Introduction
Pituitary adenomas are common benign neoplasms,
which may cause disorders of growth, reproductive function and
cortisol production (1). Surgical
resection is the treatment of choice for the majority of
symptomatic pituitary adenomas. However, due to extensive local
invasion, removal of the complete tumour is difficult (2). Although not malignant, invasive
pituitary adenomas are able to infiltrate surrounding tissues,
including the dura mater, the cranial bone, or the sphenoid sinus
(2). The invasions observed at the
time of surgery account for up to 35% of pituitary adenomas
(1). By contrast, the metastases
to the pituitary are rare, accounting for 0.1–0.2% of pituitary
tumours (2). Invasive pituitary
adenomas remain clinically indistinguishable prior to
identification of metastases. Thus, numerous attempts have been
made to define molecular markers associated with invasive adenoma.
For example, there is a significant association between the
expression of the tumour protein p53 (TP53) gene and the behaviour
of the pituitary tumour. The overexpression of TP53 was observed in
100% of pituitary carcinomas, 15% of invasive adenomas and 0% of
non-invasive adenomas examined in a previous study (3). The fibroblast growth factor receptor
4 gene has been revealed to induce pituitary tumour invasion in
in vivo animal models in association with reduced N-cadherin
expression (4). The gene
expression of matrix metalloproteinase 9 was higher in invasive
tumours compared with non-invasive pituitary tumours (5). Notably, no single marker has been
identified to reliably predict the behaviour of the tumour thus far
(6), and whether such a biomarker
may be able to improve clinical management and the ultimate outcome
remains to be elucidated.
DNA microarray technology has been developed to
measure the expression of thousands of genes simultaneously in one
single experiment. Over the past few years, a number of raw
datasets from DNA microarray experiments have been deposited in
public primary databases, such as the National Center for
Biotechnology Information (NCBI) Gene Expression Omnibus (GEO)
(7) and the European Molecular
Biology Laboratory-European Bioinformatics Institute ArrayExpress
(8). Therefore, researchers are
able to reuse the resources by conducting comprehensive
computational analysis. In the present study, the DNA microarray
expression profiles of invasive pituitary adenomas were retrieved
from the NCBI GEO database and invasion-associated genes were
identified using a computational bioinformatics analysis pipeline.
This gene signature provided novel diagnostic biomarkers and
therapeutic targets for the treatment of invasive pituitary
adenomas.
Materials and methods
DNA microarray expression datasets
The gene expression datasets of invasive and
non-invasive pituitary adenomas were retrieved from a public
functional genomics data repository, the NCBI GEO database. As
shown in Table I, a total of 16
samples were analysed in the present study, eight non-invasive and
eight invasive pituitary adenomas. The DNA microarray experiments
were based on the GPL570 [HG-US133 Plus 2] Affymetrix Human Genome
U133 Plus 2.0 Array platform (Affymetrix, Santa Clara, CA, USA),
which included complete coverage of the Human Genome U133 Set plus
9,921 new probe sets representing ~6,500 additional genes for the
analysis of >47,000 transcripts.
 | Table IDescriptions of pituitary adenoma
samples. |
Table I
Descriptions of pituitary adenoma
samples.
| Sample ID | Sample
characteristic | NCBI GEO accession
number |
|---|
| 1 | Invasive | GSM663750 |
| 2 | Invasive | GSM663753 |
| 3 | Invasive | GSM663754 |
| 4 | Invasive | GSM663755 |
| 5 | Invasive | GSM663756 |
| 6 | Invasive | GSM663757 |
| 7 | Invasive | GSM663758 |
| 8 | Invasive | GSM96622 |
| 9 | Non-invasive | GSM663745 |
| 10 | Non-invasive | GSM663746 |
| 11 | Non-invasive | GSM663747 |
| 12 | Non-invasive | GSM663748 |
| 13 | Non-invasive | GSM663749 |
| 14 | Non-invasive | GSM663751 |
| 15 | Non-invasive | GSM663752 |
| 16 | Non-invasive | GSM96623 |
Data pre-processing
The intensity files with a CEL file extension suffix
for each sample were loaded in R by the R/Bioconductor (http://www.bioconductor.org/) package, Affy (9), to create an AffyBatch object.
