Introduction
Osteoporosis is a clinically significant problem
worldwide, the incidence of which increases with age. It is a
skeletal disorder characterized by impaired bone strength, which
predisposes to fractures without an identifiable injury or with
minimal trauma, which would be insufficient to fracture healthy
bone (1,2). Therefore, bone regeneration is also a
significant problem in osteoporosis. Bone mesenchymal stem cells
(BMSCs) are important in bone tissue repair and regeneration. For
example, these cells are known to progressively differentiate into
pre-osteoblasts and mature osteoblasts under appropriate
stimulation from regulatory factors present in the microenvironment
(3,4). Shi et al (5) reported that venous injection of BMSCs
into mice that had undergone ovariectomy (OVX), prevented or
reversed osteoporosis. These results suggested that BMSC-based
treatment of osteoporosis may be a promising choice, regardless on
the location of bone disease, or whether it is due to a systemic
cause. Postmenopausal osteoporosis is one of the clinical
manifestations of estrogen deficiency, which occurs during the
process of bone aging. Osteoporotic bone loss is the result of high
bone turnover, in which bone resorption outpaces the rate of bone
deposition (6,7). This imbalance in bone turnover is
induced by estrogen deficiency in women and female rodents and may
be ameliorated by treatment with bioavailable estrogens, including
selective estrogen receptor modulators (SERMs) (8). Estrogen and SERMs primarily act by
regulating gene transcription via estrogen receptors (ERs; ERα and
ERβ) (9,10). ERs belong to the nuclear receptor
gene superfamily and have been shown to act as ligand-inducible
transcriptional factors (11). ER
dimers directly or indirectly associate with specific DNA elements
in the target gene promoter and control transcription by
reorganizing chromatin structure and histone modifications
(10,12). The present study focused on the
association between postmenopausal osteoporosis and the osteogenic
differentiation of BMSCs or ER changes (13). These changes may be modulated by
certain small molecular compounds of estradiol and flavonoids.
Recently, Chen et al (14,15)
demonstrated that icariin (ICA) enhances osteogenic differentiation
of rat BMSCs, improves the maturation and mineralization of
osteoblasts in vitro, and suppresses osteoclastogenesis and
inhibits bone resorption activity in vivo (16–20).
10−5M ICA has been shown to be a potent stimulator of
osteogenic differentiation in vitro (19–21).
It has been demonstrated that it stimulates the proliferation of
rat BMSCs and increases the number of colony-forming units of
fibroblasts that stain positive for alkaline phosphatase (ALP). In
addition, it promotes ALP activity, osteocalcin secretion and
calcium deposition in rat BMSCs in a dose-dependent manner,
suggesting a potential anabolic effect on bone (19–22).
Mok et al (23) reported
that ICA exerts an anabolic effect in the bone possibly via the
activation of ER in a ligand-independent manner, and as a result of
its ability to prevent OVX-induced bone loss. However, the
mechanisms underlying the osteogenic differentiation of BMSCs, in
response to treatment with ICA, in an OVX-induced model of
osteoporosis remain unclear. The current study focused on the
effect of 10−5M ICA on BMSCs from an OVX-induced rat
model of osteoporosis, as well as the possible underlying
mechanisms of these effects, in vitro and in
vivo.
Materials and methods
Animal models
Twelve female Wistar rats (eight weeks old) weighing
80–100 g were obtained from the Animal Breeding Center of Gansu
College of Traditional Chinese Medicine (Lanzhou, China). All
experimental animals were housed under standard conditions (22°C,
12 h light/12 h dark cycles and 50–55% humidity), and the
experimental protocol was approved by the Animal Ethical Committee
of Lanzhou University (Lanzhou, China). All rats were randomly
divided into two group, which were subjected to either OVX or a
sham operation (Sham). At three months post-surgery, three rats
from each group were sacrificed via intraperitoneal injection of 1%
pentobarbital. The metaphysis regions of the distal femur and
femoral shaft were scanned by micro-computed tomography (Siemens
Inveon Micro CT; Siemens, Munich, Germany) with a source voltage of
80 keV, current of 500 μA and 14.97 μm isotropic
resolution. This was to ensure the successful establishment of the
OVX animal model. Following 3D reconstruction, bone volume fraction
and bone mineral density (BMD) were calculated using the built-in
software. Procedures for obtaining bone marrow samples (in order to
isolating bone marrow stromal cells) from a further six rats were
conducted according to the Guide for the Care and Use of Laboratory
Animals, published by the US National Institutes of Health.
