Introduction
Alzheimer’s disease (AD) is a neurodegenerative
disorder, which ia associated with age and is characterized by
progressive memory loss and cognitive dysfunction. Epidemiological
studies have shown that the incidence of AD double increases every
5–10 years after the age of 65, indicating that globally the total
number of patients with AD will rise to 973 million by 2030
(1). Clinically, AD is
characterized by progressive impairments in behavior, cognition and
memory (2). The presence of
extracellular amyloid plaques, which develop as a result of the
deposition of progressively increasing amyloid β (Aβ) peptide
levels in the brain, are a key marker of AD (3). The Aβ peptides are important in the
pathogenesis of AD. Therefore, inhibiting the generation of Aβ and
increasing the rate of clearance of this protein, may be potential
therapeutic strategies with which to delay the development of AD
(4). While the Aβ hypothesis
states that the β-amyloid protein is involved in the progression of
AD pathology, the etiology remains unclear. Aβ is a 39–43 residue
amyloidogenic peptide, which is derived from β-amyloid pro-protein
(APP). APP may be cleaved by β-secretase and γ-secretase
sequentially (5,6), and the resulting hydrolysate is ~4
KDa of Aβ peptide (5). With
increasing age, the efficiency of organisms to eliminate Aβ is
reduced, and the concentration of Aβ in the brain is increased,
resulting in the formation of plaques. In addition, a previous
study indicated that the soluble Aβ peptide causes severe toxicity
to nerve cells, including tau hyperphosphorylation, axonal
transport disorder and the disruption of organelle trafficking
(7). Therefore, reducing the
generation of Aβ (8) or increasing
its clearance (9) may be
beneficial in the treatment of AD.
Autophagy (macroautophagy in the current study) is
the primary approach by which cells remove abnormal proteins and
damaged organelles. When autophagy is activated, the substrates
requiring removal are surrounded by double-membrane structures,
termed autophagosomes (10,11).
Autophagosomes fuse with lysosomes to form autolysosomes for
substrate degradation. Deregulation of autophagy in AD has become
an increasing focus of research (12). The enhancement of autophagy may
slow the ageing process and reduce age-associated diseases, such as
AD (13). For example, a previous
study indicated that plaques were reduced, and cognitive deficits
significantly improved, at an early age in 3xTg-AD mice following
the administration of rapamycin, which induced autophagy (14).
Mammalian target of rapamycin (mTOR), a 289 KDa
serine/threonine protein kinase, is the principle negative
regulatory kinase of autophagy, and is also involved in cell
growth, proliferation, metabolism and survival (15,16).
In addition, mTOR may inhibit autophagy by regulating its
downstream target, p70s6 kinase (17). Adenosine monophosphate-activated
protein kinase (AMPK) and phosphoinositide 3-kinase (PI3K)/protein
kinase B (Akt) are the two upstream regulators of mTOR, each of
which are associated with autophagy (18,19).
The metabolic sensor, AMPK, has been reported to inhibit mTOR via
an effect on its downstream target, Raptor (9). Akt is the positive regulatory kinase
of mTOR.
Radix Polygalae is the root of Polygala
tenuifolia Willd. and its extract appears to be capable of
improving memory (20). In
addition, Polygala tenuifolia has been reported to improve
cognitive impairment in rat AD models (21). Radix Polygalae extract was used to
protect rat neuronal cells in vitro, which were induced by
N-methyl-D-aspartate (22). In
addition, Tenuifolin, extracted from tenuigenin, has been reported
to inhibit Aβ secretion in COS-7 cells expressing APP (23). In the present study, the molecular
mechanism underlying the induction of autophagy by Radix Polygalae
extract was investigated, as this process was hypothesized to be
associated with reductions in Aβ secretion.
