Introduction
Breast cancer is a serious threat to the health of
females worldwide. Approximately 120 million individuals develop
breast cancer each year, resulting in 500,000 fatalities (1,2).
Treatment regimens for breast cancer include chemotherapy,
endocrine therapy and molecular targeted therapy. Breast cancer
chemotherapy mainly uses drugs developed in the 1970s, including
cyclophosphamide, methotrexate, fluorouracil and taxane paclitaxel
(3,4). Endocrine treatment of breast cancer
is performed via the use of estrogen antagonists and aromatase
inhibitors (5). Breast cancer
treatment through molecular targeting agents constitute drugs that
target the human epidermal growth factor receptor (HER) family,
including trastuzumab/herceptin, lapatinib and others. Angiogenesis
inhibitors (bevacizumab/avastin) have also been used in clinical
trials (6,7).
The HER family includes HER1 (epidermal growth
factor receptor; EGFR), HER2, HER3 and HER4, which are
transmembrane tyrosine kinase receptors. They are mainly involved
in cell growth, proliferation and signal transduction (8). HER-2/neu, also known as c-erb-B2, is
a proto-oncogene, which is expressed in a variety of tumors and
metastases, including breast cancer, head and neck cancer,
colorectal cancer and ovarian cancer (9–12). A
study has confirmed that 20–30% of patients with breast cancer have
a high expression of HER-2 and that overexpression of HER-2 in
patients correlates with a poor prognosis and resistance to
cytotoxic chemotherapeutic drugs (13). The anti-HER2 monoclonal antibody
herceptin was the first to be used in breast cancer treatment and
has been widely applied in the treatment of breast cancer in China
(14). At present, herceptin is
used as a first-line treatment against breast cancer. Herceptin is
a humanized immunoglobulin (Ig) G1 monoclonal antibody, which
inhibits the signal transduction pathways of cell growth and
mediates the antibody-dependent cellular cytotoxicity mechanisms to
inhibit tumor growth (15). In
clinical trials, the remission rate of adriamycin and
cyclophosphamide (AC) chemotherapy is 42% for metastatic breast
cancer and the combinatorial therapy of herceptin with the AC
program is associated with an increased remission rate of 65%
(16). Additionally, in therapies
combining herceptin with paclitaxel, the complete remission rate is
57%, which is higher than that with therapies using only paclitaxel
(25%) (16). However, herceptin is
a chimerized murine/human anti-HER2 IgG1 antibody and therapies
involving herceptin do have disadvantages, including drug
resistance, frequent side effects and requirements of high
concentrations of the antibody (17).
An efficient, genetically engineered bispecific
antibody (BsAb) was developed in previous years, which utilizes a
combination of antibodies targeting the surface of T cells (cluster
of differentiation; CD3) and tumor surface antigens (epithelial
cell adhesion molecule, CD19 and HER2) (18). CD3 is the main component of the
T-cell receptor/CD3 complex on the T-cell surface and is the key
molecule required for the activation of T lymphocytes. The CD3
antibody binds to CD3 and induces the activation of T lymphocytes,
which is followed by the release of perforin, granzyme factor and
cytokines, which are important in eliminating tumor cells (19). Therefore, the cytotoxic T cell is
the most important effector of cellular immunity and T cells are
critical for the clearance of tumor cells in vivo. The BsAb
activates the cytotoxicity of T cells by specifically binding to
the T cells and tumor cells, thereby eliminating the tumor cells.
In addition, the required clinical dose of the BsAb is extremely
low at only 1/500 of that required for herceptin (20). Previous studies have confirmed that
BsAbs targeting CD3 and the tumor antigens may activate cytotoxic T
cells to specifically target and eliminate tumor cells (21).
In the present study, a fully human recombinant
single-chain HER2/CD3 BsAb was constructed. To the best of our
knowledge, the present study was the first to describe a fully
human HER2/CD3 BsAb with high levels of anti-tumoral activity. The
recombinant BsAb molecule was expressed and secreted in a fully
active form by mammalian cells. The binding characteristics of the
HER2/CD3 BsAb and its ability to stimulate T-cell activation and to
induce lysis of BT474 and SKBR-3 cells were assessed. In addition,
the ability of the HER2/CD3 BsAb to inhibit the growth of breast
cancer tissue and to induce the proliferation of tumor
tissue-infiltrating lymphocytes was examined. The results of the
present study indicated that HER2/CD3 BsAb may be a suitable
candidate for the treatment of breast cancer.
Materials and methods
Cell lines and breast cancer tissue
The HER2-positive cell lines BT474 and SKBR-3, the
CD3-positive T-cell line Jurkat and the HER2-negative cell line
MDA-MB-231 were all obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Chinese hamster ovary cells
(CHO) used for expressing BsAbs were also obtained from the ATCC.
Human breast cancer tissues were obtained from breast cancer
patients undergoing biopsy at the First Affiliated Hospital of
Chengdu Medical College (Chengdu, China) in march 2013. Inclusion
criteria were that the tumors were HER2 positive, first operation
patients without medication and radiation. The protocol of the
present study was approved by the Institutional Ethics Committee of
Chengdu Medical College. Informed consent for the present study was
received from all patients prior to the commencement of the
experiments.