Reagents
ICA (purity>98%) was purchased from the National
Institute for Control of Pharmaceutical and Biological Products
(Beijing, China). Dulbecco’s modified Eagle’s medium with F12
(DMEM/F12) and fetal bovine serum (FBS) were purchased from
Invitrogen Life Technologies (Grand Island, NY, USA). Penicillin
and streptomycin were obtained from Gibco BRL (Gaithersburg, MD,
USA). The majority of drugs used were purchased from Sigma-Aldrich
(Steinheim, Germany), including dexamethasone, b-glycerophosphate,
ascorbic acid phosphate and 1-naphthyl phosphate sodium salt
monohydrate. The alkaline phosphatase activity measurement kit was
purchased from Nanjing Jiancheng Company (Nanjing, China) and the
calcium colorimetric assay kit was obtained from Biovision (San
Francisco, CA, USA). All other chemicals were of analytical
grade.
Isolation and culture of sham and OVX rat
BMSCs
Following euthanasia, BMSCs were harvested from the
tibial and femoral bone marrow of Sham and OVX rats, and cultured
in low glucose DMEM, low glucose; (Gibco-BRL, Grand Island, NY,
USA) and supplemented with 10% fetal bovine serum (Hyclone, Logan,
UT, USA), containing 100 U/ml penicillin, 100 U/ml streptomycin and
2 mM L-glutamine (Sigma-Aldrich) as previously described (22). BMSCs were incubated for 24 h at
37°C in a humidified atmosphere of 95% air and 5% CO2,
following which the medium was changed in order to discard
non-adherent cells. The medium was subsequently refreshed every
three days. When confluence reached ~80%, cells were passaged for
expansion. Cells were at passage three were used for the subsequent
experiments.
Comparison of proliferation and
differentiation between Sham-BMSC and OVX-BMSC groups
The proliferation of BMSCs from each group was
determined by an MTT assay (Sigma-Aldrich). Briefly, cells were
seeded at a density of 1×103 cells/well on a 96-well
plate in triplicate in the DMEM medium containing 1% fetal bovine
serum for, 1, 3, 5 and 7 days. MTT (5 mg/ml) was added to each well
and incubated for 4 h. The medium was removed and dimethyl
sulfoxide (DMSO; Sigma-Aldrich) was then added in order to dissolve
formazan. The absorbance value was measured using a microplate
reader (BioTek Instruments, Winooski, VT, USA) at 490 nm. The
results are expressed as units of optical density absorbance
values.
The differentiation of BMSCs in each group was
determined by measuring alkaline phosphatase (ALP) activity and ALP
staining at day 7 in an osteogenic medium containing
10−8M dexamethasone, 50 μg/ml L-2-ascorbic acid,
and 10 mm β-glycerophosphate. The ALP activity was measured using a
commercial kit as instructed (Nanjing Jiancheng Company). A
modified King’s method (24) was
used in the kit and the results are expressed as nmol of phenol/15
min/mg protein. Protein concentrations were determined using a
bicinchoninic acid (BCA) protein assay kit (Nanjing Jiancheng
Company). The numbers of colonies positive for ALP (CFU-FALP) were
also compared in each group on day 12. Cells were fixed in 3.7%
formaldehyde and 90% ethanol solution (Zhong Shan Jin Qiao Company,
Beijing, China) for 5 min, washed and then stained for 15~20 min at
37°C in 20 ml Michalis buffer, pH 8.9, containing 10 mg 1-naphthyl
phosphate sodium and 10 mg fast blue RR salt (Sigma-Aldrich). In
order to detect mineralization, alizarin red staining (Zhong Shan
Jin Qiao Company) of mineralized nodules was conducted on day 14.
Briefly, the cells were fixed in 3.7% formaldehyde for 10 min and
stained by 0.1% alizarin red for 1 h at 37°C. The calcium
deposition volume was measured using a calcium colorimetric assay
kit (BioVision, Inc., Milpitas, CA, USA).
