Introduction
Dendritic cells (DCs) are types of
antigen-presenting cells that are involved in the innate and
adaptive immune responses (1–3). DCs have been used as target cells
in a number of experiments. It is therefore important to develop
DCs in larger quantities and of greater purity for use in
vitro investigations (4,5).
In vitro investigations have demonstrated
that cytokines are capable of promoting DC development and
proliferation (6,7). Granulocyte macrophage-colony
stimulating factor (GM-CSF) and interleukin-4 (IL-4) are two
commonly-used cytokine therapy protocols for the generation of DCs
(89–10).
Previous investigations have suggested that GM-CSF promotes the
differentiation of bone marrow-derived DCs, while IL-4 may support
DC development and maturation when GM-CSF is absent (11,12).
Previous investigations have demonstrated that DCs cultured with
GM-CSF alone are functionally immature (13,14).
Maturation state of DCs were previously determined by the
expression levels of the major histocompatibility complex (MHC)
class II molecule as well as co-stimulatory molecules (CD86, CD80,
and CD40) (4); the expression of
surface molecules, including MHC II, CD80 and CD86, were reported
to identify an immature phenotype of DC (14). Furthermore, Lutz et al
(15) demonstrated that IL-4
promotes DC maturation. However, the molecular mechanisms
underlying the effect of IL-4 on DC maturation remain obscure
(16).
Signal transducer and activator of transcription 6
(STAT6) is able to translocate to the nucleus and interact with
other transcription factors, such as nuclear factor (NF)-κB, to
regulate gene transcription (17,18).
In the present study, DCs from rat bone marrow
progenitors were cultured with GM-CSF, with or without IL-4. The
purpose of the present study was to compare the phenotype and
functional properties of DCs generated using the two treatment
protocols (GM-CSF and GM-CSF + IL-4). Furthermore, the involvement
of IL-4 in DC maturation was examined. The present study also aimed
to determine phosphorylated (p-) STAT6 expression in order to
elucidate the role of the STAT6 signaling pathway in the DC
phenotype.
Materials and methods
Experimental design
F344 rats (weight, 180–200 g; age, 6–8 weeks) were
obtained from the Experimental Animal Center of Shanghai Institute
for Biological Sciences (Shanghai, China). Sprague-Dawley (SD) rats
(weight, 190–210 g; age, 6–8 weeks) were obtained from the
Experimental Animal Center of Nanjing Medical University (Nanjing,
China). Animals were kept maintained under specific-pathogen-free
conditions. Experiments were performed in endotoxin-free
conditions. The present study was performed under the authority of
a license issued by the Ethics Committee of Nanjing Medical
University.
In vitro generation of DCs
DCs were generated according to a previously
described method (15). Bone
marrow cells were aspirated from F344 rat femurs and suspended in
RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA). Red blood cells
were removed using a lysis buffer (Beyotime, Shanghai, China). DCs
were cultured in 24-well plates at 6×105 cells/ml in
RPMI-1640 medium containing 10% fetal bovine serum (Gibco-BRL), 2
mM glutamine (Gibco-BRL), 100 U/ml penicillin (eBioscience, San
Diego, CA, USA) and 100 U/ml streptomycin (eBioscience). One group
of cells were treated with recombinant GM-CSF (5 ng/ml; Peprotech,
Rocky Hill, NJ, USA) and IL-4 (5 ng/ml; PeproTech, Inc.; GM-CSF +
IL-4 group). A second group of cells was treated with 5 ng/ml
GM-CSF only (GM-CSF group). Cells were cultured at 37°C with 5%
CO2. Every two days, half of the medium was removed and
an equal volume of fresh medium containing GM-CSF + IL-4 or GM-CSF
was added. Morphological features of DCs were observed on days two,
four and six, using phase-contrast inverted microscopy (IX71-DP30;
magnification, ×100; Olympus Biological Microscopes, Tokyo, Japan).
Following six days of culture and treatment, non-adherent and
semi-adherent cells were harvested.