Construction of single-chain BsAb
fragments
The single-chain variable fragment (scFv) of HER2
and CD3 antibodies were cloned from the vectors pET-26a-HER2 and
pET-26a-CD3, respectively. These two vectors have been previously
established in our laboratory (Gastroenterology Tumor and
Microenvironment Laboratory, Chengdu, China) and the gene sequences
of the HER2 and CD3 antibodies were screened from the human natural
antibody library (22). The
anti-HER2 scFv fragment and anti-CD3 scFv fragment were linked with
(G4S)3 by overlapping polymerase chain
reaction to produce the recombinant protein VH(HER2)-VL(HER
2)-(G4S)3-VL(CD3)-VH(CD3), with an inserted
interleukin (IL)-2 signal peptide at the N-terminus and a histidine
tag at the C-terminus. Subsequently, the entire BsAb molecule was
cloned into the expression vector pcDNA3.1 (Stratagene, La Jolla,
CA, USA).
BsAb expression and purification
CHO cells (2×105/1 ml) were cultured in
six-well flat-bottom plates and transfected with 2 μg
plasmid DNA and 4 μg Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). The transfection medium was
removed after 4 h and cells were incubated at 37°C with fresh
medium. CHO cells, which stably expressed HER2/CD3 BsAb were
screened using G418 antibiotics (Gibco Life Technologies, Grand
Island, NY, USA) for 48 h. The culture supernatant was collected
and purified by immobilized nickel metal affinity chromatography
(ÄKTA explorer, GE Healthcare, Little Chalfont, UK) on Ni-charged
chelating sepharose (Amersham Pharmacia Biotech, GE
Healthcare).
Flow cytometry
BT474, SKBR-3 and Jurkat cells as well as peripheral
blood mononuclear cells (PBMCs; provided by State Key Laboratory or
Biotherapy) were used for detection of antibody binding, while the
HER2/CD3 negative cell line MDA-MB-231 was used as a negative
control. A total of 1×106 cells were washed with
phosphate-buffered saline (PBS; 137 mmol/l NaCl, 2.7 mmol/l KCl, 10
mmol/l Na2HPO4 and 2 mmol/l
KH2PO4) and incubated in 100 μl
HER2/CD3 BsAb (100 μg/ml in PBS) for 30 min at room
temperature and then washed twice with PBS. Fluorescein
isothiocyanate-conjugated antibody against the His-tag (Abcam,
Cambridge, UK) was used for detecting the BsAb. The His-tag
antibody (ab1206) is a rabbit polyclonal IgG, which only reacts
with human proteins. The antibody was diluted at 1:200 and added to
the cells for 30 min at room temperature. Cells were analyzed using
fluorescence-activated cell sorting (CytoFLEX; Beckman-Coulter,
Pasadena, CA, USA).
Induction of T-cell activation
Freshly prepared PBMCs (2×106 cells/ml)
were added to each well of a six-well flat-bottom plate (Molecular
Devices, Sunnyvale, CA, USA). Each well contained 2 ml RPMI 1640
(HyClone, Logan, Utah, USA) with 10% fetal calf serum (FCS) only
(control wells), or with 10% FCS and orthoclone OKT3 (30 ng/ml;
Wuhan Institute of Biological Products, Wuhan, China) or with 10%
FCS and HER2/CD3 BsAb (10 ng/ml). PBMCs were incubated for 24 h and
the activation of PBMCs was measured using flow cytometric
analysis. The expression levels of CD25 and CD69 on T cells were
detected by flow cytometry to evaluate the T-lymphocyte activation
ability of HER2/CD3 BsAbs.
Luminex liquid chip analysis
A luminex liquid chip array was used to determine
the release of inflammatory cytokines IL-2, IL-4, tumor necrosis
factor (TNF)-α and interferon (IFN)-γ from PBMCs induced by HER
2/CD3 BsAb. A human MultiAnalyte Profiling Base kit (R&D,
Minneapolis, Minnesota, USA) was used for detection. Freshly
prepared PBMCs (2×106 cells/ml) were added to each well
of a 96-well flat-bottom plate. Each well contained 100 μl
complete media alone (control wells), or with complete media
containing 1 μg/ml CD28 Ab (TGN1412; eBioscience, San Diego,
CA, USA), CD3 Ab OKT3 or HER2/CD3 BsAb. Each assay was performed in
triplicate. The PBMCs were incubated at 37°C under 5%
CO2 for 72 h and 50-μl aliquots of media were
collected for the liquid chip array. Briefly, the diluted
microparticle mixture was resuspended and 50 μl of the
mixture was added to each well of the microplate. Subsequently, 50
μl of the standard or sample was added to each well and
incubated for 3 h at room temperature using a vacuum manifold
device designed to accommodate a microplate. Subsequently, 50
μl diluted biotin antibody cocktail was added to each well
and the plate was incubated for 1 h at room temperature, whilst
agitated at 45 × g. Diluted streptavidin-phycoerythrin (50
μl) was added to each well and incubated for 30 min at room
temperature, whilst agitated at 500 rpm. The microparticles were
resuspended by adding 100 μl wash buffer to each well and
incubated for 2 min, whilst agitated at 500 rpm. The fluorescence
signal was read using a Luminex-100 analyzer (Luminex Corp.,
Austin, TX, USA) within 90 min.