Runt-related transcription factor 2 (Runx-2) and
Osterix (OSX) are two transcription factors required for osteoblast
differentiation and bone formation (25). The expression of the Runx-2 and OSX
proteins an genes was detected by western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Sham-BMSC and OVX-BMSCs were cultured for seven days in an
osteogenic medium. The cells were washed twice with distilled water
and total protein was collected by adding lysis buffer (Zhongshan
Goldenbridge, Beijing, China). Protein concentration was measured
using the BCA protein assay kit, according to the manufacturer’s
instructions (Beyotime Institute of Biotechnology, Shanghai,
China). Total protein (50 μg) from each sample was separated
by SDS-PAGE (12% gel) and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Following incubation
in blocking solution (2% non-fat milk) for 2 h at room temperature,
membranes were incubated for overnight at 4°C with the following
primary antibodies at 1:1,000 dilution: Mouse anti-Runx2 or OSX
(Abcam, Hong Kong), and mouse anti β-actin polyclonal antibody
(Zhongshan Goldenbridge). Following three washes with Tris-buffered
saline with Tween 20 (Beyotime Institute of Biotechnology),
membranes were incubated with 1:3,000 dilution of the secondary
antibody (Zhongshan Goldenbridge) for 2 h, and the immunoreactions
signals were detected using the enhanced chemiluminescence reagent
(EMD Millipore). Total RNA was extracted from BMSCs in the OVX and
Sham groups following culture in osteogenic medium for 7 days,
using TRIzol® reagent (Invitrogen Life Technologies).
RNA (1 μg) was reverse transcribed with PrimeScript RT
reagent kit (Takara Bio, Inc., Kyoto, Japan) according to the
manufacturer’s instructions. The cDNA amplification and detection
was performed in triplicate with the Bio-Rad iQ5 real-time PCR
system (Bio-Rad, Hercules, CA, USA) using a SYBR Premix Ex Taq kit
(Takara Bio, Inc.). The relative gene expression level was
normalized to that of the reference gene, β-actin, based on the
2−ΔΔCt method. Cycling conditions were as follows:
Preincubation (95°C for 10 min), 40 cycles were the performed,
comprising denaturation (94°C for 30 sec), annealing (60°C for 1
min) and extension (72°C for 1 min). A melting curve was acquired
using 95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec.
ICA treatment the OVX-BMSC to recover the
cell osteogenic differentiation assay and the mechanisms
analysis
BMSCs from the Sham and OVX groups were planted in
12-well and six-well plates, respectively. In some culture wells,
medium was supplemented with ICA at 10−5mol/l, a
concentration that has bee shown to be osteogenic in BMSCs
(14). The final concentration of
DMSO, used as a solvent of ICA, in the culture was >0.05%, which
did not interfere with the test system (26). In the cell differentiation assay,
the OVX-BMSC cells were randomly divided into four groups: OVX
control; ICA treatment; ICA + 5 mg/l ICI 182,780, a high-affinity
ER antagonist, treatment; and Sham positive control. The osteogenic
differentiation and mineralization of each group were determined by
evaluation of ALP activity, calcium deposition, ALP staining and
alizarin red staining of mineralized nodules at days 14 and 21. All
groups were cultured in an osteogenic medium contained
10−8M dexamethasone, 50 μg/ml L-2-ascorbic acid,
and 10 mm β-glycerophosphate. The expression of the Runx-2 and OSX
expression was also detected by western blotting and RT-qPCR, the
cells were cultured for 7 days. The expression of factors involved
in the estrogen signaling pathway, ERα, progesterone receptor (PR)
and trefoil factor 1 (PS-2), was also measured by western blotting
and RT-qPCR. All materials and methods were identical to those
described in the previous section.
ICA treatment the OVX-BMSC to recover the
cell osteogenic differentiation and bone formation assay in
vivo
The 0.8% collagen scaffolds used in the study were
obtained from Rebone Biomaterial Co., Ltd. (Shanghai, China).
Confluent BMSCs from OVX rats, Sham rats and OVX rats treated with
ICA were detached from culture dishes, centrifuged to form cell
pellets, and then resuspended in 0.8% collagen at a density of
2×107 cells/ml. Cell suspensions were transplanted into
six four-week old male nude mice. Each mouse received the following
three groups of complexes: Sham-BMSC group, OVX-BMSCs and ICA +
OVX-BMSCs group. Four cases were included in each group. Mice were
anesthetized by intramuscular injection of pentobarbital following
inhalation of light ether. Longitudinal incisions were made on the
back of each mouse and three separate subcutaneous pockets were
subsequently created by blunt dissection. Finally, three complexes
were randomly implanted into the pockets and the skins were
sutured. At 12 weeks post-surgery, the implants were harvested,
fixed in 10% buffered formaldehyde for 24 h, decalcified in 10%
EDTA, embedded in paraffin, sectioned into 4-μm sections and
stained with hematoxylin and eosin (H&E). Photomicrographs of
each section were captured with a light microscope (Olympus
corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard
deviation. Each treatment group had at least three samples (n=3).