Subsequently, the Bioconductor ‘gcrma’ package was used for
background correction and normalization. This function added a step
of adjusting the expression values based on the propensity of
certain probes to undergo non-specific binding. The output of this
process was an expression set object. The data sets were log2
transformed for gene expression comparison analysis.
Obtaining the ranked list of
differentially expressed genes
To identify differentially expressed genes across
multiple datasets, a non-parametric method was employed and
implemented in the RankProd (10)
package. RankProd is a statistically rigorous, but biologically
intuitive algorithm, which has been demonstrated to be robust
against noise in microarray data. This algorithm has also been
revealed to have a higher sensitivity and specificity compared with
other types of meta-analytic tools for microarrays.
Gene set enrichment analysis
To more thoroughly characterize sets of functionally
related genes differentially expressed between non-invasive and
invasive pituitary adenoma samples, Onto-Express (11) was used to classify genes according
to the following Gene-Ontology (GO) categories: Biological process;
cellular component; and molecular function. An impact analysis was
used to identify the pathways affected by the differentially
expressed genes in the invasive pituitary adenomas. The Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis was used to identify important pathways involved in
invasive pituitary adenomas. The KEGG database is well-known for a
comprehensive database focusing on various biochemistry pathways.
This systems biology approach was implemented by the Web-based
tool, Pathway-Express (11).
Results
Identification of genes differentially
expressed under an invasive state
Although the microarray experiments were conducted
by various groups (Table I), the
gcrma function conducted background correction and normalization on
the raw data during the pre-processing stage (Fig. 1). The RankProd function yielded a
list of genes ranked by percentage of false positive prediction
(pfp) values. In the current study, a total of 194 genes, including
55 upregulated and 139 downregulated genes, were identified when
the threshold was set as pfp<0.05 and |logFC|>2 (Fig. 2).
Gene ontology analysis of differentially
expressed genes
As shown in Table
II and Fig. 3, the functional
gene groups demonstrating the most significant representation in
the selected set of differentially expressed genes appear under the
biological process ontology and map to cellular biopolymer
metabolic process, cellular macromolecule metabolic process,
macromolecule metabolic process, primary metabolic process,
cellular metabolic process and metabolic process. Functional
categories significantly represented under the cellular component
and molecular function ontologies include genes involved in the
cytoplasm, protein binding, intracellular membrane-bound organelles
and membrane-bound organelles.
 | Table IIRanked list of KEGG pathways impacted
in invasive pituitary adenomas. |
Table II
Ranked list of KEGG pathways impacted
in invasive pituitary adenomas.
| Rank | Pathway name | Impact factor | P-value |
|---|
| 1 | Leukocyte
transendothelial migration | 188.308 | 3.13E-80 |
| 2 | Cell adhesion
molecules | 143.574 | 6.41E-61 |
| 3 | Adherens
junction | 15.326 | 3.60E-06 |
| 4 | Circadian rhythm | 11.094 | 1.84E-04 |
| 5 | Phosphatidylinositol
signalling system | 7.01 | 7.23E-03 |
Pathway impact analysis
To translate the list of differentially expressed
genes into an understanding of the underlying biological phenomena,
the web-based software, Pathway-Express, was used to conduct the
pathway impact analysis. This analysis included classical
statistics and considerations on the type and position of each gene
in the specified pathways. As a result, the pathway impact analysis
produced more biologically meaningful results than other existing
techniques. In the present study, the pathway impact analysis
revealed five pathways, which had a significant effect in invasive
pituitary adenomas (Table II).
These pathways include leukocyte transendothelial migration, cell
adhesion molecules (CAMs), adherens junction, circadian rhythm and
the phosphatidylinositol signalling system.