Materials and methods
Reagents and antibodies
3-(4,5-Dimethylthiazol-2-yl)-
2,5-diphenyl-tetrazolium bromide (MTT), dansylcadaverine (MDC) and
the rabbit monoclonal anti-light chain 3 (LC3) B antibody (1:1,000;
cat. no. L7543) were purchased from Sigma-Aldrich (Shanghai,
China). The rabbit monoclonal anti-mTOR (1:1,000; cat. no. 2983),
anti-phospho-mTOR (Ser2448; 1:1,000; cat. no. 2971), anti-p70s6k
(1:1,000; cat. no. 2708), anti-phospho-p70s6k (Thr389; 1:1,000;
cat. no. 9205), anti-AMPK (1:1,000; cat. no. 2532),
anti-phospho-AMPK (Thr172; 1:1,000; cat. no. 2535), anti-Raptor
(1:500; cat. no. 2280), anti-phospho-Raptor (Ser792 (1:500; cat.
no. 2083), anti-Akt (1:1,000; cat. no. 4685) and anti-phospho-Akt
(Ser473; 1:1,000; cat. no. 4058) antibodies were obtained from Cell
Signaling Technology, Inc. (Shanghai, China). The sheep anti-rabbit
IgG antibody conjugated with horseradish peroxidase (IgG-HRP),
SDS-PAGE, phenylmethanesulfonyl fluoride, loading buffer,
chemiluminescence kit, and penicillin and streptomycin were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Fetal bovine serum (FBS), G418 sulfate, DMEM/F12 and F12 basic
culture medium were purchased from Life Technologies (Shanghai,
China). Paraformaldehyde, Tris-buffered saline (TBS) and TBS
supplemented with 0.05% Tween-20 (TBST), were obtained from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Preparation of Radix Polygalae
extract
Radix Polygalae (Pudong New District Medicine and
Medicinal Materials Co., Ltd., Shanghai, China) was extracted using
a spin steaming process with a rotary evaporator (Yarong
Biochemical Instrument Plant, Shanghai, China) (24).
Cell culture
The SH-SY5Y human neuroblastoma cell line and
Chinese hamster ovary (CHO) cells were obtained from Shanghai
Institute of Materia Medica (Shanghai, China). SH-SY5Y cells were
cultured in DMEM/F12, supplemented with 10% FBS, 100 IU/ml
penicillin and 100 μg/ml streptomycin. CHO cells, stably
transfected with APP and BACE1, were cultured in F12 supplemented
with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 400
μg/ml G418 sulfate. Cells were maintained in an incubator at 37°C,
with an atmosphere of 95% air and 5% CO2.
MTT assay for drug toxicity
SH-SY5Y cells were seeded in 96-well plates and
treated with different concentrations (100, 40, 20, 10, 5 or 0
μg/ml) of Radix Polygalae. Following incubation for 24 h, 10 μl MTT
(5 mg/ml) was added, and the cells were maintained at 37°C for an
additional 4 h. The liquid was then discarded, crystals were
dissolved in 100 μl DMSO and the light absorbance was read at 570
nm using a Multiskan FC Microplate Reader (Thermo Fisher
Scientific, Shanghai, China), with 620 nm as the reference
wavelength. Cells treated with 0 μg/ml Radix Polygalae served as
controls (CTL). Radix Polygalae was dissolved in DMSO and while
preparing a working solution, isometric DMSO was used in accordance
with the drug treatment group.
Measurement of Aβ1-40 secretion in the
supernatant
CHO-APP/BACE1 cells were seeded into 24-well plates
at a density of 80,000 cells/well. Following treatment with (100,
40, 20, 10, 5 or 0 μg/ml) Radix Polygalae for 24 h, the Aβ1-40
ELISA kit (Shanghai ExCell Biology, Inc., Shanghai, China) and the
Bicinchoninic Acid Protein Assay Reagent (Thermo Fisher Scientific)
were used to measure the Aβ1-40 concentration in the supernatant
and the total protein respectively. The experiments were conducted
in accordance with the manufacturer’s instructions. The ratio of
Aβ1-40 level and total protein in the DMSO group was similar to the
control.
MDC-labeled autophagosomes detected in
SH-SY5Y cells
SH-SY5H cells were treated with 3 different doses
(0, 10 and 100 μg/ml) of Radix Polygalae with in the 6-well plate
for 24 h, followed by incubation with 50 μmol/l MDC at 37°C for 30
min. Following incubation, the cells were washed with
phosphate-buffered saline and fixed with 4% paraformaldehyde.