Detection of cell surface molecules
Expression levels of cell surface molecules (MHC II,
CD80 and CD86) (14) were
determined using a FACScan flow cytometer (BD FACSCanto II™; BD
Biosciences, San Jose, CA, USA). Following six days of culture, DCs
were harvested and mixed with mouse anti-rat monocloncal
fluorescein isothiocyanate (FITC)-conjugated anti-MHC II (1:400;
cat. no. 11-0920-82), mouse anti-rat monoclonal phycoerythrin
(PE)-conjugated anti-CD80 (1:400; cat. no. 12-0800-82) or mouse
anti-rat monoclonal ITC-conjugated anti-CD86 (1:400; cat. no.
11-0860-81) antibodies (eBioscience), as previously described
(19). Following 30 min of
incubation at 5°C, cells were fixed in ethanol for 10 min and
analyzed using flow cytometry (BD FACSCanto™ II; BD
Biosciences). In order to investigate the effect of
lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO, USA) on DC
morphology, LPS (1 μg/ml) was added to the medium for 48 h
at the end of 6 days of culture treatment. Subsequently,
LPS-stimulated DCs were harvested, labeled with antibodies for MCH
II, CD90 and CD86 (as above) and analyzed using flow cytometry.
Endocytosis assays
In order to assay endocytic activity, DCs
(5×105) were incubated with FITC-dextran (Sigma-Aldrich)
at a final concentration of 1 mg/ml at 37°C for 45 min.
Subsequently, cells were collected, resuspended in 250 μl of
flow cytometry buffer and analyzed using flow cytometery.
Experiments were performed at 4°C.
Pro-inflammatory cytokine
measurements
DCs (2×105 cells/ml) were incubated with
or without 1 μg/ml of LPS for 48 h, and supernatants were
collected. IL-12p70 and TNF-α concentrations were measured using
enzyme-linked immunosorbent assay kits (ELISA; R&D
systems™, Minneapolis, MN, USA) according to the
manufacturer’s instructions. Streptavidin-horseradish peroxidase
and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)
(Sigma-Aldrich) were used as enzyme and substrate, respectively.
Optical density was measured at 405 nm using an ELISA reader
(Bio-Rad 680; Bio-Rad, Hercules, CA, USA).
Mixed lymphatic reaction (MLR)
MLR was conducted in order to measure the level of
naïve allogeneic T cell stimulation. Cell proliferation was
detected using a Cell Counting kit (Dojindo, Kumamoto, Japan)
(20). Allogeneic T cells from SD
rat splenic cells were isolated as responder cells using a Nylon
Fiber Column (Wako, Osaka, Japan). Different numbers of DCs
(2.5×103, 5×103, 10×103 and
20×103) were treated with 25 μg/ml mitomycin C
(Sigma-Aldrich) for 30 min at 37°C and added to the allogeneic T
cells (2×105). Following 72 h of incubation, 10
μl WST-8 solution (Dojindo, Osaka, Japan) was added to each
well, which contained 100 μl cell suspension in a 96-well
plate for 2 h. Absorbance was then measured at 450 nm using the
Bio-Rad 680 (Bio-Rad).
Western blotting
DCs were lysed on ice using Triton X-100 buffer,
containing a protein inhibitor cocktail (Cell Biolabs, Inc., San
Diego, CA, USA). Cytosolic proteins were extracted and separated
using 10% SDS-PAGE gel electrophoresis (ZomAnBio, Beijing, China).
Separated proteins were transferred onto a nitrocellulose membrane
(Amersham Biosciences, Inc., Pittsburgh, PA, USA). Bovine serum
albumin (3%; Pierce, Rockford, IL, USA) was added in order to
prevent non-specific binding. Primary mouse anti-rat monoclonal
anti-STAT6 (ab130235) or rabbit anti-rat anti-p-STAT6 (ab195701)
antibodies (Abcam, San Francisco, CA, USA) were incubated with the
membrane for 18 h. The membranes were washed three times and
exposed to polyconal goat anti-mouse (ab101063) or goat anti-rabbit
(ab102279) immunoglobulin G secondary antibody conjugated to
horseradish peroxidase (Absin, Shanghai, China). Bands were then
visualized using enhanced chemiluminescence staining (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). β-actin was used as the
reference gene.
