatment alone (0.1–100 μM) for 24 h had no effect on NRC
viability, suggesting that no GSRg3-induced toxicity occurred at
concentrations up to 10 μM (n=8; *P<0.05, vs.
control). (B) Cellular viability was determined using an MTT assay
following SI/R (3 h hypoxia followed by 2 h reoxygenation). (C)
SI/R-induced apoptosis was determined by annexin V-FITC/PI flow
cytometry in control and vehicle groups. (D) GSRg3 (10 mM)
significantly reduced SI/R-induced apoptosis as determined by
annexin V-FITC/PI flow cytometry. Data are expressed as the mean ±
standard error of the mean (#P<0.05 and
##P<0.01, vs. control; *P<0.01, vs.
SI/R.) These experiments were performed in triplicate with similar
results. SI/R, simulated ischemia/reperfusion; GSRg3, ginsenoside
Rg3; NRC, neonatal rat cardiomyocyte; FITC, fluorescein
isothiocyanate; PI, propidium iodide; Con, control; Veh, vehicle
(0.2% (v/v) dimethyl sulfoxide treatment 30 min prior to SI/R). |
Flow cytometric analysis was perfomed to assess the
cellular apoptosis (Fig. 5C).
Annexin V/PI double staining revealed a significant increase in
apoptosis in the sham group compared with the control post-SI/R
(8.6±0.3%, vs. 3.1±0.2%; P<0.05), and treatment with 10 mM GSRg3
markedly decreased cellular apoptosis (4.6±0.1%; P<0.01;
Fig. 5D). Overall, these in
vitro results suggested that GSRg3 protected cardiomyocytes,
which was in accordance with the in vivo data.
GSRg3 modulates the expression levels of
Bcl-2 and Bax in NRCs subjected to SI/R
The present study aimed to determine whether GSRg3
inhibited the apoptosis of NRCs induced by SI/R by modulating the
Bcl-2 family proteins. SI/R treatment reduced the expression of
Bcl-2 and increased the expression of Bax, therefore,
downregulating the Bcl-2/Bax ratio (Fig. 6A). Pretreating NRCs with 10 mM
GsRg3 prior to SI/R induced the expression of Bcl-2 and inhibited
the expression of Bax, therefore, increasing the Bcl-2/Bax ratio
(Fig. 6A).
 | Figure 6GSRg3 inhibits mitochondrial-mediated
apoptosis in NRCs subjected to SI/R. (A) Representative western
blot analysis demonstrating the expression of Bcl-2 and Bax
following various treatments. Densitometric analysis demonstrated
that SI/R reduced the ratio of Bcl-2/Bax, however, treatment with
GSRg3 increased the Bcl-2/Bax ratio. (B) Representative western
blot analysis of the SI/R-induced activation of caspase-3 and
caspase-9. Densitometric analysis demonstrated that 10 mM GSRg3
reduced the expression levels of cleaved caspase-9 and caspase-3.
Data are expressed as the mean ± standard error of the mean (n=6;
##P<0.01, vs. control, #P<0.05 and
**P<0.01, vs. SI/R.) SI/R, simulated
ischemia/reperfusion; GSRg3, ginsenoside Rg3; NRC, neonatal rat
cardiomyocyte; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 associated X
protein; Con, control; Veh, vehicle (0.2% (v/v) dimethyl sulfoxide
treatment 30 min prior to SI/R). |
GSRg3 decreases the activities of
caspase-3 and caspase-9 in NRCs following SI/R
The caspase family of proteins regulate cellular
apoptosis. Caspase-9 is activated by cytochrome c, which
activates caspase-3, causing cell apoptosis (4). SI/R significantly increased the
protein expression levels of cleaved caspase-9 and caspase-3,
however, pretreating NRCs with 10 mM GsRg3 significantly attenuated
the expression levels of cleaved caspase-9 and caspase-3 (Fig. 6B).