Autophagosomes were observed by fluorescence photometry (Olympus,
Tokyo, Japan) at the excitation wavelength of 380 nm and emission
filter of 525 nm (25).
Western blot analysis
SH-SH5Y cells were treated with different doses of
Radix Polygalae (100, 40, 20, 10, 5 and 0 μg/ml) for 24 h in a
6-well plate. The total proteins were collected by the addition of
radioimmunoprecipitation assay buffer containing 1 mM
phenylmethanesulfonyl fluoride, mixed with loading buffer and
boiled at 95°C for 15 min. Total proteins were separated by
SDS-PAGE (20 μg/lane) and transferred to nitrocellulose membranes
(Merck Millipore, Boston, MA, USA). The membranes were blocked for
2 h in TBS (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) containing 5% non
fat milk, then incubated with LC3,(p)-mTOR, (p)-p70s6k, (p)-AMPK,
(p)-Raptor and (p)-Akt primary antibodies at 4°C overnight.
Membranes were washed three times with TBST, and incubated with
horseradish peroxidase-linked anti-rabbit IgG for a further 2 h,
followed by washing with TBST again. Blots were detected by
enhanced chemiluminescence (ECL) and band intensity was analyzed by
ImageJ software 1.48 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data for multiple variable comparisons were analyzed by one-way
analysis of variance using GraphPad Prism v5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Radix Polygalae exerts no significant
effects on the viability of SH-SY5Y cells
The cell viability of human neuroblastoma cells with
and without exposure to Radix Polygalae, was measured using an MTT
assay. Following 24 h treatment, cell viability was not
significantly different between the control group and the Radix
Polygalae-treated groups (P>0.05). These results implied that
the drug is not cytotoxic at doses <100 μg/ml (Fig. 1).
Aβ1-40 peptides level is markedly lower
in the supernatant of cells treated with Radix Polygalae
The level of the Aβ1-40 peptide in the supernatant
of CHO-APP/BACE1 cells was measured using an ELISA assay. The data
showed that there was a reduction in the level of Aβ1-40 following
treatment with Radix Polygalae, and that this effect occurred in a
dose-dependent manner. The Aβ1-40 level in the 5 μg/ml group was
reduced compared with DMSO group, although this result was not
statistically significant. However, the reduction in Aβ1-40 was
significantly different from the control group at doses of ≥10
μg/ml (P<0.01; Fig. 2).
Autophagy is induced by Radix
Polygalae
In order to demonstrate that the Aβ peptide is
degraded by autophagy, autophagy vesicles were detected using the
MDC method, and he autophagy protein marker, LC3, was measured by
western blotting analysis. Intracellular autophagy vesicles were
labeled by MDC, and were observed as green dots. Few vesicles were
observed in the control group (Fig.
3A), while an increase in the number of labeled vesicles
occurred in the 10 μg/ml group (Fig.
3B) and a marked increase was observed in the 100 μg/ml group
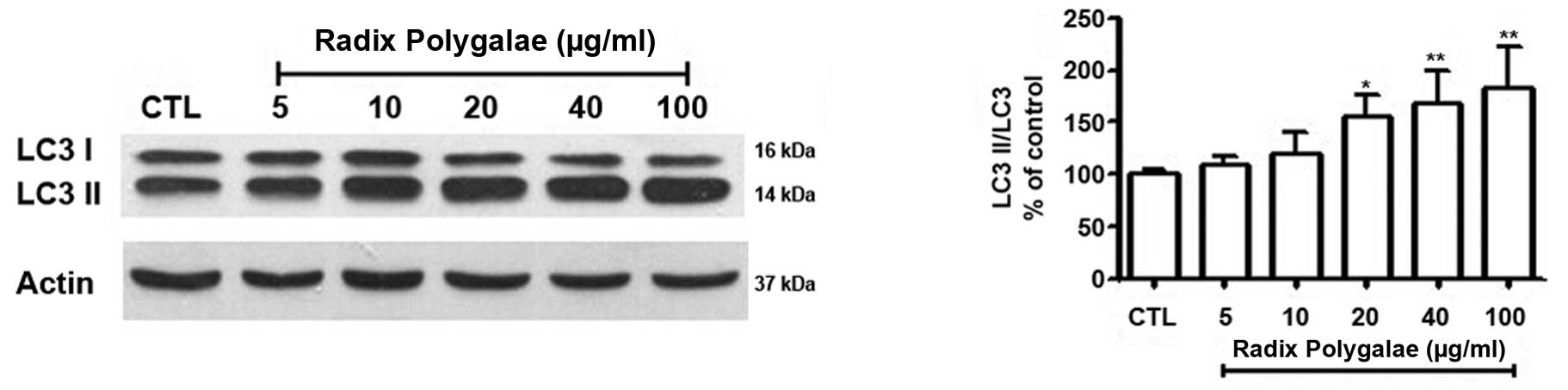
(Fig. 3C). LC3I and LC3II are the
two forms of the microtubule-associated protein light chain 3
(LC3). When autophagy occurs, LC3I, located in the cytoplasm, is
modified and processed by the ubiquitin-proteasome system and
converted into LC3II (9,26). Western blot analysis indicated that
LC3II/LC3I gradually increased, in accordance with the
concentration of Radix Polygalae (Fig.