Statistical analysis
Data are presented as the mean ± standard error.
Experiments were repeated three times. An independent samples
t-test was performed in order to compare means. SPSS, Inc. (version
11.0, Chicago, IL, USA) was used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Graphs were produced using GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
DC morphology and cell surface molecule
expression
On day two, DCs were small, round and adherent to
the plate surface. DCs exhibited increased volume, reduced
adherence to the plate surface and morphological changes, in a
time-dependent manner. On day four, DCs were more irregular in
shape, larger in size and were suspended in the medium, compared
with cells on day two. On day six, DCs were less adherent to the
plate surface compared with those on day four. Morphological
changes are shown in Fig. 1A.
Higher numbers of GM-CSF + IL-4 DC than GM-CSF DC clusters were
observed. Expression of surface molecules in GM-CSF DCs and GM-CSF
+ IL-4 DCs was detected using flow cytometry. Expression levels of
CD80/86 and MHC II surface molecules were significantly greater in
GM-CSF + IL-4 DCs than those in GM-CSF DCs (P<0.01; Fig. 1B and C). In order to compare
responses to LPS treatment, DCs were treated with LPS for 48 h.
Following LPS treatment, GM-CSF + IL-4 DCs expressed significantly
higher levels of CD80/86 and MHC II cell surface molecules than
GM-CSF DCs (P<0.01; Fig.
2).
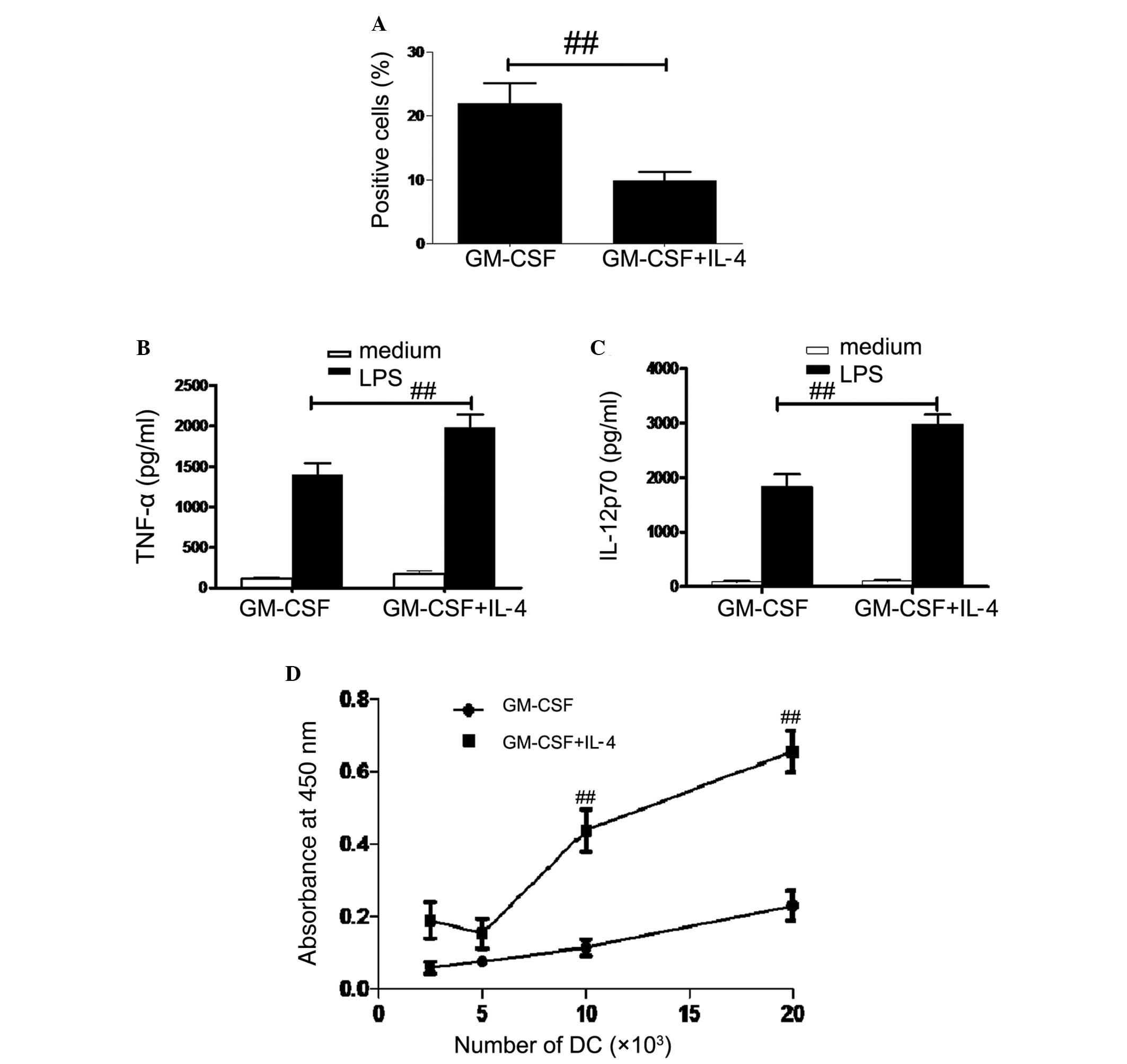
DC endocytic activity
In order to measure the endocytic activity, DCs were
incubated with FITC-dextran and analyzed using flow cytometry.