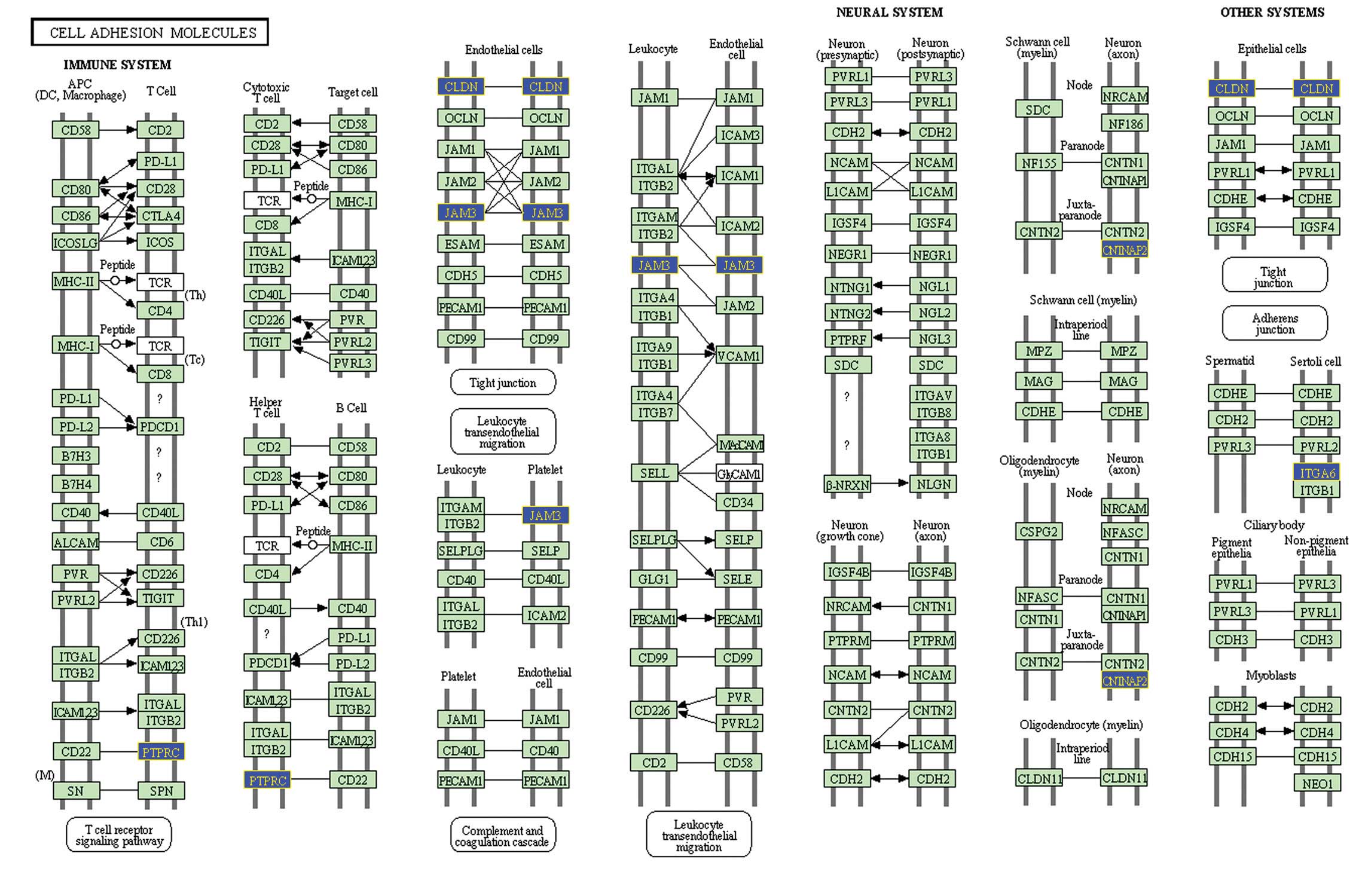
Invasion-associated gene
identification
As shown in Table
II, there are two pathways with significantly higher impact
factors, leukocyte transendothelial migration (Fig. 4) and CAMs (Fig. 5). The genes, which were
differentially expressed in the invasive pituitary adenomas were
identified as invasion-associated genes. These genes include
claudin 7 (CLDN7), contactin associated protein-like 2 (CNTNAP2),
integrin, α6 (ITGA6), junctional adhesion molecule 3 (JAM3),
protein tyrosine phosphatase, receptor type, C (PTPRC) and catenin
(cadherin-associated protein), α1 102 kDa (CTNNA1).
Discussion
In the present study, the raw intensity files of
microarray experiments were retrieved from the NCBI GEO databases.
Following pre-processing, RandProd was used to identify
differentially expressed genes in invasive pituitary adenomas.