All data were analyzed using one-way analysis of variance followed
by the least significant difference post hoc test using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
OVX-BMSC proliferation capacity is
significantly decreased compared with Sham-BMSCs
At three months post-surgery, three rats from the
OVX group and three from the Sham group were sacrificed. Micro-CT
3D reconstruction images showed that OVX rats had less trabecular
bone, as well as disorganized trabecular architecture, expanded
marrow cavities and thinning cortical bones compared with the bones
of Sham rats (Fig. 1A). These
results demonstrated that the creation of the osteoporotic animal
model had been successful. BMSCs were isolated from Sham and OVX
rats. Results of the colony-forming assay demonstrated that the
Sham-BMSCs clone formation rate was ~38.6%, compared with the
OVX-BMSCs clone formation rate of ~22.8. The Sham-BMSCs exhibited a
stronger proliferation ability and began the proliferative process
without a stagnation period. The logarithmic growth phase began at
day 3, the growth peak was reached on day 7 and on day the growth
plateau phase was entered (Fig.
1B).
OVX-BMSC osteogenic differentiation
capacity is significantly decreased compared with Sham-BMSCs
In order to further evaluate osteogenic
differentiation, the ALP activity and calcified tubercle red
staining in the two groups was compared. The expression of
osteogenic-associated genes and proteins was also detected in the
Sham-BMSCs and OVX-BMSCs using RT-qPCR and western blotting
analysis. The results showed that the Sham-BMSCs had significantly
larger CFU-FALP than OVX-BMSCs (Fig
2A), The ALP activity was also higher in the Sham group than
that in the OVX group (Fig 2B).
Similarly, with ALP staining, the mineralized nodule formation
assay demonstrated more and larger areas of mineralized nodules in
the Sham-BMSCs group compared with the OVX-BMSCs group (Fig. 2C). The calcium deposition volume
was also significantly higher in the Sham group than the OVX group
(Fig. 2D). The expression of the
osteogenic-associated genes, Runx-2 and OSX, was significantly
higher in the Sham-BMSCs group than in the OVX-BMSCs group
(Fig. 2E and F). The expression of
the Runx-2 and OSX proteins was also stronger in the Sham-BMSCs
group compared with the OVX-BMSCs group (Fig. 2G and H).
ICA treatment recovers the osteogenic
differentiation of OVX-BMSCs through the estrogen pathway
The effects of ICA on the differentiation and
mineralization of OVX-BMSCs were investigated by staining of
mineralized bone nodules and ALP histochemical staining at days 14
and 21. ALP activity and mineralized bone nodules were used as a
phenotypic marker for osteogenic differentiation, and were detected
simultaneously. Following treatment of the OVX-BMSCs with 10P-5PM
ICA for 14 days under the osteogenic inducing medium, the ICA
treatment groups exhibited a significant increase in cellular ALP
activity and in the area positive for ALP histochemical staining,
compared with the OVX-BMSCs group. No significant difference was
detected between the Sham-BMSCs and ICA treatment groups (Fig. 3A and B). However, when ICI was
added to the ICA treatment group, the ALP activity and area
positive for ALP histochemical staining was significantly lower
than in the ICA treatment group alone. Furthermore, no significant
difference was detected between the OVX-BMSCs and the ICI+ICA
groups (Fig. 3A and B). The
alizarin red staining of bone nodules and the volume of calcium
sediment demonstrated that ICA stimulated the differentiation of
OVX-BMSCs into mature mineralized osteoblastic cells. The same
pattern was observed with the results of the ALP assay. No
difference was detected between the Sham-BMSC and ICA treatment
groups. However, these groups each exhibited more bone nodule and a
greater volume of calcium sediment than the OVX-BMSCs group. In
addition, these values were lower in the ICI+ICA group compared
with the Sham-BMSC and ICA treatment groups (Fig. 3C and D).
