Introduction
Resveratrol, a natural polyphenol found in grapes
and red wine, is known to have various effects on antioxidant
activity (1) and can induce
apoptosis and inhibit angiogenesis in various types of cancer
(2,3). In previous studies, resveratrol has
been observed to induce apoptosis in glioma cells (4) and to inhibit the expression of
hypoxia-inducible factor 1α and vascular endothelial growth factor
(VEGF) via multiple mechanisms, including inhibition of protein
kinase B and mitogen-activated protein kinase (MAPK) in human
ovarian cancer cell lines (5). In
addition, resveratrol inhibits tumor growth and decreases
angiogenesis by suppressing capillary-like tube formation by human
umbilical vein endothelial cells in mouse models of lung cancer
(6). Its potential as an
antiangiogenic agent is further supported by its dose-dependent
inhibition of tumor-induced neovascularization (7).
5-Fluorouracil (5-FU), a drug that induces apoptosis
by inhibiting thymidylate synthase, has been used therapeutically
in various types of cancer (8).
However, its long-term use leads to resistance, limiting its
clinical use (9). To overcome drug
resistance in tumor cells, several studies have examined novel
treatments or combination therapies with 5-FU. Previous studies
have demonstrated that co-treatment with genistein and 5-FU induces
apoptosis more efficiently compared with either drug alone in colon
cancer and co-treatment with 5-FU and resveratrol is more efficient
compared with controls in suppressing tumor cell growth in a murine
model of liver cancer (4,10).
Cyclooxygenase-2 (COX-2) is an inducible enzyme,
which catalyzes the synthesis of prostaglandin E2
(PGE2) (11). The
COX-2/PGE2 pathway is important in inflammation,
angiogenesis and tumorigenesis and its overexpression is found in
various cancer cell lines (12,13).
It has been observed that downregulation of COX-2 suppresses
angiogenesis and tumor growth via regulation of VEGF,
angiopoietin-1, tie-2 and matrix metalloproteinase-2 (MMP2) in an
in vivo model of gastric cancer (14). Furthermore, COX-2 binds directly to
resveratrol and controls PGE2 and this complex inhibits
the proliferation of colon cancer cells (15).
VEGF is overexpressed in a variety of cancer cells
and is considered an important angiogenic factor, which is
upregulated by hypoxia inducible factor-1 (16). Hypoxia-simulated VEGF regulates
proliferation, migration and vascular permeability via various
signaling pathways, including MAPK, phosphoinositide 3-kinase and
protein kinase C, in endothelial cells (17). Previous studies have demonstrated
that VEGF-C-knockdown decreases proliferation and actinmediated
stress fiber formation in endothelial cells through the RhoA
pathway (18) and that inhibition
of the expression of VEGF suppresses tumor growth and vessel
density in vivo (19).
Vasodilator-stimulated phosphoprotein (VASP) is
expressed in vascular endothelial cells and smooth muscle cells and
is important in the formation of endothelial cell substrates and
contacts between cells by binding to actin, profilin, zyxin and
vinculin (20). VASP regulates
carcinoma cell invasion and metastasis in vivo and in
vitro via epidermal growth factor (EGF) signaling (21).
The present study investigated the effect of
resveratrol on the proliferation and metastatic potential of cancer
cells via multiple pathways. In addition, the effect of combined
treatment of resveratrol and 5-FU on the expression of VEGF, COX-2
and VASP as well as cell growth and migration was assessed on B16
melanoma cells. The present study indicated that co-treatment of
melanoma cells with resveratrol and 5-FU was more efficient
compared with either drug alone.
Materials and methods
Cells and reagents
B16 murine melanoma cells were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 with 10% fetal bovine serum (Gibco-BRL, Grand Island,
NY, USA) at 37°C in a 5% CO2 atmosphere. Resveratrol and
5-FU were purchased from Sigma (St. Louis, MO, USA). MTT and
celecoxib were also obtained from Sigma. Monoclonal antibodies
specific for phosphorylated (p)-AMP-activated protein kinase [AMPK;
monoclonal rabbit immunoglobulin G (IgG)], COX-2 (polyclonal rabbit
IgG), VASP (polyclonal rabbit IgG) and β-actin (polyclonal rabbit
IgG) were purchased from Cell Signaling Technology, Inc. (Beverly,
MA, USA) and the VEGF (monoclonal mouse IgG) antibody was obtained
from Santa Cruz Biotechnology, Inc. (San Diego, CA, USA).
Cell proliferation measurements
Cell proliferation was assessed using an MTT assay.
The cells were seeded into a 12-well plate (1×106
cells/well) for 24 h and were incubated with resveratrol (10–50
μM) and 5-FU (5–20 μM) for 24 h. Following incubation
with the test compounds, the cells were incubated with 30 μl
MTT solution (5 mg/ml) in phosphate-buffered saline (PBS) for 1 h.