Subsequently, Pathway-Express was used to conduct pathway impact
analysis. Compared with other gene set enrichment techniques, this
approach took consideration of important biological aspects,
including the magnitude of each gene’s change in expression and
their positions in the specified pathways. This provides a greater
level of pathway-specific biological analysis than other existing
techniques, which render the results more meaningful. The pathway
impact analysis revealed two important pathways, leukocyte
transendothelial migration and CAMs.
CAMs are important in all aspects of cell growth,
cell migration and cell differentiation in vertebrate cells
(12). They have been implicated
in numerous cellular functions, including signal transduction,
cellular communication and recognition, embryogenesis, inflammatory
and immune responses, and apoptosis (12). Dysregulation of CAMs is often
associated with carcinogenesis (13), particularly tumour invasion. For
instance, Cadherin genes are members of the CAMs and are considered
to be tumour suppressor genes (14). A member of the Cadherin family,
E-cadherin, mediates cell-cell contacts and acts as an important
suppressor of epithelial tumour cell invasiveness and metastasis
(15). In another study,
E-cadherin was observed to be deregulated in the poorly
differentiated human squamous cell carcinomas of the head and neck
and its expression was inversely correlated with lymph node
metastasis (16). In addition to
the identification that the E-cadherin-catenin complex is vital in
epithelial cell-cell adhesion and in the maintenance of tissue
architecture, the expression of the complex was found to be
inversely correlated with the invasion of tumour cells (17). These data may support the findings
of the present study, which demonstrated that the gene CTNNA1,
termed α-catenin, was identified as one of the invasion-associated
genes in pituitary adenomas.
CLDN7, CNTNAP2, ITGA6, JAM3, PTPRC and CTNNA1 have
been identified as invasion-associated genes in pituitary adenomas.
In previous studies, it has been reported that reduced expression
of CLDN7 correlated with tumour invasion in oesophageal squamous
cell carcinoma, colorectal cancer, lung cancer and oral squamous
cell carcinoma (18–22). Silencing of ITGA6 was able to
significantly inhibit cell migration of head and neck squamous cell
carcinoma cells (23). ITGA6 may
serve as a potential therapeutic target in oesophageal squamous
cell carcinoma (24). In gliomas,
the interaction between JAM2 and JAM3 activates the SRC
proto-oncogene, which is a central upstream molecule in the
pathways that regulated cell migration and invasion (25). The gene JAM3 is also important in
the adhesion of cancer cells to extracellular matrices and the
subsequent invasion in HT1080 human fibrosarcoma cells (26). Therefore, these six
invasion-associated genes identified in the present study may serve
as novel diagnostic or therapeutic biomarkers in pituitary
adenomas. However, further studies are required.
In conclusion, pathway impact analysis was used to
identify an invasion-associated molecular signature of six genes,
CLDN7, CNTNAP2, ITGA6, JAM3, PTPRC and CTNNA1. These genes were
significantly deregulated in invasive pituitary adenomas and may
serve as potential diagnostic or therapeutic biomarkers in the
treatment of invasive pituitary adenomas. However, further studies
are required to validate the present findings.
References
|
1
|
Oruçkaptan HH, Senmevsim O, Ozcan OE and
Ozgen T: Pituitary adenomas: results of 684 surgically treated
patients and review of the literature. Surg Neurol. 53:211–219.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheithauer BW, Kurtkaya-Yapicier O,
Kovacs KT, Young WF Jr and Lloyd RV: Pituitary carcinoma: a
clinicopatho-logical review. Neurosurgery. 56:1066–1074. 2005.
|
|
3
|
Thapar K, Scheithauer BW, Kovacs K,
Pernicone PJ and Laws ER Jr: p53 expression in pituitary adenomas
and carcinomas: correlation with invasiveness and tumor growth
fractions. Neurosurgery. 38:765–770. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ezzat S, Zheng L and Asa SL: Pituitary
tumor-derived fibroblast growth factor receptor 4 isoform disrupts
neural cell-adhesion molecule/N-cadherin signaling to diminish cell
adhesiveness: a mechanism underlying pituitary neoplasia. Mol
Endocrinol. 18:2543–2552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawamoto H, Kawamoto K, Mizoue T, Uozumi
T, Arita K and Kurisu K: Matrix metalloproteinase-9 secretion by
human pituitary adenomas detected by cell immunoblot analysis. Acta
Neurochir (Wien). 138:1442–1448. 1996. View Article : Google Scholar
|
|
6
|
Gadelha MR, Trivellin G, Hernández Ramírez
LC and Korbonits M: Genetics of pituitary adenomas. Front Horm Res.