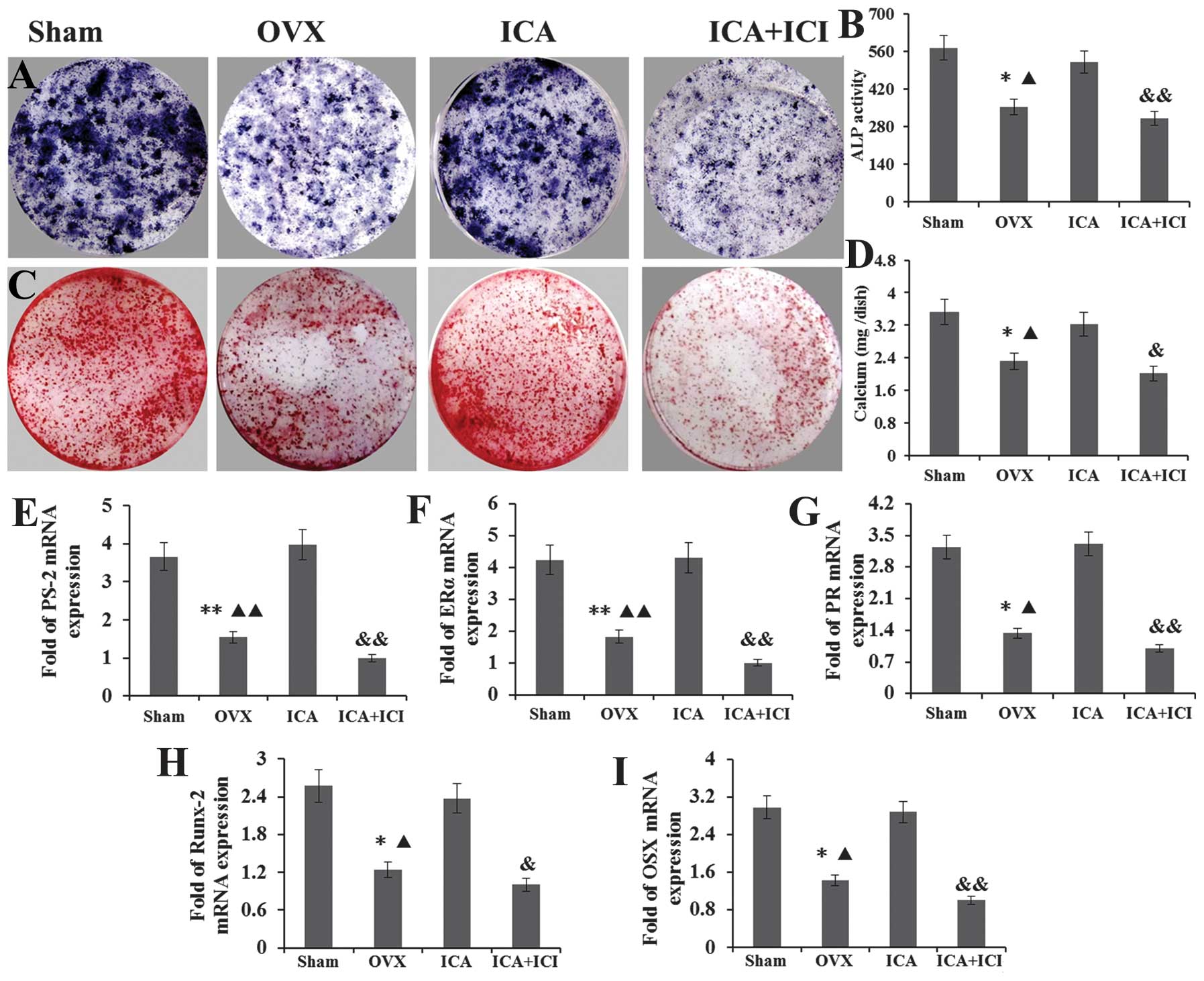 | Figure 3ICA treatment groups exhibited a large
area positive for (A) ALP histochemical staining and (B) ALP
activity compared with the OVX-BMSCs group. No significant
difference was detected between the Sham-BMSCs and ICA treatment
groups. When ICI was added to the ICA treatment group, the ALP
activity and area positive for ALP histochemical staining was
significantly lower than that in the ICA treatment group, whilst no
difference was detected between the OVX-BMSCs and ICI + ICA groups.