GSRg3 increases the phosphorylation of
Akt and eNOS in NRCs subjected to SI/R
To further investigate the molecular mechanism
underlying GsRg3-mediated cardioprotection, western blot analysis
was performed to determine the protein expression levels of
phosphorylated (p)-Akt/Akt and p-eNOS in NRCs following SI/R. No
significant differences were observed in the expression levels of
Akt and eNOS between the treatment groups at the baseline (Fig. 7A and B). Consistent with previous
reports, pretreatment with 10 mM GSRg3 significantly increased the
expression levels of p-Akt and p-eNOS, and consequently increased
the ratios of p-Akt/Akt and p-eNOS/eNOS (P<0.01). Treatment with
the phosphoinositide 3-kinase inhibitor, LY294002, inhibited the
GSRg3-mediated phosphorylation of Akt (Fig. 7B).
 | Figure 7GSRg3 increases the phosphorylation of
Akt and eNOS in NRCs subjected to SI/R. Densitometric analysis
demonstrated that GSRg3 increased the ratio of (A) p-eNOS/eNOS and
(B) p-Akt/Akt, and the increasing ratio of p-Akt/Akt was
significantly inhibited by the Akt inhibitor, LY294002. Data are
expressed as the mean ± standard error of the mean (n=6;
#P<0.05 and ##P<0.05, vs. SI/R;
*P<0.01, vs. SI/R+GSRg3.) SI/R, simulated
ischemia/reperfusion; GSRg3, ginsenoside Rg3; NRC, neonatal rat
cardiomyocyte; eNOS, endothelial nitric oxide synthase; Con,
control; Veh, vehicle(0.2% (v/v) dimethyl sulfoxide treatment 30
min prior to SI/R); p, phosphorylated. |
Discussion
The present study revealed that GSRg3 significantly
attenuated MI/R injury in the rat model, as demonstrated by the
reduced myocardial infarct size, improved rat cardiac functions,
CK/LDH levels in blood following MI/R and decreased NRC apoptosis.
The in vitro investigation revealed that treatment with
GSRg3 (10 mM) reduced the NRC apoptotic response by inhibiting the
activation of caspase-3 and caspase-9 and by increasing the
phosphorylation of Akt/eNOS and the Bcl-2/Bax ratio.
As one of the most popular Chinese herbal medicines,
ginseng has been used for the treatment of diabetes, cancer and
cardiovascular diseases for thousands of years (7,8).
Over 40 ginsenosides have been isolated and identified (9). Previous studies have demonstrated
that ginsenosides exerts significant protective effects on the
cardiovascular system (9–11). MI/R injury is predominantly caused
by ardiomyocyte apoptosis (12)
and GSRg3 is able to directly depress cardiomyocytes contraction by
increasing the production of nitric oxide (NO) (13). Yang et al demonstrated that
the NO produced by eNOS has a direct impact on cardiac remodeling
(14). In addition, it has also
been suggested that GSRg1 is important in the improvement of the
cardiovascular system. In a tumor necrosis factor-α stimulated
HUVEsl culture model, GSRg1 increases the production of NO and the
mRNA expression of eNOS (15).
In vitro, GSRg1 is capable of reducing homocysteine-induced
endothelial dysfunction and free radical production in porcine
coronary arteries (16–18). Therefore, the present study aimed
to determine whether pretreatment with GSRg1 reduced myocardial
infarction following MI/R, and whether this had significant
clinical importance. A previous study suggested that GSRg1 may
induce the production of NO and regulate the acute activation of
eNOS in human aortic endothelial cells (19).
GSRg3, an important ginsenoside in the extract of
ginseng, is used in herbal medicine as a tonic and restorative
agent. However, the molecular mechanism underlying the beneficial
effects of GSRg3 remains to be elucidated. The present study
demonstrated, using an in vivo rat model, that pretreatment
with GSRg3 significantly decreased the infarct size and plasma
levels of CK/LDH. The levels of CK/LDH and oxidative stress in the
myocardium were also significantly suppressed in the GSRg3 treated
group, whereas the level of SOD was improved. In vitro, SI/R
treatment increased the expression of Bax, a pro-apoptotic protein,
decreased the expression of Bcl-2, an anti-apoptotic protein,
reduced the Bcl-2/Bax ratio and activated caspase-3 and caspase-9.
GSRg3 upregulated the phosphorylation of eNOS and increased the
expression of p-Akt.
In conclusion, GSRg3 exerted cardioprotective
effects in MI/R injury and may have a positive significance for
clinical treatment.
References
|
1
|
Terman A and Brunk UT: The effect of
Polbax extract on lipofuscin accumulation in cultured neonatal rat
cardiac myocytes. Phytother Res. 16:180–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chlopcíková S, Psotová J, Miketová P,
Sousek J, Lichnovský V and Simánek V: Chemoprotective effect of
plant phenolics against anthracycline-induced toxicity on rat
cardiomyocytes. Part II caffeic, chlorogenic and rosmarinic acids.