41:111–140. 2013.PubMed/NCBI
|
|
7
|
Barrett T, Wilhite SE, Ledoux P, et al:
NCBI GEO: archive for functional genomics data sets – update.
Nucleic Acids Res. 41:D991–D995. 2013. View Article : Google Scholar
|
|
8
|
Rustici G, Kolesnikov N, Brandizi M, et
al: ArrayExpress update-trends in database growth and links to data
analysis tools. Nucleic Acids Res. 41:D987–D990. 2013. View Article : Google Scholar
|
|
9
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong F, Breitling R, McEntee CW, Wittner
BS, Nemhauser JL and Chory J: RankProd: a bioconductor package for
detecting differentially expressed genes in meta-analysis.
Bioinformatics. 22:2825–2827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarca AL, Draghici S, Khatri P, et al: A
novel signaling pathway impact analysis. Bioinformatics. 25:75–82.
2009. View Article : Google Scholar :
|
|
12
|
Cohen MB, Griebling TL, Ahaghotu CA,
Rokhlin OW and Ross JS: Cellular adhesion molecules in urologic
malignancies. Am J Clin Pathol. 107:56–63. 1997.PubMed/NCBI
|
|
13
|
Okegawa T, Pong RC, Li Y and Hsieh JT: The
role of cell adhesion molecule in cancer progression and its
application in cancer therapy. Acta Biochim Pol. 51:445–457.
2004.PubMed/NCBI
|
|
14
|
Okegawa T, Li Y, Pong RC and Hsieh JT:
Cell adhesion proteins as tumor suppressors. J Urol. 167:1836–1843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
16
|
Behrens J: The role of cell adhesion
molecules in cancer invasion and metastasis. Breast Cancer Res
Treat. 24:175–184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Usami Y, Chiba H, Nakayama F, et al:
Reduced expression of claudin-7 correlates with invasion and
metastasis in squamous cell carcinoma of the esophagus. Hum Pathol.
37:569–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lioni M, Brafford P, Andl C, et al:
Dysregulation of claudin-7 leads to loss of E-cadherin expression
and the increased invasion of esophageal squamous cell carcinoma
cells. Am J Pathol. 170:709–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshima T, Kunisaki C, Yoshihara K, et al:
Reduced expression of the claudin-7 gene correlates with venous
invasion and liver metastasis in colorectal cancer. Oncol Rep.
19:953–959. 2008.PubMed/NCBI
|
|
21
|
Lu Z, Ding L, Hong H, Hoggard J, Lu Q and
Chen YH: Claudin-7 inhibits human lung cancer cell migration and
invasion through ERK/MAPK signaling pathway. Exp Cell Res.
317:1935–1946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshizawa K, Nozaki S, Kato A, et al: Loss
of claudin-7 is a negative prognostic factor for invasion and
metastasis in oral squamous cell carcinoma. Oncol Rep. 29:445–450.
2013.
|
|
23
|
Kinoshita T, Nohata N, Hanazawa T, et al:
Tumour-suppressive microRNA-29 s inhibit cancer cell migration and
invasion by targeting laminin-integrin signalling in head and neck
squamous cell carcinoma. Br J Cancer. 109:2636–2645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon J, Lee TS, Lee HW, et al: Integrin
alpha 6: a novel therapeutic target in esophageal squamous cell
carcinoma. Int J Oncol. 43:1523–1530. 2013.PubMed/NCBI
|
|
25
|
Tenan M, Aurrand-Lions M, Widmer V, et al:
Cooperative expression of junctional adhesion molecule-C and -B
supports growth and invasion of glioma. Glia. 58:524–537. 2010.
|
|
26
|
Fuse C, Ishida Y, Hikita T, Asai T and Oku
N: Junctional adhesion molecule-C promotes metastatic potential of
HT1080 human fibrosarcoma. J Biol Chem. 282:8276–8283. 2007.
View Article : Google Scholar : PubMed/NCBI
|