Cytotoxicity assay
The HER2-positive cell lines BT474 and SKBR-3 were
used as target cells and the MDA-MB-231 cells were used as negative
controls. Cytotoxicity was measured using a CytoTox 96®
Non-Radioactive Cytotoxicity assay kit (Promega, Madison,
Wisconsin, USA) using RPMI 1640 complete medium with 5% FCS in a
round-bottom 96-well plates. Briefly, PBMCs were added as effector
cells to each well at gradient concentrations, followed by the
addition of the target cells (1×104). HER2/CD3 BsAb (100
ng/ml) was then added to achieve final effector cell to target cell
(E:T) ratios of 100:1, 50:1, 10:1 and 1:1. The cell mixtures were
incubated at 37°C under 5% CO2 for 4 h, following which
50 μl aliquots of media were transferred to fresh 96-well
flat-bottom plates for the LDH-release assay. The percentage of
cell lysis was calculated as the specific release (%) =
(experimental release − effector spontaneous release − target
spontaneous release) / (target maximum release − target spontaneous
release) × 100. Each assay was performed in triplicate.
Primary culture of HER2-positive breast
cancer tissue with HER2/CD3 BsAb
Primary cultures of breast cancer tissue samples for
detecting the activity of HER2/CD3 BsAb were initiated by
collecting tissue samples of HER2-positive breast cancer from six
patients under sterile conditions. The tumor tissues were washed
with saline and the fatty tissues and necrotic tissues surrounding
the tumor tissue were removed, following which the samples were cut
into pieces of 4–8 mm3. The tissue samples were weighed
and divided into three groups of equal weight randomly. One group
was inoculated with RPMI 1640 medium alone and the other group was
inoculated with RPMI 1640 medium containing 0.1 μg/ml
HER2/CD3 BsAb or 1 μg/ml HER2/CD3. The tissue samples were
incubated at 37°C under 5% CO2 for five days. On the
third day of the incubation period, one tissue sample each was
removed from the control group and the experimental groups for
hematoxylin and eosin (HE; Beyotime Institue of Biotechnology,
Inc., Shanghai, China) staining to determine the proliferation of
tumor-infiltrating T cells. On the fifth day, images were captured
(Nikon D90 camera; Nikon, Tokyo, Japan) of the remaining tissue
samples and their weights were measured. The changes in the volume
and weight of the tissue samples were used as measures of
therapeutic efficacy.
Statistical analysis
Values are expressed as the mean ± standard
deviation of at least three independent experiments. Differences
between the treatment groups in the cytotoxicity assays and tumor
tissue weight were analyzed using analysis of variance. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were calculated using SPSS 16.0 (SPSS,
Inc., Chicago, IL, USA) software.
Results
Preparation and binding properties of
HER2/CD3 BsAb
In the recombinant plasmid pcDNA3.1, the scFv
fragments were linked with the (G 4S)3 linker
in the format VH