Phytother Res. 18:408–413. 2004. View
Article : Google Scholar
|
|
3
|
Li SY, Wang XG, Ma MM, Liu Y, Du YH, et
al: Ginsenoside-Rd potentiates apoptosis induced by hydrogen
peroxide in basilar artery smooth muscle cells through the
mitochondrial pathway. Apoptosis. 17:113–120. 2012. View Article : Google Scholar
|
|
4
|
Wang Y, Li X, Wang X, Gao F, et al:
Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury
via Akt/GSK-3β signaling and inhibition of the
mitochondria-dependent apoptotic pathway. PLoS One. 8:e709562013.
View Article : Google Scholar
|
|
5
|
Zhu JH, Qiu YG, Wang QQ, Zhu YJ, Hu SJ,
Zheng LG, et al: Low dose cyclophosphamide rescues myocardial
function from ischemia-reperfusion in rats. Eur J Cardiothorac
Surg. 34:661–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Gao Y, Tian J, Shen J, Xing Y, Liu
Z, et al: Sophocarpine administration preserves myocardial function
from ischemia-reperfusion in rats via NF-κB inactivation. J
Ethnopharmacol. 135:620–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang YS, Seo EK, Gyllenhaal C and Block
KI: Panax ginseng: a role in cancer therapy? Integr Cancer Ther.
2:13–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attele AS, Zhou, Yuan CS, et al:
Antidiabetic effects of Panax ginseng berry extract and the
identification of an effective component. Diabetes. 51:1851–1858.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Han B, Yu X, Qu S and Sui D:
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury
in rats. Pharm Biol. 49:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng L, Sun S, Xie LH, Wicks SM and Xie
JT: Ginsenoside Re: pharmacological effects on cardiovascular
system. Cardiovasc Ther. 30:e183–188. 2012. View Article : Google Scholar
|
|
11
|
Xia R, Zhao B, Wu Y, Hou JB, Zhang L, et
al: Ginsenoside Rb1 preconditioning enhances eNOS expression and
attenuates myocardial ischemia/reperfusion injury in diabetic rats.
J Biomed Biotechnol. 2011:7679302011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji L, Fu F, Zhang L, Liu W, Cai X, et al:
Insulin attenuates myocardial ischemia/reperfusion injury via
reducing oxidative/nitrative stress. Am J Physiol Endocrinol Metab.
298:871–880. 2010. View Article : Google Scholar
|
|
13
|
Scott GI, Colligan PB, Ren BH and Ren J:
Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat
ventricular myocytes: role of nitric oxide. Br J Pharmacol.
134:1159–1165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XP, Liu YH, Shesely EG, Bulagannawar
M, Liu F and Carretero OA: Endothelial nitric oxide gene knockout
mice: cardiac phenotypes and the effect of angiotensinconverting
enzyme inhibitor on myocardial ischemia/reperfusion injury.
Hypertension. 34:24–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lü JP, Ma ZC, Yang J, Huang J, Wang SR and
Wang SQ: Ginsenoside Rg1-induced alterations in gene expression in
TNF-α-stimulated endothelial cells. Chin Med J (Engl). 117:871–876.
2004.
|
|
16
|
Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q
and Chen C: Ginsenoside Rb1 blocks homocysteine-induced endothelial
dysfunction in porcine coronary arteries. J Vasc Surg. 41:861–868.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu W, Conklin BS, Lin PH, Lumsden AB, Yao
Q and Chen C: Red wine prevents homocysteine-induced endothelial
dysfunction in porcine coronary arteries. J Surg Res. 115:82–91.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spencer TA, Chai H, Fu W, et al: Estrogen
blocks homocysteine-induced endothelial dysfunction in porcine
coronary arteries (1,2). J Surg Res. 118:83–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, Eto M, Akishita M, Kaneko A, Ouchi Y
and Okabe T: Signaling pathway of nitric oxide production induced
by ginsenoside Rb1 in human aortic endothelial cells: a possible
involvement of androgen receptor. Biochem Biophys Res Commun.
353:764–769. 2007. View Article : Google Scholar : PubMed/NCBI
|





