The sample was then solubilized in dimethyl sulfoxide and the
absorbance of purple formazan dye, the product of MTT converted by
the viable cells, was quantified at 565 nm (Microplate Reader;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of apoptosis
Following stimulation of the B16 murine melanoma
cells with resveratrol and 5-FU, apoptosis was measured using a
fluorescein isothiocyanate (FITC)-Annexin V apoptosis detection kit
(BD Pharmingen, San Diego, CA, USA). The B16 cells, including
floating cells, were collected following trypsinization
(Trypsin-EDTA; Welegene, Inc., Deagu, Korea). The trypsinized cells
were washed with PBS and resuspended in a mixture containing 100
μl Annexin binding buffer mixed and 5 μl
FITC-conjugated Annexin V and phycoerythrin-conjugated propidium
iodide (PI). The resuspended cells were then incubated in the dark
at room temperature for 15 min, followed by analysis of the labeled
cells by flow cytometry (FACS Calibur; Becton-Dickinson, Franklin
Lakes, NJ, USA).
Wound healing migration assay
The B16 murine melanoma cells (1×105
cells/ml) were seeded into a six-well plate for 24 h. The confluent
monolayer was starved using serum-free medium for 12 h and wounded
by scratching with a 200 μl pipette tip (Axygen, Union City,
CA, USA). The cells were incubated in serum-free medium containing
the test compound at 37°C and images of the movement of cells into
the wound area were captured at 0, 24 and 48 h using a microscope
(magnification, ×100; CK40-F200, Olympus Corp., Tokyo, Japan).
Western blot analysis
The cells (1×106 cells/ml) were incubated
with resveratrol (10–50 μM) and 5-FU (5–20 μM) for 6
h on a six-well plate. The total proteins were extracted using
radioimmunoprecipitation assay lysis buffer containing 50 mM
Tris-HCl (pH 8.0), 1% NP 40, 0.5% sodium deoxycholate, 150 mM NaCl
and 1 mM phenylmethylsulfonyl fluoride. The mixture was centrifuged
at 19,326xg for 20 min at 4°C and the protein concentration was
measured using a Bradford protein assay kit (Bio-Rad Laboratories,
Inc.). Equal quantities of the protein were separated by SDS-PAGE
and were then electrophoretically transferred onto nitrocellulose
transfer membranes (Whatman, Pittsburgh, PA, USA). Following
inhibition (BSA; Bovogen Biologicals Pty, Ltd, VIC, Australia), the
membranes were incubated with phospho-AMPK (1:1,000), COX-2
(1:1,000), VASP (1:1,000), β-actin (1:1,000) (Cell Signaling
Technology, Inc.) and VEGF (1:1,000; Santa Cruz Biotechnology,
Inc.) antibodies overnight at 4°C with gentle agitation. Following
incubation with the primary antibodies, the membrane was incubated
with anti-mouse or anti-rabbit IgG secondary antibodies (Enzo Life
Sciences, Farmingdale, NY, USA) for 1.5 h at room temperature with
gentle agitation. Following washing with TBST containing 20 mM
Tris, 500 mM NaCL, pH 7.4 and 1 ml Tween20 (Bio-Rad Laboratories,
Inc.), the bands were visualized (Autoradiography cassette; Fisher
Scientific, Inc., Pittsburgh, PA, USA) using enhanced
chemiluminescence detection reagents (WEST-ZOL; iNtRON
Biotechnology, Inc., Seoul, Korea).
Tumor formation
Male five-week-old Balb/c nu/nu mice were
obtained from SLC, Inc. (Tokyo, Japan) and housed in sterile
filer-topped cages. Mice were kept in an air-conditioned barrier
facility at an ambient temperature of 25±2°C, a relative humidity
of 50±5% and a 12-h on/off light cycle. Diets were purchased from
Dyets Inc. (Bethlehem, PA, USA; AIN-76A Rodent Purified diet).
Health was monitored daily by gross observation. For tumor
induction, the B16 murine melanoma cells (2.5×105
cells/0.1 ml) were subcutaneously injected into the left flank of
the mice (n=5/group). Subsequently, one week after injection of the
B16 murine melanoma cells, co-treatment with resveratrol and 5-FU
was performed via injection into the left flank of Balb/c nu/nu
mice for 10 days. The control animals were injected with a vehicle
of PBS alone. The tumor size was measured using calipers at two-day
intervals and the tumor volume was calculated using the modified
formula V = 1/2 (length × width2). After 10 days of
treatment, the tumors were excised and either frozen in liquid
nitrogen for western blot analysis or fixed with formalin for
immunohis-tochemical analysis. All surgery was performed under
ether anesthesia, and efforts were made to minimize suffering. All
animal experiments were approved by the Ethics Committee for Animal
Experimentation, Hannam National University (Hannam, Korea).