(HER2)-VL(HER2)-(G4S)3-VL(CD3)-VH(CD3)-6xHis.
The IL-2 signal peptide upstream of the HER2/CD3 BsAb directed the
HER2/CD3 BsAb to be secreted into the supernatant. The CHO cell
culture supernatant was passed through an immobilized nickel metal
affinity chromatography column and the HER2/CD3 BsAb eluted from
the Ni-NTA column at 300 mM imidazole as a distinct peak. The
purified HER2/CD3 BsAb was subjected to 10% SDS-PAGE and analyzed
using western blotting. The protein migrated with an apparent
molecular mass of 57 kDa, consistent with the theoretical molecular
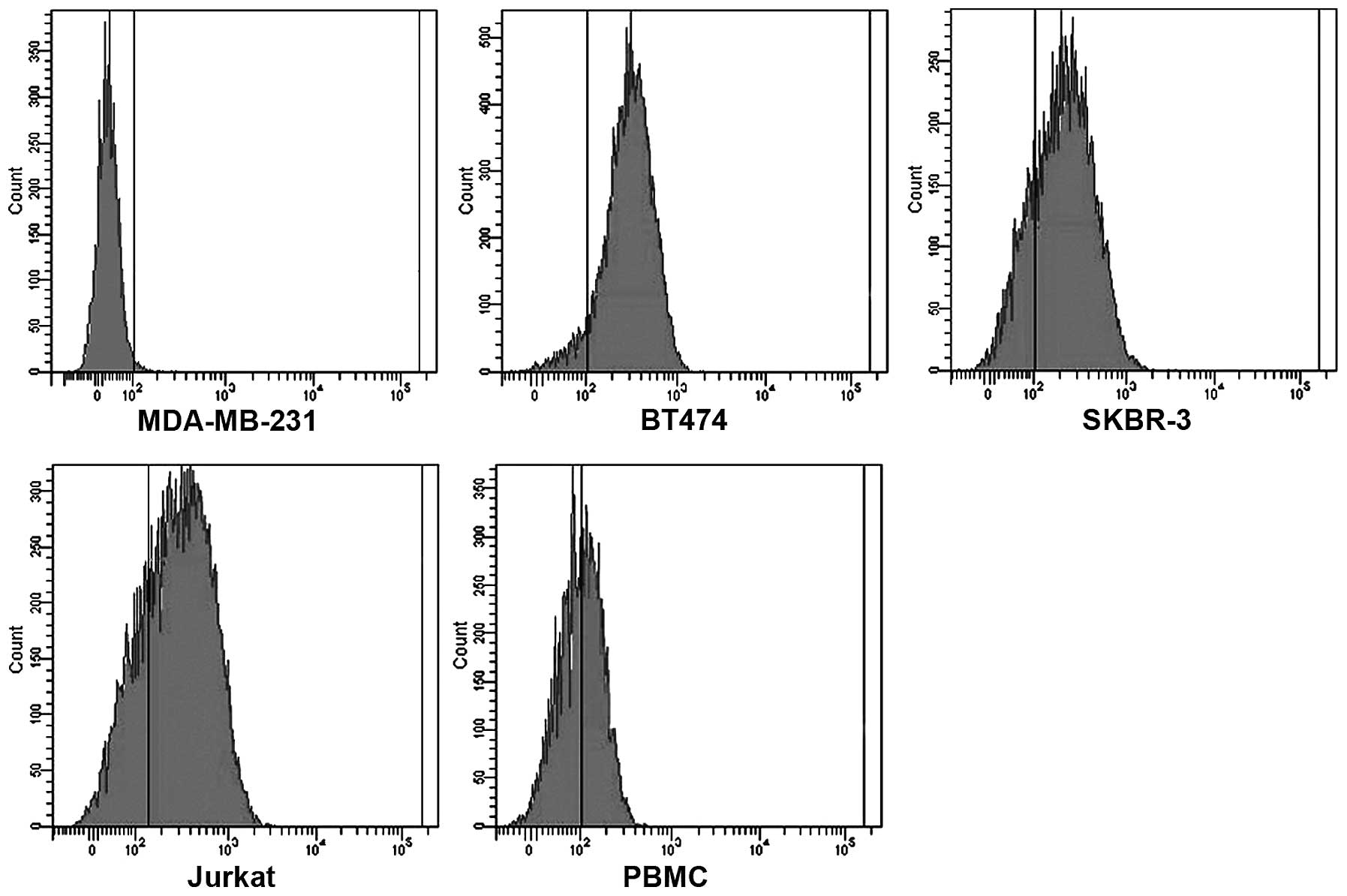
weight. In addition, HER2/CD3 BsAb bound specifically to the
HER2-positive BT474 and SKBR-3 cells, as well as CD3-positive
Jurkat and PBMCs cells; however, there was no detectable binding to
MDA-MB-231 cells that express neither HER2 nor CD3 (Fig. 1). These results indicated that the
HER2/CD3 BsAb specifically bound to HER2 and CD3.
HER2/CD3 BsAb induces T-cell
activation
The expression of CD25 and CD69 on T cells is
rapidly upregulated upon activation. To evaluate the ability of
HER2/CD3 BsAb to activate T lymphocytes, the expression of CD25 and
CD69 on T cells was monitored using flow cytometry. The results
demonstrated that the rate of CD25-expressing cells was 8.4% and
the rate of CD69-expressing cells was 36.7% among cells treated
with HER2/CD3 BsAb, compared with those in the PBS-treated group.
In CD4 and CD8 T cells from the HER2/CD3 BsAb-treated group, the
rate of CD25-expressing cells was 4.0 and 3.9%, respectively, while
the rate of CD69-expressing cells was 19.7 and 16.5%, respectively.
In cells treated with OKT3, the rate of CD25-expressing cells was
6.5% and the rate of CD69-expressing cells was 37.5%. In CD4 or CD8
T cells from the OKT3-treated group, the expression proportion of
CD25 was 3.7 and 2.5%, respectively, and that of CD69 was 20.6 and
15.3%, respectively (Fig. 2).
These data indicated that T-cell activation by HER2/CD3 BsAb was
similar to that by OKT3, without any pre-stimulus to induce T-cell
activation.
HER2/CD3 BsAb induces release of
cytokines from PBMCs
Secretion of cytokines, including TNF-α, IFN-γ, IL-4
and IL-2 from PBMCs induced by HER2/CD3 BsAb, CD3-Ab OKT3 and
CD28-Ab were determined under similar conditions to those described
above (2×106 cells/ml PBMCs, 1 μg/ml CD28 Ab, CD3
Ab OKT3 or HER2/CD3 BsAb incubated for 72 h). The results revealed
that the release of TNF-α, IFN-γ and IL-2 induced by CD28-Ab was
significantly higher than that induced by OKT3 and HER2/CD3 BsAb,
whereas the secretion of cytokines induced by OKT3 and HER2/CD3
BsAb were comparable. No significant differences were identified
between OKT3, CD28-Ab and HER2/CD3 BsAb in their ability to induce
the release of IL-4 (Fig. 3).
 | Figure 3Liquid chip analysis of cytokines
released by activated T lymphocytes. PBMCs were treated with PBS, 1
μg/ml CD28 Ab, CD3 Ab OKT3 or HER2/CD3 BsAb for 72 h.