4).
Radix Polygalae induces autophagy by
activating AMPK/mTOR signaling
In order to investigate the molecular mechanisms
underlying the autophagy induced by Radix Polygalae in neurones,
the negative regulator of autophagy, mTOR, in addition to its
associated kinases, was examined by western blot analysis. The
phosphorylation level of mTOR was reduced in a dose-dependent
manner in the Radix Polygalae-treated groups. This indicates that
Radix Polygalae may increase the level of autophagy. In addition,
the downstream signaling kinase of mTOR, p70s6k, which also
participates in the regulation of autophagy, was also inhibited by
Radix Polygalae (Fig. 5).
In order to investigate the upstream pathway of
mTOR, which may be affected by Radix Polygalae, the expression of
AMPK and its downstream target, Raptor, was measured (27). With increasing doses of Radix
Polygalae, the phosphorylation level of AMPK at Thr172 was
increased, as was the phosphorylation of Raptor at Ser792 (Fig. 6).
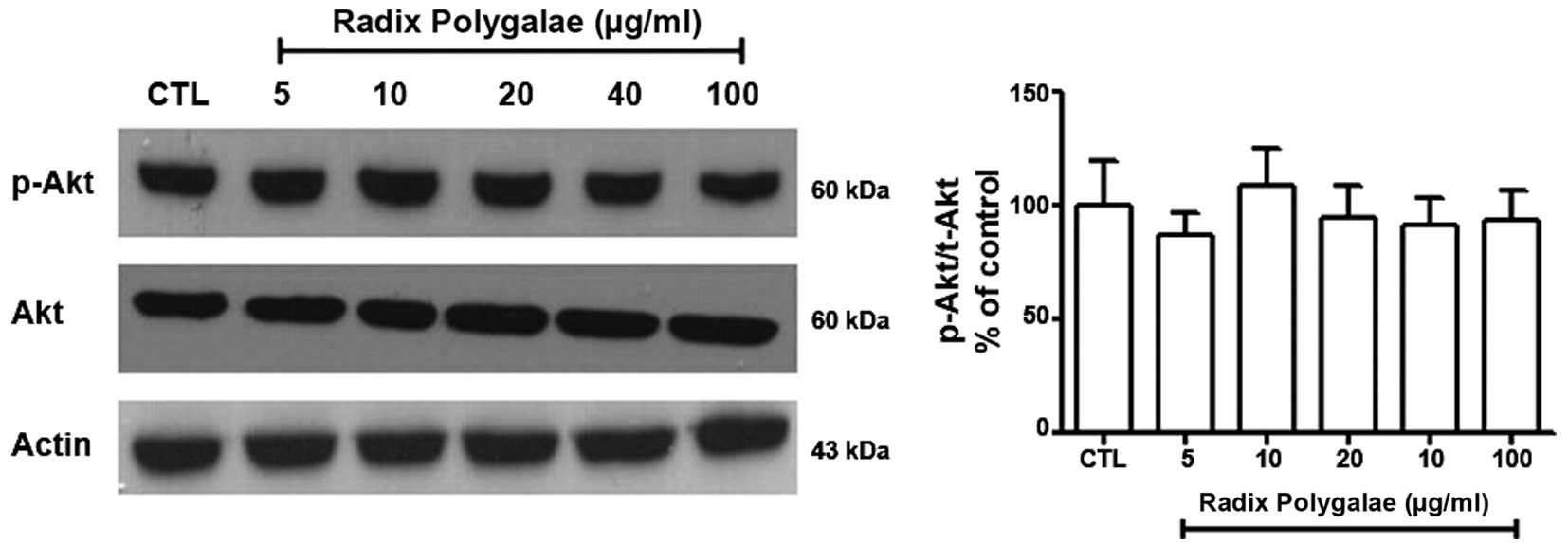
Akt is another kinase that is known to activate mTOR
(15). The results of the present
study demonstrated that the phosphorylation level of Akt at Ser473
in the different groups was not significantly altered (Fig. 7).