Immunohistochemistry
The tumor specimens from the mice were fixed in 10%
formaldehyde, embedded in paraffin and sectioned into 5-μm
slices. Consecutive 5-μm cryosections of optimum cutting
temperature compound-embedded (Sakura Finetek, Torrance, CA, USA)
tumor tissues were fixed in acetone at 4°C for 10 min. Following
washing in PBS, the sections were treated with 3%
H2O2 for 10 min to inhibit endogenous
peroxidase activity and the sections were inhibited with normal
rabbit serum. The sections were then washed in PBS and incubated
with anti-CD31 antibody (rat-anti-mouse; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. Negative controls were incubated with the
primary normal serum immunoglobulin G for the species from which
the primary antibody was obtained. The number of CD31-stained sites
in the B16 murine melanoma tissues were counted, which corresponded
to the microvessel density.
Statistical analysis
The microvessel density data were statistically
analyzed using Student’s t-test using SPSS 20 software (IBM SPSS,
Armonk, NY, USA). Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Resveratrol and 5-FU inhibit cell
proliferation via the regulation of levels of AMPK, COX-2, VASP and
VEGF in B16 murine melanoma cells
To examine whether resveratrol and 5-FU exerted
antiproliferative activity, the present study examined the effects
of resveratrol and 5-FU on the growth of the B16 cells. The cells
were treated with different concentrations (10, 25 and 50
μM) of resveratrol for 24 h and the cell viability was
evaluated using an MTT assay. As shown in Fig. 1A, the cell viability at
concentrations of 10, 25 and 50 μM were 85, 60 and 50%,
respectively. The cells were also treated with 5, 10 and 20
μM 5-FU for 24 h, resulting in a cell viability of 79, 70
and 60%, respectively (Fig. 1B).
To investigate changes in the expression levels of AMPK, COX-2,
VASP and VEGF, the B16 cells were treated with resveratrol and 5-FU
for 6 h and the proteins were analyzed using western blot analysis.
Resveratrol (Fig. 1C) and 5-FU
(Fig. 1D) activated AMPK in a
dose-dependent manner and decreased the expression levels of COX-2,
VASP and VEGF, also in a dose-dependent manner.
 | Figure 1Resveratrol and 5-FU inhibit cell
proliferation and regulate the expression levels of COX-2, VEGF and
VASP in B16 cells. (A) Cells were treated with resveratrol (10–50
μM) for 24 h and the cell viability was measured using an
MTT assay. (B) Cells were treated with 5-FU (5–20 μM) for 24
h and cell viability was measured using an MTT assay. (C) Cells
were treated with resveratrol (10–50 μM) for 6 h and total
protein was subjected to western blot analysis using p-AMPK, COX-2,
VEGF, VASP and β-actin (loading control) antibodies. (D) Cells were
treated with 5-FU (5–20 μM) for 6 h and total protein was
subjected to western blot analysis using p-AMPK, COX-2, VEGF, VASP
and β-actin (loading control) antibodies. p-AMPK, phosphorylated
AMP-activated protein kinase; COX-2, cyclooxygenase-2; VASP,
vasodilator-stimulated phosphoprotein; VEGF, vascular endothelial
growth factor; FU, fluorouracil; N, none. |
Growth-inhibitory effects of combined
resveratrol and 5-FU treatment on B16 cells are based on regulation
of AMPK, COX-2 VASP and VEGF
To investigate the effects of co-treatment with
resveratrol and 5-FU on cell growth, B16 cells were treated with
resveratrol and 5-FU either alone or in combination and the cell
viability was measured using an MTT assay. When the cells were
treated with 25 μM resveratrol, 20 μM 5-FU or 25
μM resveratrol in combination with 20 μM 5-FU, the
viability of the cells was 51, 45 and 37%, respectively (Fig. 2A). Thus, co-treatment with
resveratrol and 5-FU inhibited cell growth more efficiently
compared with either resveratrol or 5-FU alone. To understand
whether resveratrol- and 5-FU-induced cell death are mediated by
apoptosis or necrosis, the apoptotic cell death was measured using
Annexin V/PI staining. As Fig. 1B
shows, 25 μM resveratrol and 20 μM 5-FU alone induced
apoptosis; however, in combination, apoptosis was induced more
efficiently. To determine the effects of co-treatment with
resveratrol and 5-FU on the expression levels of AMPK, COX-2, VASP
and VEGF, protein was extracted from the B16 cells treated with
resveratrol, 5-FU or a combination of the two. The results
demonstrated that phosphorylation of AMPK was increased to a
greater extent following co-treatment of resveratrol and 5-FU and
that the expression levels of COX-2, VASP and VEGF were decreased
more effectively by the co-treatment (Fig. 2C).