(C) Alizarin red staining of bone nodule mineralization and (D) the
volume of calcium sediment yield exhibited the same pattern as that
of the ALP assay. No difference was detected between the Sham-BMSCs
and ICA treatment groups, whilst those two groups exhibited more
bone nodule mineralization and calcium volume than the OVX-BMSCs
group. The results for the ICI + ICA group were lower than those
for the Sham-BMSC and ICA treatment groups. The expression of the
(E) PS-2, (F) ERα, (G) PR, (H) Runx-2 and (I) OSX genes exhibited
the same pattern. ICA, icariin; ALP, alkaline phosphatase; OVX,
ovariectomy; BMSCs, bone marrow stromal cells; ICI, ICI 182,780;
PS-2, trefoil factor 1; ERα, estrogen receptor α; PR, progesterone
receptor; OSX, osterix; Runx-2, runt-related transcription factor
2. |
Following culture with an osteogenic inducing medium
for seven days, total mRNA was isolated from the BMSCs in each
group. The expression of the PS-2, ERα, PR, Runx-2 and OSX genes
was detected by RT-qPCR. For PS-2, the relative levels of mRNA were
consistently higher in the cultures grown in the presence of ICA
compared with those in the OVX-BMSCs and ICI+ICA groups. also
almost higher than Sham-BMSCs group (Fig. 3E). For ERα, the relative levels of
mRNA were higher in cultures grown in the presence of ICA compared
with those in the Sham-BMSCs and OVX-BMSCs groups. However, when
ICI was added to the ICA treatment group, the ERα expression was
significantly reduced compared with that in the other groups
(Fig. 3F). For PR, the relative
levels of mRNA were higher following ICA treatment of OVX-BMSCs,
but this increase was abrogated by the administration of ICI. No
significant difference was detected between the between the Sham
and ICA treatment groups (Fig.
3G). For Runx-2 and OSX, as the lower reaches of the osteogenic
differentiation marker gene was also detected, and they have the
same expression pattern, the results showed that treatment with ICA
significantly increased the expression of these genes compared with
that of the OVX-BMSCs and ICI+ICA groups (Fig. 3H and I).
Secretion of the PS-2, ERα, PR, Runx-2 and OSX
proteins was assessed by western blot analysis of cultured BMSCs in
each group. Under osteogenic-inducing conditions, the protein
expression was detected as an immunoreactive blot and the area of
blots was analyzed by IPP imaging software. The β-actin protein was
analyzed in the same samples as an reference protein. Based on the
area of the blots, the expression of these proteins in the
OVX-BMSCs was compared with the Sham-BMSCs group. The expression of
the estrogen pathway marker protein PS-2, ERα and PR was reduced in
the OVX-BMSCs group, in particular that of ERα expression. However,
when the OVX-BMSCs were treated with ICA, the expression of PS-2,
ERα and PR was restored. By contrast, when the ICA and ICI were
administered simultaneously, the expression of ERα was not
significantly increased compared with the OVX-BMSCs group, and it
was significantly lower than that in the Sham-BMSCs and ICA
treatment group s (Fig. 4A–C). The
expression of the Runx-2 and OSX proteins in the OVX-BMSCs group
was also lower than that in the Sham-BMSCs group and ICA treatment
group following culture in an osteogenic-inducing medium for seven
days. Furthermore, the levels of these proteins decreased when ICI
was added to the ICA treatment group (Fig. 4D and E).
 | Figure 4Expression of the (A) PR, (B) PS-2,
(C) Runx-2, (D) ERα and (E) OSX proteins was assessed by western
blot analysis of cultured BMSCs in the Sham, OVX, ICA treatment and
ICI + ICA treatment groups. PS-2, trefoil factor 1; ERα, estrogen
receptor α; PR, progesterone receptor; BMSCs, bone marrow stromal
cells; OVX, ovariectomy; ICA, icariin; ICI, ICI 182,780; OSX,
osterix; Runx-2, runt-related transcription factor 2. |
ICA restores the ability for osteogenic
differentiation and bone formation in BMSCs in vivo
At 12 weeks post-surgery, the implants were
harvested, embedded in paraffin, sectioned into 4-μm
sections and stained with H&E. The results demonstrated new
bone formation in all three groups. However, there were marked
differences among them. The OVX-BMSC group exhibited a smaller
quantity of bone formation than the Sham-BMSC group. The LCA
treatment group exhibited more bone formation than the OVX-BMSC
group, and slightly less than the Sham-BMSC group (Fig. 5). Thus, the H&E staining
results showed that OVX-BMSC osteogenic differentiation and bone
formation ability was also restored by treatment with ICA in
vivo.