Discussion
Aβ is an important protein in the pathogenesis of
AD, and has become an increasing focus of the research into the
development of novel anti-AD treatments (28–31).
Reducing the levels of Aβ in the brain, with the aim of improving
cognitive function and quality of life for patients with AD, are
currently key aims in AD research. However, a specific and
effective drug with which to treat AD remains to be developed,
although the understanding of AD has advanced rapidly (20,32).
In previous years, traditional Chinese medicine (TCM) products have
gained attention for their potential use in AD treatment, due to
the low toxicity and the fact that they are easy to obtain
(20). Radix Polygalae, a TCM that
is used to improve memory, has been previously studied, however the
mechanisms involved remain to be elucidated (21–23).
In the current study, the effect of Radix Polygalae on Aβ peptide
levels was investigated in vitro.
Serious damage to neurons by Aβ peptides results in
the formation of amyloid plaques and also leads to induction of the
inflammatory response (33) and
hyperphosphorylation of Tau (34,35),
resulting in neuronal death. In the present study, secreted Aβ was
detected in the CHO-APP/BACE1 supernatant, and the levels were
significantly reduced following treatment with Radix Polygalae,
suggesting that this compound simulates the removal of the Aβ
peptide. Autophagy, which is the primary method of removing
abnormal proteins and organelles from cells, was hypothesized to
have been involved in this process. In order to confirm this
hypothesis, MDC staining was used to detect autophagosomes. In
addition, levels of the autophagy marker protein, LC3, were
measured. The results from these two experiments supported the
hypothesis.
In order to investigate the signaling mechanism
underlying the induction of autophagy by Radix Polygalae, the mTOR
pathway was assessed, due to its association with autophagy. In a
previous study, mTOR was shown to be inhibited by rapamycin, while
autophagy was increased, with the result that β amyloid plaque in a
mouse model of AD decreased and cognitive function improved
(14,36). p70s6k is the downstream target of
mTOR. It is regulated by mTOR and participates in the inhibition of
autophagy (17,37). In the current study, it was
observed that the levels of mTOR and p70s6k were reduced by the
addition of Radix Polygalae, indicating that autophagy had been
induced. AMPK and PI3K/Akt are the two upstream regulators of mTOR,
and AMPK is the key energy-sensing kinase that regulates cellular
energy homeostasis in eukaryotes (27). AMPK regulates mTOR by
phosphorylating Raptor at Ser792 (9,38).
The Akt pathway is an additional upstream regulator of mTOR,
exerting its action by phosphorylating Akt at Ser473 (16). In the current study, the
phosphorylation of AMPK/Raptor increased. By contrast, Akt was not
observed to be activated. These results support the hypothesis that
Radix Polygalae induces autophagy via the activation
AMPK/Raptor/mTOR signaling.
In conclusion, autophagy is involved in the removal
of Aβ peptide by Radix Polygalae. The basic signaling mechanism
underlying this effect was detected, and the results of the current
study may provide a basis for further investigation into the use of
this compound in the treatment of AD.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81373421 and
81270650).
References
|
1
|
Feng Y and Wang X: Antioxidant therapies
for Alzheimer’s disease. Oxid Med Cell Longev. 2012:4729322012.