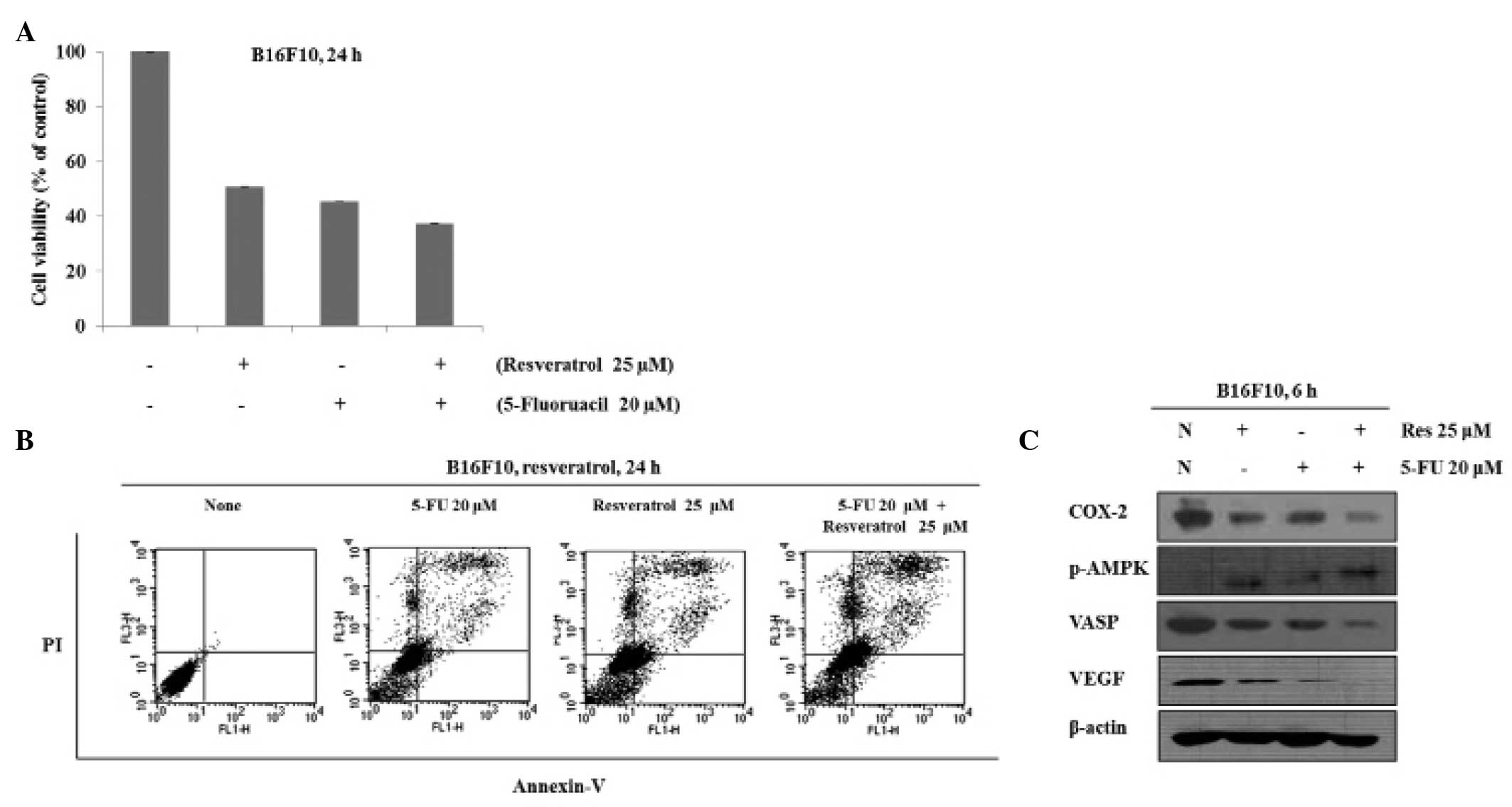 | Figure 2Combination treatment with Res and
5-FU suppresses cell proliferation and induces apoptosis. (A) Cells
were treated with 5-FU (20 μM), Res (25 μM) or
co-treated with Res and 5-FU for 24 h and cell viability was
measured using an MTT assay. (B) Cells were treated with 5-FU (20
μM), Res (25 μM) or co-treated with Res and 5-FU for
24 h. Apoptosis was measured by Annexin V-fluorescein
isothiocyanate + PI staining. (C) Cells were treated with Res (25
μM) and 5-FU (20 μM) or Res in combination with 5-FU
for 6 h. The expression levels of p-AMPK, COX-2, VEGF, VASP and
β-actin were examined by western blot analysis. Res, resveratrol;
FU, fluorouracil; p-AMPK, phosphorylated AMP-activated protein
kinase; COX-2, cyclooxygenase-2; VASP, vasodilator-stimulated
phosphoprotein; VEGF, vascular endothelial growth factor; N, none;
PI, propidium iodide. |
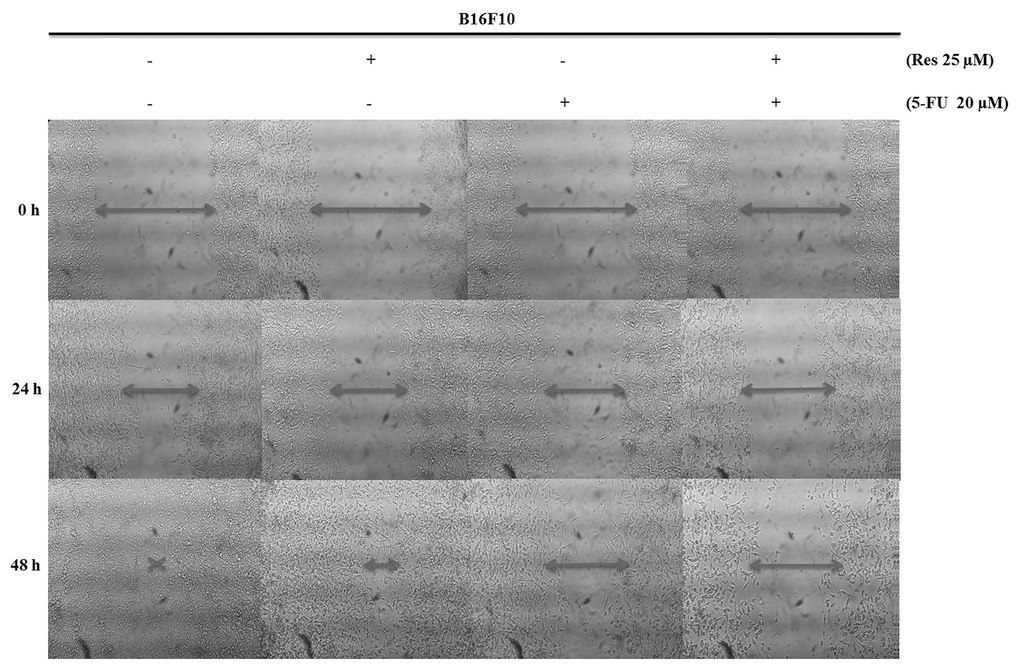
Resveratrol and 5-FU inhibit the
migration of B16 murine melanoma cells
The present study examined the effects of
resveratrol and 5-FU on the migration of B16 cells. The cells were
treated with 25 μM resveratrol, 20 μM 5-FU and 25
μM resveratrol in combination with 20 μM 5-FU and the
cell migration was measured using a wound healing assay. The
untreated B16 cells readily migrated to the wound, whereas cells
treated with resveratrol and 5-FU exhibited cell flattening and the
cell migration was inhibited in a dose- and time-dependent manner.
The migration of cells treated with a combination of resveratrol
and 5-FU was inhibited to a greater extent compared with either
drug alone (Fig. 3).
Growth inhibitory effects of combined
resveratrol and 5-FU treatment in vivo via regulation of AMPK, VASP
and VEGF
To investigate the effect of combined resveratrol
and 5-FU treatment in vivo, a B16 xenograft animal model was
established. A combination of resveratrol and 5-FU (10 mg/kg
resveratrol + 10 mg/kg 5-FU/day) was injected intraperitoneally
once a day for 10 days, starting one week after the initial
injection of B16 cells. Following treatment of the mice with a
combination of resveratrol and 5-FU for 10 days, tumor growth
decreased compared with the control group, although body weights
were unchanged (Fig. 4A). Protein