Release of the cytokines (A) IFN-γ, (B) TNF-α, (C) IL-4 and (D)
IL-2 was measured. *P<0.01, vs. other three groups.
Ab, antibody; BsAb, bispecific antibody; CD, cluster of
differentiation; HER, human epidermal growth factor receptor; PBMC,
peripheral blood mononuclear cell; IFN, interferon; TNF, tumor
necrosis factor; IL, interleukin; PBS, phosphate-buffered
saline. |
HER2/CD3 BsAb mediates specific
cytotoxicity against breast cancer cells
The cytotoxic activity of HER2/CD3 BsAb against the
breast cancer cell lines BT474 and SKBR-3 were measured using the
LDH-release assay. Unstimulated PBMCs were added as effector cells
to target BT474 and SKBR-3 cells at E:T ratios of 100:1, 50:1, 10:1
and 1:1. In parallel wells, HER2-negative breast cancer MDA-MB-231
cells were used as a negative control. HER2/CD3 BsAb may
significantly induce BT474 and SKBR-3 cell death at all E:T ratios
without pre-stimulation, while no cytotoxic activity was observed
when MDA-MB-231s were used as target cells. The cytotoxic activity
of HER2/CD3 BsAb was significantly increased at higher E:T ratios
(Fig. 4). These results suggested
that HER2/CD3 BsAb-mediated cytotoxic effects were HER2-specific
and dependent on T cells without pre-stimulation.
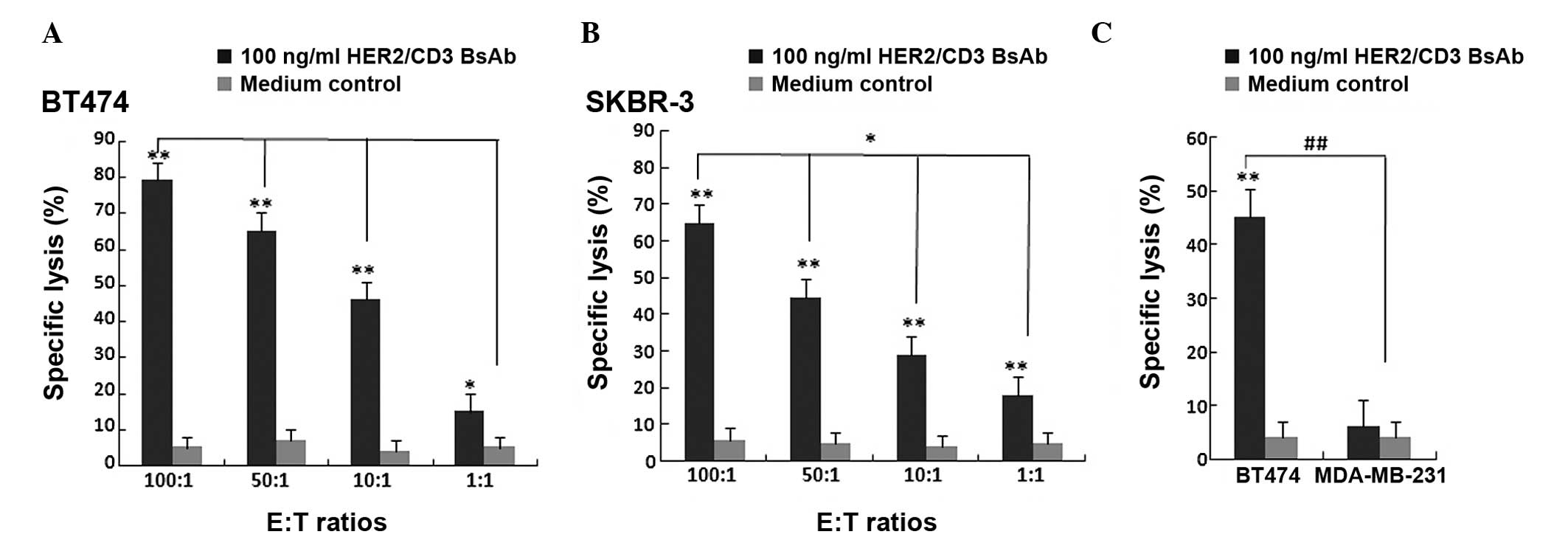 | Figure 4Cytotoxic effect of HER2/CD3 BsAb.