View Article : Google Scholar
|
|
2
|
Huang Y and Mucke L: Alzheimer mechanisms
and therapeutic strategies. Cell. 148:1204–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bondareff W: Age-related changes in brain
extracellular space affect processing of amyloid-β peptides in
Alzheimer’s disease. J Alzheimers Dis. 35:1–6. 2013.
|
|
4
|
Shi TY, Zhao DQ, Wang HB, et al: A new
chiral pyrrolyl α-nitronyl nitroxide radical attenuates β-amyloid
deposition and rescues memory deficits in a mouse model of
Alzheimer disease. Neurotherapeutics. 10:340–353. 2013. View Article : Google Scholar :
|
|
5
|
Cappai R and White AR: Amyloid β. Int J
Biochem Cell Biol. 31:885–889. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cavallucci V, D’Amelio M and Cecconi F: Aβ
toxicity in Alzheimer’s disease. Mol Neurobiol. 45:366–378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanchez-Varo R, Trujillo-Estrada L,
Sanchez-Mejias E, et al: Abnormal accumulation of autophagic
vesicles correlates with axonal and synaptic pathology in young
Alzheimer’s mice hippocampus. Acta Neuropathol. 123:53–70. 2012.
View Article : Google Scholar :
|
|
8
|
Zhu Z, Li C, Wang X, Yang Z, Chen J, Hu L,
Jiang H and Shen X: 2,2′,4′-trihydroxychalcone from Glycyrrhiza
glabra as a new specific BACE1 inhibitor efficiently ameliorates
memory impairment in mice. J Neurochem. 114:374–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arsikin K, Kravic-Stevovic T, Jovanovic M,
Ristic B, Tovilovic G, Zogovic N, Bumbasirevic V, Trajkovic V and
Harhaji-Trajkovic L: Autophagy-dependent and -independent
involvement of AMP-activated protein kinase in 6-hydroxydopamine
toxicity to SH-SY5Y neuroblastoma cells. Biochim Biophys Acta.
1822:1826–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H,
Huang WP and Liao YF: Autophagy: a double-edged sword in
Alzheimer’s disease. J Biosci. 37:157–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Periyasamy-Thandavan S, Jiang M,
Schoenlein P and Dong Z: Autophagy: Molecular machinery, regulation
and implications for renal pathophysiology. Am J Physiol Renal
Physiol. 297:F244–F256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung ZH and Ip NY: Autophagy
deregulation in neurode-generative diseases-recent advances and
future perspectives. J Neurochem. 118:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaushik S and Cuervo AM: Autophagy as a
cell-repair mechanism: Activation of chaperone-mediated autophagy
during oxidative stress. Mol Aspects Med. 27:444–454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majumder S, Richardson A, Strong R and
Oddo S: Inducing autophagy by rapamycin before, but not after, the
formation of plaques and tangles ameliorates cognitive deficits.
PLoS One. 6:e254162011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chong ZZ, Shang YC, Zhang L, Wang S and
Maiese K: Mammalian target of rapamycin: Hitting the bull’s-eye for
neurological disorders. Oxid Med Cell Longev. 3:374–391. 2010.
View Article : Google Scholar
|
|
16
|
Wu X, Kihara T, Akaike A, Niidome T and
Sugimoto H: PI3K/Akt/mTOR signaling regulates glutamate transporter
1 in astrocytes. Biochem Biophys Res Commun. 393:514–518. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klionsky DJ, Meijer AJ, Codogno P, Neufeld
TP and Scott RC: Autophagy and p70S6 kinase. Autophagy. 1:59–61.
2005. View Article : Google Scholar
|
|
18
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activated protein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–15.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saiki S, Sasazawa Y, Imamichi Y, et al:
Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar :
|
|
20
|
Lin Z, Gu J, Xiu J, Mi T, Dong J and
Tiwari JK: Traditional chinese medicine for senile dementia. Evid
Based Complement Alternat Med. 2012:6926212012. View Article : Google Scholar
|
|
21
|
Lee HJ, Ban JY, Koh SB, Seong NS, Song KS,
Bae KW and Seong YH: Polygalae radix extract protects cultured rat
granule cells against damage induced by NMDA. Am J Chin Med.