was extracted from the tumors and, as shown in Fig. 4B, the phosphorylation of AMPK was
increased by combined resveratrol and 5-FU treatment, while the
expression levels of VASP and VEGF were reduced.
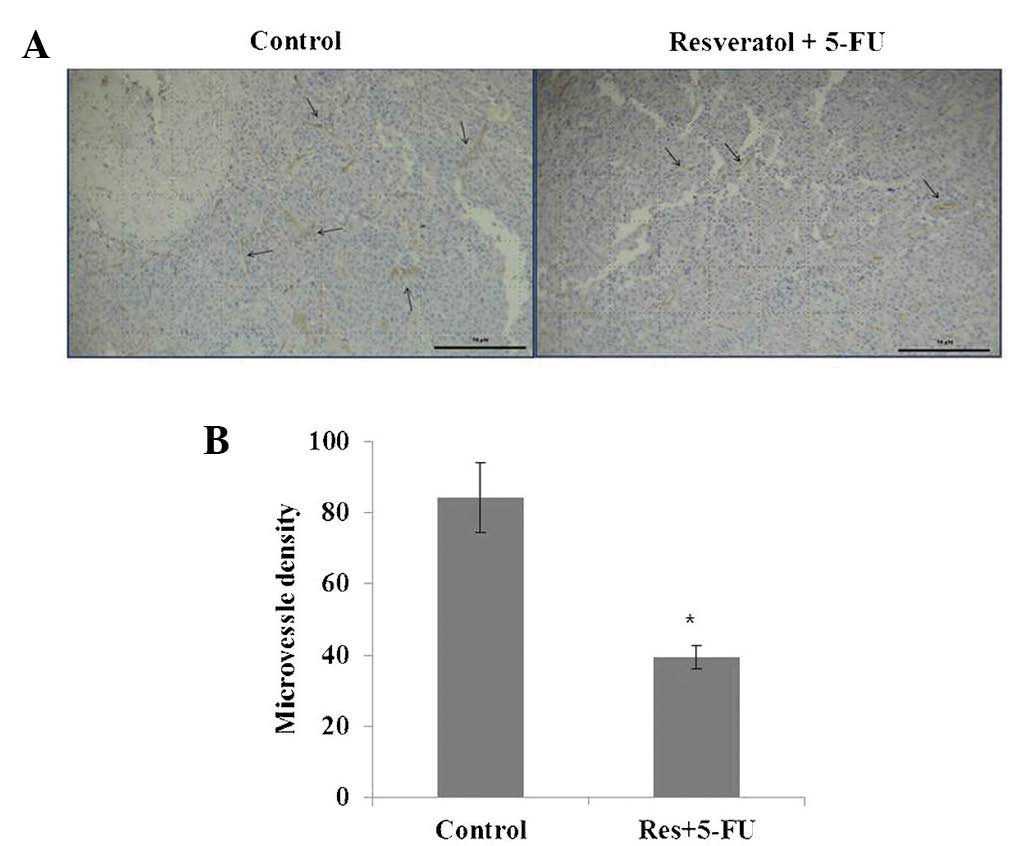
Decreased microvessel density in B16
tumors treated with a combination of resveratrol and 5-FU
To examine the effect of combined resveratrol and
5-FU treatment on angiogenesis in the present study, morphometric
analysis of immunohistochemical staining for CD31, a marker for
microvessel density, was performed. Images of the CD31 staining of
microvessels revealed that tumors treated with resveratrol and 5-FU
combined had fewer microvessels compared with those in the control
group (Fig. 5). These results
suggested that combined treatment with resveratrol and 5-FU
effectively suppressed angiogenesis.
Discussion
Angiogenesis, the growth of new vessels from the
pre-existing vascular network, is essential for solid tumor growth
and metastasis (10) and depends
on specific growth factors (2).
Therefore, the inhibition of growth factors is widely considered as
one of the most effective strategies for the efficient suppression
of tumor growth. A key gene involved in tumor growth is COX-2,
which is required for angiogenesis, cell migration and invasion and
is overexpressed in various cancer cells (12). A previous study demonstrated that
the COX-2-selective inhibitors, JTE-522 and NS-398, significantly
reduced tumor mass and vascular density (22). VEGF is another pro-angiogenic
factor, which can promote tumor progression, metastasis and cancer
cell dissemination (18). In
B16F10 melanoma, VEGF inhibition by anti-VEGF agents decreases
tumor growth and microvessel density (23). Another protein involved in
angiogenesis is VASP, which regulates cell-cell and cell-matrix
interactions by regulating actin filament networks and is
phosphorylated by AMPK (24).
5-FU is used to treat various cancer cells; however,
several types of tumor have developed resistance to it (8). Therefore, several studies have
evaluated novel treatments or 5-FU combination treatments to
overcome this drug resistance. Previous studies have demonstrated
that co-treatment of cells with genistein and 5-FU reduces the