Cytotoxicity of HER2/CD3 BsAb as measured using a lactate
dehydrogenase-release assay. Cells were incubated for 4 h with 100
ng/ml HER2/CD3 BsAb. Primary human PBMCs and (A) BT474 or (B)
SKBR-3 cells were incubated at various E:T ratios (100:1, 50:1,
10:1 and 1:1). Values are expressed as the mean ± standard
deviation. *P<0.01, **P<0.001, compared
with medium control. (C) Primary human PBMCs and BT474 cells or
MDA-MB-231 cells were incubated at an E:T ratio of 10:1. Values are
expressed as the mean ± standard deviation.
##P<0.001, **P<0.001, compared with
medium control. BsAb, bispecific antibody; CD, cluster of
differentiation; HER, human epidermal growth factor receptor; E:T,
effector cell to target cell; PBMC, peripheral blood mononuclear
cell. |
Inhibition of breast cancer cell growth
by HER2/CD3 BsAb
In order to detect the anti-tumoral activity of
HER2/CD3 BsAb, the HER2-positive breast cancer tissue samples from
six patients were treated with RPMI 1640 medium alone or RPMI 1640
medium containing 0.1 μg/ml or 1 μg/ml HER2/CD3 BsAb
and the tumor growth was determined using volume and weight
measurements. The colon cancer tissues selected for incubation with
HER2/CD3 BsAb were HER2-positive (Fig.
5A). The results demonstrated that treatment with 0.1
μg/ml or 1 μg/ml HER2/CD3 BsAb significantly
inhibited breast tumor cell growth compared with that of
vehicle-treated cancer tissue samples. A significant reduction in
the growth of HER2-positive breast cancer cells from six patients
was observed. The volume of the tissue samples treated with
HER2/CD3 BsAb was significantly lower than that of the
vehicle-treated tissue. With the increase in the concentration of
HER2/CD3 BsAb, the weight of the tissue samples decreased. The
results revealed that the HER2/CD3 BsAb significantly inhibited the
growth of HER2-positive breast tumor cells (Fig. 5B and C). HE staining demonstrated
that HER2/CD3 BsAb was able to induce the proliferation of tumor
tissue-infiltrating lymphocytes (Fig.
5D). This indicated that the HER2/CD3 BsAb-mediated
anti-tumoral effects were HER2-specific and dependent on the tumor
tissue-infiltrating lymphocytes.
Discussion
The use of BsAbs (mouse origin) for the treatment of
breast cancer has been observed to be effective in vitro and
in vivo (23,24). In the present study, a fully human
recombinant single chain BsAb, which targeted CD3 and HER2, was
constructed. Recombinant HER2/CD3 BsAb acted as a powerful
stimulator of T-cell activation and induced cytotoxicity in breast
tumor BT474 and SKBR-3 cells in the presence of T cells. HER2/CD3
BsAb may also efficiently inhibit the growth of HER2-positive
breast tumor samples by activating and inducing the proliferation
of tumor tissue-infiltrating lymphocytes. The anti-tumoral effects
of HER2/CD3 BsAb required no pre-stimulation with human PBMCs, even
at low doses of HER2/CD3 BsAb (0.1 μg/ml). Furthermore, the
cytokine release assay revealed that HER2/CD3 BsAb was not similar
to the anti-CD28 agonist antibody (TGN1412). These results
indicated that the HER2/CD3 BsAb is a potent candidate treatment
for patients with HER2 positive breast cancer.
The pharmacodynamic evaluation of BsAbs in
vivo is a complex process. Conventionally, the evaluation is
mainly performed through the establishment of tumor animal models
followed by treatment with BsAbs and lymphocytes. In addition, the
changes in tumor weight and survival time may be used as measures
of therapeutic efficacy (25,26).
However, this method does have certain limitations. Firstly, the
type of animal model and treatment method may markedly affect the
treatment efficacy of BsAbs and therefore, it is difficult to
isolate the effects of the clinical condition of the tumor from the
animal model and treatment method. Secondly, a large volume of
fresh blood is necessary for extracting the lymphocytes required
for the experiment. In the present study, fresh breast cancer
tissue culture was used to evaluate the anti-tumoral activity of
BsAbs. Samples of breast cancer tissue which had been surgically
removed were collected and inoculated with HER2/CD3 BsAb. Changes
in the volume and weight of the tissue samples were used as
measures of therapeutic efficacy. It was observed that with an
increase in the concentration of HER2/CD3 BsAb, the weight of the
tissue samples decreased. The advantage of this method is the
relatively simple procedure, reproducibility, controllability and a
more accurate reflection of the in vivo physiological
condition in patients.
The anti-CD28 agonist antibody (TGN1412) has
received attention due to its marked adverse reactions in Phase I
clinical trials (27). TGN1412 is
able to induce T-cell activation to further activate the immune
system by combining with CD28 on the cell surface of T cells. In
the first human clinical trial, within 12–16 h following injection
with TGN1412, all subjects developed symptoms of pulmonary
infiltration, acute lung injury, diffuse intravascular coagulation
and renal failure. In the first six to eight days after TGN1412
injection, two subjects exhibited intense cardiovascular injury,
acute respiratory distress syndrome and multiple organ failure.