Discussion
ICA, a prenylated flavonol glycoside isolated from
the Epimedium herb, stimulates osteogenic differentiation of
BMSCs and inhibits the bone resorption activity of osteoclasts. The
existence of a prenyl group on C-8 of the ICA molecule has been
shown to lead to an increased potency of ICA with regard to
osteogenic activity (13,21,22).
It has been reported that ICA induces osteogenic differentiation in
a BMP-2-, SMAD 4-, Runx-2-, or ER-dependent manner (23,27)
and that it inhibits osteoclast differentiation and bone resorption
via suppression of MAPKs/NF-κB synthesis (15). In addition, ICA is a PDE5 inhibitor
(28,29) and is known to enhance the
production of bioactive nitric oxide, as well as mimicking the
effects of testosterone (26).
In the present study, it was shown that cell
proliferation, cell differentiation, mineralization capacity, and
the expression of osteogenesis-related genes and proteins was
significantly decreased in the OVX-BMSCs compared with the
Sham-BMSCs. ICA was shown to improve the differentiation,
mineralization, and expression of osteogenesis-related genes and
protein in OVX-BMSCs. Furthermore, these effects were completely
blocked by administration of the ER inhibitor, ICI 182780,
demonstrating that the effects on proliferation of icaritin and
desmethylicaritin were mediated by its action on the ER. Bian et
al demonstrated that ICA may have different effects on BMSCs
isolated from rats in which osteoporosis had been induced by
corticosterone from that in those in which it had been induced by
OVX. Although ICA treatment promoted osteogenic differentiation in
BMSCs from corticosterone-treated and OVX rats, microarray studies
of the isolated BMSCs showed that ICA treatment produced a marked
shift in the expression of genes involved in cell communication,
cell adhesion, the cell cycle and the secretion of cytokines, with
this shift being more significant in the corticosterone-treated
rats. Notably, there was little overlap between the differentially
expressed genes induced by ICA treatment in these two models,
suggesting that the effects, and molecular mechanisms underlying
these effects, of ICA in reducing bone loss and prevention of
osteoporosis may be pathogenesis-dependent (30). However, there is currently a lack
of data on the influence on the ER of ICA, and the associated
mechanisms. In the present study, the expression of ERα, SP-2 and
PR was detected following ICA treatment of OVX-BMSCs. The PS-2 and
PR genes are estrogen responsive in hepatocarcinoma cells (HepG2)
in the presence of the ER (31–33).
The result from the current study showed that the expression of the
ERα, SP-2 and PR genes and proteins in OVX-BMSCs was upregulated by
ICA treatment, and that the ER inhibitor, ICI 182 780, blocked the
upregulation of these molecules. Therefore, it may be that ICA
improves the osteogenic differentiation and mineralization of
OVX-BMSCs in vitro via the ER pathway. Osteogenic
differentiation and bone formation ability, which had been damaged
by estrogen deficiency and increased age, was also restored in
vivo.
References
|
1
|
McGarry KA and Kiel DP: Postmenopausal
osteoporosis. Strategies for preventing bone loss, avoiding
fracture. Postgrad Med. 108:79–82. 85–88. 912000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hertrampf T, Gruca MJ, Seibel J, et al:
The bone-protective effect of the phytoestrogen genistein is
mediated via ER alpha-dependent mechanisms and strongly enhanced by
physical activity. Bone. 40:1529–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Negishi-Koga T, Shinohara M, Komatsu N, et
al: Suppression of bone formation by osteoclastic expression of
semaphorin 4D. Nat Med. 17:1473–1481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
5
|
Liu Y, Wang L, Kikuiri T, et al:
Mesenchymal stem cell-based tissue regeneration is governed by
recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 17:1594–1601.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teitelbaum SL: Osteoclasts: what do they
do and how do they do it? Am J Pathol. 170:427–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riggs BL and Hartmann LC: Selective
estrogen-receptor modulators - mechanisms of action and application
to clinical practice. N Engl J Med. 348:618–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Couse JF and Korach KS: Estrogen receptor
null mice: what have we learned and where will they lead us? Endocr
Rev. 20:358–417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang Y and Brown M: Molecular
determinants for the tissue specificity of SERMs. Science.
295:2465–2468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mangelsdorf DJ, Thummel C, Beato M, et al:
The nuclear receptor superfamily: the second decade. Cell.