proliferation of colon cancer cells more effectively compared with
genistein or 5-FU alone (4), and
the co-treatment of colon cancer cells with resveratrol and 5-FU
inhibited cell growth more effectively compared with resveratrol or
5-FU alone (25). In the present
study, the combination treatment of 5-FU and resveratrol was
investigated in order to develop modalities to overcome drug
resistance. Resveratrol, a natural polyphenol present in grapes and
red wine, has been observed to suppress the proliferation and
survival of cancer cells via several mechanisms, including the
inhibition of angiogenesis, induction of apoptosis and cell cycle
arrest (1,3,26).
The present study demonstrated the synergistic
effects of combined resveratrol and 5-FU treatment on B16 cell
proliferation and angiogenesis by regulating the expression levels
of COX-2, VASP and VEGF in the B16 cells. The combined resveratrol
and 5-FU treatment reduced B16 cell proliferation in a
dose-dependent manner. Therefore, resveratrol and 5-FU were
identified as compounds effectively inhibiting the growth of B16
cells.
The present study also determined whether the
resveratrol and 5-FU-induced inhibition of cell proliferation
involved changes in COX-2, VEGF, VASP and p-AMPK. Treatment with
resveratrol and 5-FU alone significantly reduced the levels of
COX-2, VEGF and VASP and increased AMPK. These results indicated
that downregulation of COX-2, VEGF and VASP and upregulation of
AMPK may have a significant role in the resveratrol- and
5-FU-induced inhibition of B16 cell proliferation.
To examine the effect of combined resveratrol and
5-FU treatment, B16 cells were treated with the two drugs either
alone or in combination. The combination of the two drugs inhibited
cell proliferation more effectively compared with either drug
alone. To further understand whether this inhibition of cell
proliferation was due to apoptosis, Annexin V/PI staining was
performed. Treatment with resveratrol or 5-FU alone induced
apoptosis in the B16 cells; however, the combination of the two was
more effective. These results indicated that the inhibition of cell
proliferation and the induction of apoptosis was greater when
resveratrol and 5-FU were used in combination rather than alone.