Antigen uptake was significantly higher in GM-CSF DCs compared with
that of GM-CSF + IL-4 DCs (P<0.01; Fig. 3A). In order to investigate cytokine
profiles, DCs were incubated with or without 1 μg/ml LPS for
48 h. IL-12p70 and TNF-α concentrations were then measured using
ELISA. IL-12p70 and TNF-α concentrations were significantly higher
in GM-CSF + IL-4 DCs and GM-CSF DCs following LPS treatment
compared with those not treated with LPS (P<0.01; P<0.05;
Fig. 3B and C).
Naïve allogenic T cell stimulation
The capacity of two DC populations to stimulate
allogeneic T cells was investigated using MLR. As shown in Fig. 3D, GM-CSF DCs had a weaker capacity
to stimulate the proliferation of allogeneic T cells, while
GM-CSF+IL-4 DCs showed enhanced proliferation at all ratios tested,
with significantly increased results at 10×103 and
20×103 DCs (P<0.01).
STAT6 expression
Western blotting was performed in order to detect
STAT6 expression as well as p-STAT6 expression, which is the active
form of STAT6, in the DCs. p-STAT6 was not in GM-CSF DCs; however,
it was expressed in GM-CSF + IL-4 DCs (Fig. 4A and B). Mature DCs were treated
with IL-4 and p-STAT6 expression was then detected in GM-CSF DCs
without IL-4 (Fig. 4C), GM-CSF DCs
with IL-4 (Fig. 4D), GM-CSF + IL-4
DC without IL-4 (Fig. 4E) and
GM-CSF + IL-4 DC with IL-4 (Fig.
4F). As p-STAT6 is the active form of STAT6, it was not
obviously expressed in the mature state (Fig. 4C and E); however, following the
addition of IL-4, p-STAT6 was highly expressed. These observations
indicate that IL-4 treatment during DC maturation resulted in the
activation of STAT6 signaling (Fig. 4D
and F).