Serum analyses of volunteers injected with TGN1412 revealed a
significant increase in the levels of inflammatory cytokines,
including TNF-α and IFN-γ as well as IL-1β, −2, −4, −6, −8 and −10
levels. Cytokines direct the function and activity of the immune
system. When the expression levels of cytokines show sudden and
marked changes, a series of emergency commands are sent to the
lymphocytes, which leads to an immediate induction of T-cell
activation. Activated lymphocytes migrate to the various tissues
and organs, triggering an acute inflammatory reaction, attacking
the system and organs, finally causing multiple organ failure,
which was observed within the subjects in the TGN1412 trial.
Simultaneously, as the bone marrow and the hematopoietic system are
not able to produce a sufficient number of lymphocytes in a short
period of time, peripheral blood lymphocyte depletion occurs.
HER2/CD3 BsAb belongs to the same category of immune
agonist antibodies as TGN1412 and identifies and activates the
immune cells to eliminate tumor cells. Due to the adverse reaction
of TGN1412, it is important to detect inflammatory cytokines. In
the present study, the quantity of TNF-α, IFN-γ, IL-4 and IL-2
induced by HER2/CD3 BsAb, monoclonal antibody to CD3-OKT3 and
monoclonal antibody to CD28 were determined under the same
conditions. The results demonstrated that the release of TNF-α,
IFN-γ and IL-2 induced by the CD28 monoclonal antibody were
significantly higher than that induced by OKT3 and HER2/CD3 BsAb,
while the release of TNF-α, IFN-γ and IL-2 induced by OKT3 was
similar to that induced by HER2/CD3 BsAb. No significant difference
was identified between OKT3, CD28 monoclonal antibody and HER2/CD3
BsAb in stimulating the release of IL-4. Considering that OKT3 is
listed as a drug that is safe and reliable in clinical treatment
and that the CD19/CD3 BsAb antibody has exhibited a potent
anti-tumoral effect and qualified as safe in Phase I clinical
trials (20), HER2/CD3 BsAb is
also expected to be safe in clinical treatment.
Currently, the antibody drugs available for cancer
treatment are either chimeric antibodies or humanized antibodies,
including rituxan and herceptin (28,29).
The main limitation of these antibodies is the marked
immunogenicity that induces a human anti-mouse antibody (HAMA)
response. The HAMA response may cause allergic reactions and the
neutralization of the exogenously administered antibodies, reducing
their efficacy. In the present study, a fully human BsAb was
constructed, which may effectively decrease the immunogenicity of
the BsAb and thus enhance its efficacy and reduce the side
effects.
In conclusion, the fully human recombinant scFv BsAb
against HER2 and CD3 was constructed in the present study, which
was shown to be a highly potent inducer of T-cell activation.
HER2/CD3 BsAb may induce the lysis of cultured SKBR-3 and BT474
cells in the presence of unstimulated T lymphocytes. In addition,
the HER2/CD3 BsAb efficiently inhibited the growth of breast cancer
tissue samples by activating and inducing the proliferation of
tumor tissue infiltrating lymphocytes. Furthermore, when incubated
with PBMCs, HER2/CD3 BsAb did not act similarly to the anti-CD28
agonist antibody (TGN1412), which previously led to a
life-threatening cytokine storm in the first human trials. This
HER2/CD3 BsAb is fully human and only a low dose is required for
significant therapeutic efficacy. It is thus a potential candidate
for the clinical treatment of patients with HER2-positive breast
cancer.
Acknowledgments
The present study was supported by National Clinical
Medicine Research Foundation of China (grant no. L2012055), the
Natural Science Foundation of Chengdu Medical College (grant no.
CYZ12-005), the National Natural Science Foundation of China (grant
no. 81302170) and the National Science and Technology Major
Projects of New Drugs (grant no. 2012ZX09103301-037).
References
|
1
|
El Saghir NS and Anderson BO: Breast
cancer early detection and resources: where in the world do we
start? Breast. 21:423–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu ZG, Jia CX, Liu LY, et al: The
prevalence and correlates of breast cancer among women in Eastern
China. PLoS One. 7:e377842012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliva S, Cioffi G, Frattini S, et al:
Administration of angiotensin-converting enzyme inhibitors and
β-blockers during adjuvant trastuzumab chemotherapy for
nonmetastatic breast cancer: marker of risk or cardioprotection in
the real world? Oncologist. 17:917–924. 2012. View Article : Google Scholar :
|
|
4
|
de Hoon JP, Veeck J, Vriens BE, Calon TG,
van Engeland M and Tjan-Heijnen VC: Taxane resistance in breast
cancer: a closed HER2 circuit? Biochim Biophys Acta. 1825.197–206.
2012.
|
|
5
|
Arapantoni-Dadioti P, Valavanis C,
Gavressea T, Tzaida O, Trihia H and Lekka I: Discordant expression
of hormone receptors and HER2 in breast cancer. A retrospective
comparison of primary tumors with paired metachronous recurrences
or metastases. J BUON. 17:277–283. 2012.PubMed/NCBI
|
|
6
|
Baselga J, Bradbury I, Eidtmann H, et al:
Lapatinib with trastuzumab for HER2-positive early breast cancer
(NeoALTTO): a randomised, open-label, multicentre, phase 3 trial.