32:599–610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park CH, Choi SH, Koo JW, Seo JH, Kim HS,
Jeong SJ and Suh YH: Novel cognitive improving and neuroprotective
activities of Polygala tenuifolia Willdenow extract, BT-11. J
Neurosci Res. 70:484–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv J, Jia H, Jiang Y, Ruan Y, Liu Z, Yue
W, Beyreuther K, Tu P and Zhang D: Tenuifolin, an extract derived
from tenuigenin, inhibits amyloid-β secretion in vitro. Acta
Physiol (Oxf). 196:419–425. 2009. View Article : Google Scholar
|
|
24
|
Melo MC, Gadelha DN, Oliveira TK and
Brandt CT: Alcohol extract of Schinu sterebinthifolius raddi
(anacardiaceae) as a local antimicrobial agent in severe
autogenously fecal peritonitis in rats. Acta Cir Bras. 29(Suppl 1):
52–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munafó DB and Colombo MI: A novel assay to
study autophagy: Regulation of autophagosome vacuole size by amino
acid deprivation. J Cell Sci. 114:3619–36291. 2001.PubMed/NCBI
|
|
26
|
Hung SY, Huang WP, Liou HC and Fu WM:
Autophagy protects neuron from Abeta-induced cytotoxicity.
Autophagy. 5:502–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai Z, Yan LJ, Li K, Quazi SH and Zhao B:
Roles of AMP-activated protein kinase in Alzheimer’s disease.
Neuromolecular Med. 14:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arbel M and Solomon B: Immunotherapy for
Alzheimer’s disease: Attacking amyloid beta from the inside. Trends
Immunol. 28:511–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeon S, Bose S, Hur J, Jun K, Kim YK, Cho
KS and Koo BS: A modified formulation of Chinese traditional
medicine improves memory impairment and reduces Aβ level in the
Tg-APPswe/PS1dE9 mouse model of Alzheimer’s disease. J
Ethnopharmacol. 137:783–789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai AY and McLaurin J: Inhibition of
amyloid-beta peptide aggregation rescues the autophagic deficits in
the TgCRND8 mouse model of Alzheimer disease. Biochim Biophys Acta.
1822:1629–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenberg D and Jucker M: The amyloid
state of proteins in human diseases. Cell. 148:1188–1203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao J, Inagaki Y, Li X, Kokudo N and Tang
W: Research progress on natural products from traditional Chinese
medicine in treatment of Alzheimer’s disease. Drug Discov Ther.
7:46–57. 2013.PubMed/NCBI
|
|
33
|
Meraz-Ríos MA, Toral-Rios D,
Franco-Bocanegra D, Villeda-Hernández J and Campos-Peña V:
Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci.
7:592013. View Article : Google Scholar
|
|
34
|
Cuchillo-Ibáñez I, Balmaceda V,
Botella-López A, Rabano A, Avila J and Sáez-Valero J: Beta-amyloid
impairs reelin signaling. PLoS One. 8:e722972013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tokutake T, Kasuga K, Yajima R, Sekine Y,
Tezuka T, Nishizawa M and Ikeuchi T: Hyperphosphorylation of Tau
induced by naturally secreted amyloid-β at nanomolar concentrations
is modulated by insulin-dependent Akt-GSK3β signaling pathway. J
Biol Chem. 287:35222–35233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spilman P, Podlutskaya N, Hart MJ, Debnath
J, Gorostiza O, Bredesen D, Richardson A, Strong R and Galvan V:
Inhibition of mTOR by rapamycin abolishes cognitive deficits and
reduces amyloid-beta levels in a mouse model of Alzheimer’s
disease. PLoS One. 5:e99792010. View Article : Google Scholar
|
|
37
|
Lafay-Chebassier C, Paccalin M, Page G,
Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L and Hugon J:
mTOR/p70S6k signalling alteration by Ab exposure as well as in
APP-PS1 transgenic models and in patients with Alzheimer’s disease.
J Neurochem. 94:215–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JW, Park S, Takahashi Y and Wang HG:
The association of AMPK with ULK1 regulates autophagy. PLoS One.
5:e153942010. View Article : Google Scholar : PubMed/NCBI
|