Discussion
In the present study, surface molecule expression
was significantly higher, antigen uptake was significantly lower
and the capacity to stimulate allogeneic T cells was significantly
higher in GM-CSF + IL-4 DCs compared with GM-CSF DCs. Therefore,
GM-CSF DCs were phenotypically and functionally immature compared
with GM-CSF + IL-4 DCs. Following LPS treatment, GM-CSF + IL-4 DCs
matured more quickly and expressed higher levels of
pro-inflammatory cytokines compared with GM-CSF DCs.
Wu et al (10) cultured bone marrow DCs with GM-CSF
and recombinant chicken IL-4. DCs generated using this method
exhibited typical DC morphological characteristics, and the
presence or absence of the following cell surface molecules was
observed: MHC class II (high), CD11c (high), CD40 (moderate), CD1.1
(moderate), CD86 (low), CD83 (not present) and DEC-205 (not
present). The presence of these markers indicates whether or not
the cells are functionally mature, thus, GM-CSF + IL4 treatment
promotes DC growth and maturation. In the present study, following
LPS treatment GM-CSF + IL-4 DCs expressed significantly higher
levels of cell surface molecules than GM-CSF DCs. Labeur et
al (13) demonstrated that DCs
cultured with GM-CSF alone were functionally immature, whereas
GM-CSF DCs that were subsequently incubated with CD40L or LPS were
functionally mature.
Typically, DCs take up extracellular antigens via
endocytosis, which they subsequently process and present to T cells
(20). During DC maturation,
endocytosis is downregulated (21). In the present study, endocytosis
levels were significantly lower in GM-CSF + IL-4 DCs compared with
those of GM-CSF DCs. Furthermore, the production of
pro-inflammatory cytokines was significantly greater in GM-CSF +
IL-4 DCs than in GM-CSF DCs. Pro-inflammatory cytokines produced by
DCs are important immunomodulatory factors which are capable of
influencing immune responses (22).
In the present study, p-STAT6 was expressed in
GM-CSF + IL-4 DCs but not in GM-CSF DCs. STAT6 is a downstream
transcriptional activator involved in IL-4 signaling (17). Following treatment with IL-4 of the
two DC groups (GM-CSF + IL-4 and GM-CSF), STAT6 was shown to be
activated. This result indicated that STAT6 signaling is activated
when DCs are treated with IL-4 during the maturation period. Once
activated, STAT6 translocates into the nucleus and interacts with
other transcription factors, such as NF-κB, which regulates gene
transcription (18). Therefore,
IL-4 treatment during the DC maturation phase resulted in the
activation of IL-4 signaling and STAT6.
In conclusion, the present study demonstrates that
IL-4 affects DC phenotype and function, and that it may be
associated with the activation of STAT6.
Acknowledgments
This study was supported by the Major Science and
Technique Program of Changzhou Health Bureau (grant no. ZD201011)
and the Key Development Program of Nanjing Medical University
(grant no. 2011NJMU229).
References
|
1
|
Adema GJ: Dendritic cells from bench to
bedside and back. Immunol Lett. 122:128–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezzelarab M and Thomson AW: Tolerogenic
dendritic cells and their role in transplantation. Semin Immunol.
23:252–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidt SV, Nino-Castro AC and Schultze
JL: Regulatory dendritic cells: there is more than just immune
activation. Front Immunol. 3:2742012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalantari T, Kamali-Sarvestani E, Zhang
GX, et al: Generation of large numbers of highly purified dendritic
cells from bone marrow progenitor cells after co- culture with
syngeneic murine splenocytes. Exp Mol Pathol. 94:336–342. 2013.
View Article : Google Scholar :
|
|
5
|
Xiong X, Meng Y, Wang X, et al: Mice
immunized with bone marrow- derived dendritic cells stimulated with
recombinant Coxiella burnetii Com1 and Mip demonstrate enhanced
bacterial clearance in association with a Th1 immune response.
Vaccine. 30:6809–6815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerkar SP, Chinnasamy D, Hadi N, et al:
Timing and intensity of exposure to interferon-γ critically
determines the function of monocyte-derived dendritic cells.