83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Belandia B and Parker MG: Nuclear
receptors: a rendezvous for chromatin remodeling factors. Cell.
114:277–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ming LG, Chen KM and Xian CJ: Functions
and action mechanisms of flavonoids genistein and icariin in
regulating bone remodelling. J Cell Physiol. 228:513–521. 2013.
View Article : Google Scholar
|
|
14
|
Chen KM, Ge BF, Ma HP, et al: Icariin, a
flavonoid from the herb Epimedium enhances the osteogenic
differentiation of rat primary bone marrow stromal cells.
Pharmazie. 60:939–942. 2005.
|
|
15
|
Chen KM, Ma HP, Ge BF, et al: Icariin
inhibits the osteoclast formation induced by RANKL and
macrophage-colony stimulating factor in mouse bone marrow culture.
Pharmazie. 62:388–391. 2007.PubMed/NCBI
|
|
16
|
Yamaza T, Miura Y, Bi Y, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One. 3:e26152008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011. View Article : Google Scholar
|
|
18
|
Huang J, Yuan L, Wang X, et al: Icaritin
and its glycosides enhance osteoblastic, but suppress osteoclastic,
differentiation and activity in vitro. Life Sci. 81:832–840. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai YK, Ge BF and Ma HP: Comparative
study on the osteogenic differentiation of rat bone marrow stromal
cells effected by icariin and icariside II. Zhong Yao Cai.
33:1896–1900. 2010.In Chinese.
|
|
20
|
Zhai YK, Ge BF and Ma HP: Icariin promotes
osteogenic differentiation of rat bone marrow stromal cells in
vitro. Zhongguo Zhong Yao Za Zhi. 35:3219–3222. 2010.In
Chinese.
|
|
21
|
Ma HP, Ming LG and Ge BF: Icariin is more
potent than genistein in promoting osteoblast differentiation and
mineralization in vitro. J Cell Biochem. 112:916–923. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ming LG, Zhou J, Cheng GZ, et al: Osthol,
a coumarin isolated from common cnidium fruit, enhances the
differentiation and maturation of osteoblasts in vitro.
Pharmacology. 88:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mok SK, Chen WF and Lai WP: Icariin
protects against bone loss induced by oestrogen deficiency and
activates oestrogen receptor-dependent osteoblastic functions in
UMR 106 cells. Br J Pharmacol. 159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Powell ME and Smith MJ: The determination
of serum acid and alkaline phosphatase activity with
4-aminoantipyrine (A.A.P.). J Clin Pathol. 7:245–248. 1954.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang ZB and Yang QT: The testosterone
mimetic properties of icariin. Asian J Androl. 8:601–605. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning H, Xin ZC, Lin G, et al: Effects of
icariin on phosphodies-terase-5 activity in vitro and cyclic
guanosine monophosphate level in cavernous smooth muscle cells.
Urology. 68:1350–1354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dell’Agli M, Galli GV, Dal Cero E, et al:
Potent inhibition of human phosphodiesterase-5 by icariin
derivatives. J Nat Prod. 71:1513–1517. 2008. View Article : Google Scholar
|
|
29
|
Xu HB and Huang ZQ: Icariin enhances
endothelial nitric-oxide synthase expression on human endothelial
cells in vitro. Vascul Pharmacol. 47:18–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bian Q, Huang JH, Liu SF, et al: Different
molecular targets of icariin on bMSCs in CORT and OVX-rats. Front
Biosci (Elite Ed). 4:1224–1236. 2012. View
Article : Google Scholar
|
|
31
|
Barkhem T, Haldosén LA, Gustafsson JA and
Nilsson S: Transcriptional synergism on the pS2 gene promoter
between a p160 coactivator and estrogen receptor-alpha depends on
the coactivator subtype, the type of estrogen response element, and
the promoter context. Mol Endocrinol. 16:2571–2581. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zampese E, Fasolato C, Pozzan T and Pizzo
P: Presenilin-2 modulation of ER-mitochondria interactions: FAD
mutations, mechanisms and pathological consequences. Commun Integr
Biol. 4:357–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh TP, Sheu SY, Sun JS, et al: Icariin
isolated from Epimedium pubescens regulates osteoblasts anabolism
through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine.
17:414–423. 2011. View Article : Google Scholar
|



