The present study also investigated whether a combination of
resveratrol and 5-FU regulated the expression levels of COX-2,
VEGF, VASP and p-AMPK. Resveratrol and 5-FU increased p-AMPK
activation and decreased the expression levels of COX-2, VEGF and
VASP and, again, this effect was increased when the drugs were used
in combination. These results indicated that treatment with a
combination of resveratrol and 5-FU had synergistic effects on the
induction of apoptosis and on regulation of the expression of
COX-2, VEGF, VASP and p-AMPK in the B16 cells.
To evaluate the effect of the combination of
resveratrol and 5-FU on cell migration in the present study, a
wound healing assay was used. Resveratrol and 5-FU in combination
effectively inhibited the migration of B16 cells, which was more
marked compared with the effect of either drug alone. In a previous
study, resveratrol was found to repress the migration and invasion
of LoVo cells by inhibiting the expression levels of VEGF and MMP-9
(27). Furthermore, glioma tissues
treated with resveratrol exhibited a reduction in angiogenesis
(28). These results indicated
that combination treatment with resveratrol and 5-FU suppressed
metastasis by inhibiting cell migration.
The present study investigated the synergistic
effect of resveratrol and 5-FU on angiogenesis in vitro and
demonstrated that the combination of resveratrol and 5-FU was more
effective than either drug alone in preventing cell proliferation,
migration and apoptosis. In a previous study, the use of
resveratrol and 5-FU in combination to treat liver tumors led to
more marked inhibition of tumor growth compared with that of either
drug alone (10). Furthermore, in
an in vivo model of cholangiocarcinoma, co-treatment with
resveratrol and 5-FU decreased tumor growth significantly more
compared with treatment with either drug alone and a terminal
deoxynucleotidyl transferase dUTP nick end labeling assay
demonstrated that this combination was also more effective in
inducing apoptosis (29). The
results of the present study demonstrated that resveratrol or 5-FU
alone decreased tumor growth; however, co-treatment with
resveratrol and 5-FU was even more effective. However, the
mechanisms underlying the enhanced antitumor activity of combined
resveratrol and 5-FU treatment remain to be fully elucidated.
Therefore, the present study also examined the synergistic effect
of combined resveratrol and 5-FU on the regulation of angiogenic
factors and B16 tumor growth in vivo.
To assess the effects of co-treatment with
resveratrol and 5-FU on B16 tumor growth, mice were treated with
resveratrol and 5-FU. Co-treatment with resveratrol and 5-FU
reduced tumor growth significantly compared with that in the
control group. Changes in the protein expression levels of p-AMPK,
VASP and VEGF were also examined and the expression levels of
p-AMPK increased, while the expression levels of VASP and VEGF
decreased in the mice treated with a combination of resveratrol and
5-FU compared with levels in the control group. In order to
evaluate the association between the resveratrol- and 5-FU-mediated
inhibition of tumor growth and angiogenesis, immunohistochemical
staining for CD31 was used, which specifically stains microvessels.
The staining revealed that co-treatment with resveratrol and 5-FU
reduced microvessel density compared with that in the control
group. These results suggested that the decrease in microvascular
vessels in tumor angiogenesis coincided with the decrease in tumor
size when using a combination of resveratrol and 5-FU.
In conclusion, the present study indicated that
resveratrol and 5-FU have antiproliferative and proapoptotic
effects in B16 cells, which are greater when the drugs are used in
combination. These effects were mediated, at least in part, by
downregulation of the expression levels of COX-2, VEGF and VASP and
the resultant inhibition of angiogenesis.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (no. KRF-2012-0021402).
References
|
1
|
Belguendouz L, Fremont L and Linard A:
Resveratrol inhibits metal ion-dependent and independent
peroxidation of porcine low-density lipoproteins. Biochem
Pharmacol. 53:1347–1355. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Emmett MS, Dewing D and Pritchard-Jones
RO: Angiogenesis and melanoma - from basic science to clinical
trials. Am J Cancer Res. 1:852–868. 2011.PubMed/NCBI
|
|
3
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesiss in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar
|
|
4
|
Hwang JT, Ha J and Park OJ: Combination of
5-fluorouracil and genistein induces apoptosis synergistically in
chemo-resistant cancer cells through the modulation of AMPK and
COX-2 signaling pathways. Biochem Biophys Res Commun. 332:433–440.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Z, Fang J, Xia C, Shi X and Jiang BH:
trans-3,4,5′-trihydroxystibene inhibits hypoxia-inducible factor
1alpha and vascular endothelial growth factor expression in human
ovarian cancer cells. Clin Cancer Res. 10:5253–5263. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura Y and Okuda H: Resveratrol isolated
from Polygonumcuspidatum root prevents tumor growth and metastasis
to lung and tumor-induced neovascularization in Lewis lung
carcinoma-bearing mice. J Nutr. 131:1844–1849. 2001.PubMed/NCBI
|
|
7
|
Bråkenhielm E, Cao R and Cao Y:
Suppression of angiogenesis, tumor growth, and wound healing by
resveratrol, a natural compound in red wine and grapes. FASEB J.