Immunology. 143:96–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno A, Jijon H, Traves S, et al: Opposing
effects of smoking in ulcerative colitis and Crohn’s disease may be
explained by differential effects on dendritic cells. Inflamm Bowel
Dis. 20:800–810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tiurbe G, Matuschek A, Kämmerer U, et al:
Inhibitory effects of rat bone marrow-derived dendritic cells on
naïve and alloantigen-specific CD4+ T cells: a comparison between
dendritic cells generated with GM-CSF plus IL-4 and dendritic cells
generated with GM-CSF plus IL-10. BMC Res Notes. 2:122009.
View Article : Google Scholar
|
|
9
|
Globisch T, Steiner N, Fülle L, et al:
Cytokine- dependent regulation of dendritic cell differentiation in
the splenic microenvironment. Eur J Immunol. 44:500–510. 2014.
View Article : Google Scholar
|
|
10
|
Wu Z, Rothwell L, Young JR, Kaufman J,
Butter C and Kaiser P: Generation and characterization of chicken
bone marrow- derived dendritic cells. Immunology. 129:133–145.
2010. View Article : Google Scholar :
|
|
11
|
Lutz MB and Rössner S: Factors influencing
the generation of murine dendritic cells from bone marrow: the
special role of fetal calf serum. Immunobiology. 212:855–862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koike E, Takano H, Inoue K and Yanagisawa
R: Accelerated differentiation of bone marrow- derived dendritic
cells in atopic prone mice. Int Immunopharmacol. 8:1737–1743. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Labeur MS, Roters B, Pers B, et al:
Generation of tumor immunity by bone marrow- derived dendritic
cells correlates with dendritic cell maturation stage. J Immunol.
162:168–175. 1999.PubMed/NCBI
|
|
14
|
Zhu C, Xu H, Zhang G, Lu C, Ji M and Wu W:
Myeloid differentiation factor 88- silenced bone marrow- derived
dendritic cells exhibit enhanced tolerogenicity in intestinal
transplantation in rats. Transplant Proc. 40:1625–1628. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lutz MB, Suri RM, Niimi M, et al: Immature
dendritic cells generated with low doses of GM- CSF in the absence
of IL- 4 are maturation resistant and prolong allograft survival in
vivo. Eur J Immunol. 30:1813–1822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hettihewa LM: Prolonged expression of MHC
class I -peptide expression in bone marrow derived retrovirus
transfected matured dendritic cells by continuous centrifugation in
the presence of IL- 4. Indian J Med Res. 134:672–678. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiroi M, Sakaeda Y, Yamaguchi H and Ohmori
Y: Anti-inflammatory cytokine interleukin-4 inhibits inducible
nitric oxide synthase gene expression in the mouse macrophage cell
line RAW264.7 through the repression of octamer-dependent
transcription. Mediators Inflamm. 2013:3696932013. View Article : Google Scholar
|
|
18
|
Zhuang S: Regulation of STAT signaling by
acetylation. Cell Signal. 25:1924–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roelen DL, Schuurhuis DH, van den
Boogaardt DE, et al: Prolongation of skin graft survival by
modulation of the alloimmune response with alternatively activated
dendritic cells. Transplantation. 76:1608–1615. 2003. View Article : Google Scholar
|
|
20
|
Chatterjee B, Smed-Sörensen A, Cohn L, et
al: Internalization and endosomal degradation of receptor- bound
antigens regulate the efficiency of cross presentation by human
dendritic cells. Blood. 120:2011–2020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Platt CD, Ma JK, Chalouni C, et al: Mature
dendritic cells use endocytic receptors to capture and present
antigens. Proc Natl Acad Sci USA. 107:4287–4292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Chu N, Rostami A and Zhang GX:
Dendritic cells transduced with SOCS- 3 exhibit a tolerogenic/DC2
phenotype that directs type 2 Th cell differentiation in vitro and
in vivo. J Immunol. 177:1679–1688. 2006. View Article : Google Scholar : PubMed/NCBI
|