Lancet. 379:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boulaamane L, Boutayeb S and Errihani H:
Bevacizumab based chemotherapy in first line treatment of HER2
negative metastatic breast cancer: results of a Moroccan
observational institutional study. BMC Res Notes. 5:1622012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blok EJ, Kuppen PJ, van Leeuwen JE and
Sier CF: Cytoplasmic overexpression of HER2: a key factor in
colorectal cancer. Clin Med Insights Oncol. 7:41–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conti M, Hsieh M, Park JY and Su YQ: Role
of the epidermal growth factor network in ovarian follicles. Mol
Endocrinol. 20:715–723. 2006. View Article : Google Scholar
|
|
11
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. Arch Pathol Lab Med.
131:18–43. 2007.PubMed/NCBI
|
|
12
|
Larbouret C, Robert B, Navarro-Teulon I,
et al: In vivo therapeutic synergism of anti-epidermal growth
factor receptor and anti-HER2 monoclonal antibodies against
pancreatic carcinomas. Clin Cancer Res. 13:3356–3362. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burstein HJ: The distinctive nature of
HER2-positive breast cancers. N Engl J Med. 353:1652–1654. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bayoudh L, Afrit M, Daldoul O, et al:
Trastuzumab (herceptin) for the medical treatment of breast cancer.
Tunis Med. 90:6–12. 2012.PubMed/NCBI
|
|
15
|
Wang CX, Koay DC, Edwards A, Lu Z, Mor G,
Ocal IT and Digiovanna MP: In vitro and in vivo effects of
combination of Trastuzumab (Herceptin) and Tamoxifen in breast
cancer. Breast Cancer Res Treat. 92:251–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rugo H, Brammer M, Zhang F and Lalla D:
Effect of trastuzumab on health-related quality of life in patients
with HER2-positive metastatic breast cancer: data from three
clinical trials. Clin Breast Cancer. 10:288–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shepard HM, Jin P, Slamon DJ, et al:
Herceptin. Handb Exp Pharmacol. 181:183–219. 2008.
|
|
18
|
Wolf E, Hofmeister R, Kufer P, et al:
BiTEs: bispecific antibody constructs with unique anti-tumor
activity. Drug Discovery Today. 10:1237–1244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seimetz D, Lindhofer H and Bokemeyer C:
Development and approval of the trifunctional antibody catumaxomab
(anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer
Treat Rev. 36:458–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bargou R, Leo E, Zugmaier G, et al: Tumor
regression in cancer patients by very low doses of a T
cell-engaging antibody. Science. 321:974–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto K, Trad A, Baumgart A, et al: A
novel bispecific single-chain antibody for ADAM17 and CD3 induces
T-cell-mediated lysis of prostate cancer cells. Biochem J.
445:135–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu G, Fan X, Wu H, et al: Bioscreening of
phage display antibody library and expression of a humanized
single-chain variable fragment antibody against human connective
tissue growth factor (CTGF/CCN2). Biotechnol Appl Biochem.
56:95–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petsch S, Gires O, Rüttinger D, Denzel S,
Lippold S, Baeuerle PA and Wolf A: Concentrations of EpCAM
ectodomain as found in sera of cancer patients do not significantly
impact redirected lysis and T-cell activation by
EpCAM/CD3-bispecific BiTE antibody MT110. MAbs. 3:31–37. 2011.
View Article : Google Scholar :
|
|
24
|
Jäger M, Schoberth A, Ruf P, et al:
Immunomonitoring results of a phase II/III study of malignant
ascites patients treated with the trifunctional antibody
catumaxomab (anti-EpCAM × anti-CD3). Cancer Res. 72:24–32. 2012.
View Article : Google Scholar
|
|
25
|
Reusch U, Sundaram M, Davol PA, et al:
Anti-CD3 anti-epidermal growth factor receptor (EGFR) bispecific
antibody redirects T-cell cytolytic activity to EGFR-positive
cancers in vitro and in an animal model. Clin Cancer Res.
12:183–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasuya K, Shimazu M, Suzuki M, Itoi T,
Aoki T and Tsuchida A: Bispecific anti-HER2 and CD16 single-chain
antibody production prolongs the use of stem cell-like cell
transplantation against HER2-overexpressing cancer. Int J Mol Med.
25:209–215. 2010.PubMed/NCBI
|
|
27
|
Stebbings R, Findlay L, Edwards C, et al:
‘Cytokine storm’ in the phase I trial of monoclonal antibody
TGN1412: better understanding the causes to improve preclinical
testing of immunotherapeutics. J Immunol. 179:3325–3331. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McLaughlin P, Grillo-López AJ, Link BK, et
al: Rituximab chimeric anti-CD20 monoclonal antibody therapy for
relapsed indolent lymphoma: half of patients respond to a four-dose
treatment program. J Clin Oncol. 16:2825–2833. 1998.PubMed/NCBI
|
|
29
|
Finn RS and Slamon DJ: Monoclonal antibody
therapy for breast cancer: herceptin. Cancer Chemother Biol
Response Modif. 21:223–233. 2003.
|