15:1798–1800. 2001.PubMed/NCBI
|
|
8
|
Borralho PM, Moreira da Silva IB, Aranha
MM, et al: Inhibition of Fas expression by RNAi modulates
5-fluorouracil-induced-apoptosis in HCT116 cells expressing
wild-type p53. Biochim Biophys Acta. 1772:40–47. 2007. View Article : Google Scholar
|
|
9
|
Ortiz R, Prados J, Melguizo C, et al:
5-Fluorouracil-loaded poly (ε-caprolactone) nanoparticles combined
with phage E gene therapy as a new strategy against colon cancer.
Int J Nanomedicine. 7:195–107. 2012.
|
|
10
|
Wu SL, Sun ZJ, Yu L, et al: Effect of
resveratrol and in combination with 5-FU on murine liver cancer.
World J Gastroenterol. 10:3048–3052. 2004.PubMed/NCBI
|
|
11
|
Subbaramaiah K and Dannenberg AJ:
Cyclooxygenase 2: a moleculartarget for cancer prevention and
treatment. Trends Pharmacol Sci. 24:96–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao X, Shi D, Liu L, et al: Quercetin
suppresses cyclooxygenase-2 expression and angiogenesis through
inactivation of P300 signaling. PLoS One. 6:e229342011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenhough A, Smartt HJ, Moore AE, et al:
The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and
adaptation to the tumour microenvironment. Carcinogenesis.
30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao L, Liu F, Hong L, et al: The function
and mechanism of COX-2 in angiogenesis of gastric cancer cells. J
Exp Clin Cancer Res. 30:2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zykova TA, Zhu F, Zhai X, et al:
Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol
Carcinog. 47:797–805. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HN, Kim H, Kong JM, et al: Vitamin C
down-regulatesVEGF production in B16F10 murine melanoma cells via
the suppression of p42/44 MAPK activation. J Cell Biochem.
112:894–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu G, Luo J, Rana JS, et al: Involvement
of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in
vascular endothelial cells. Cardiovasc Res. 69:512–519. 2006.
View Article : Google Scholar
|
|
18
|
Kumar B, Chile SA, Ray KB, et al: VEGF-C
differentially regulates VEGF-A expression in ocular and cancer
cells; promotes angiogenesis via RhoA mediated pathway.
Angiogenesis. 14:371–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KJ, Li B, Winer J, et al: Inhibition
of vascular endothelial growth factor-induced angiogenesis
suppresses tumour growth in vivo. Nature. 362:841–844. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Price CJ and Brindle NP:
Vasodilator-stimulated phosphoprotein is involved in stress-fiber
and membrane ruffle formation in endothelial cells. Arterioscler
Thromb Vasc Biol. 20:2051–2056. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Philippar U, Roussos ET, Oser M, et al: A
Mena invasion isoform potentiates EGF-induced carcinoma cell
invasion and metastasis. Dev Cell. 15:813–828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amano H, Hayashi I, Endo H, et al: Host
prostaglandin E(2)-EP3 signaling regulates tumor-associated
angiogenesis and tumor growth. J Exp Med. 197:221–232. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S and Maity P: Augmented antitumor
effects of combination therapy with VEGF antibody and cisplatin on
murine B16F10 melanoma cells. Int Immunopharmacol. 7:1598–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blume C, Benz PM, Walter U, et al:
AMP-activated protein kinase impairs endothelial actin cytoskeleton
assembly by phosphorylating vasodilator-stimulated phosphoprotein.
J Biol Chem. 282:4601–4612. 2007. View Article : Google Scholar
|
|
25
|
Colin D, Gimazane A, Lizard G, et al:
Effects of resveratrol analogs on cell cycle progression, cell
cycle associated proteins and 5fluoro-uracil sensitivity in human
derived colon cancer cells. Int J Cancer. 127:2780–2788. 2009.
View Article : Google Scholar
|
|
26
|
Zhang W, Fei Z, Zhen HN, Zhang JN and
Zhang X: Resveratrol inhibits cell growth and induces apoptosis of
rat C6 glioma cells. J Neurooncol. 81:231–240. 2007. View Article : Google Scholar
|
|
27
|
Wu H, Liang X, Fang Y, et al: Resveratrol
inhibit hypoxia-induced metastasis potential enhance ment by
restricting hypoxia-induced factor-1 alpha expression in colon
carcinoma cells. Biomed Pharmacother. 62:613–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JC, Chen Y, Lin JH, Wu JM and Tseng
SH: Resveratrol suppresses angiogenesis in gliomas: evaluation by
color Doppler ultrasound. Anticancer Res. 26:1237–1245.
2006.PubMed/NCBI
|
|
29
|
Frampton GA, Lazcano EA, Li H, Mohamad A
and DeMorrow S: Resveratrol enhances the sensitivity of
cholangiocarcinoma to chemotherapeutic agents. Lab Invest.
90:1325–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|