Introduction
The fetal membrane (FM) is a complex consisting of
the amnion and the chorion, constituting the fetal components and
the decidua, a maternal component. It surrounds the fetus and
amniotic fluid throughout the gestational period and is critical in
providing a protective screen for the fetus and in regulating the
amniotic fluid volume (1,2). Throughout pregnancy, the increasing
area of the FM, the development and maintenance of permeability and
tension of the FM are required to fulfill the demands for
increasing fetal weight, fetal size and amniotic fluid volume
(1). Evidence from human studies
demonstrated the presence of five aquaporins (AQPs; AQP1, 3, 4, 8
and 9) and water transport channels in epithelial cells of the
human FM. In addition, the intramembranous amniotic fluid
regulation is controlled by alterations in AQP expression, which is
associated with oligohydramnios and polyhydramnios (2). At present, it is accepted that
extracellular matrix (ECM) proteins and a fibrous mesh structure
from an assemblage of collagen fibers in the FM’s mesoderm are
major contributors to the permeability and the tension of the FM
mesoderm (3,4). The increased thickness and density of
connective tissues, which predominantly consists of collagen
components, have been implicated in the elevated tension of the FM
but also reduce the permeability of the FM (3). Thus, the two contradictory properties
of the FM were not completely explained by changes in thickness and
density of the FM connective tissue (3,4).
Fibroblasts and myofibroblasts (MFBB), two main cell
components in the FM mesoderm (5,6),
possess a potential for producing collagen and other ECM proteins
in connective tissues (6). In the
FM mesoderm cells, the fibroblasts are relatively quiescent under
normal conditions and function to maintain tissue homeostasis by
regulating the turnover of the ECM. MFBBs are a more active cell
type, producing more abundant collagen, other ECM proteins and
matrix metalloproteinases (MMPs) and are thus important in tissue
remodeling (5,7). In addition to producing ECM proteins,
the highly contractile properties of MFBBs have been noted
(7). Wang and Schneider (6) suggested that the MFBB in the human
amniotic membrane and the chorionic membrane may contribute to the
protection of the FM from overdistension. Previous studies have
elucidated that the differentiation and the increase in the numbers
of MFBB in the FM may implicate FM rupture and premature labor
(5).
α-smooth muscle actin (α-SMA), a cytoskeletal
protein component in MFBBs and smooth muscle cells, is perceived as
a potential mechanosensitive protein (8,9),
closely associated with the contraction and the stretch effects of
MFBBs and smooth muscle cells (8).
However, MFBBs are hypothesized to have a hybrid phenotype between
fibroblasts and smooth muscle cells. Therefore, it is difficult to
identify MFBBs among the stromal cells based upon cell phenotype.
Of note, a feature unique to free human chorioamniotic membranes is
the absence of fetal vessels and other tissues containing smooth
muscle cells during all stages of development (10). Thus, α-SMA serves as a specific
biomarker of MFBBs in FM mesoderm.
Phenotypic switching between fibroblasts and MFBBs
is a common event (11). MFBBs are
mainly derived from fibroblast differentiation under conditions of
mechanical microenvironmental change (12), tissue injury (13) and hypoxia (11). In addition, MFBBs may be derived
from macrophage transdifferentiation and epithelial-mesenchymal
transition (14,15). In addition, MFBBs may also
dedifferentiate into fibroblasts as apoptosis occurs (15).
With the advance of gestation, the FM goes through
stages of development and proliferation prior to reaching mature
stages which occur simultaneously with apoptosis. Pre-eclampsia is
a serious complication of gestation associated with placental
hypoxia (16). Oligohydramnios and
polyhydramnios are also gestational complications associated with
abnormal amniotic fluid volume. However, the numbers and the
distribution of MFBBs and α-SMA expression in the FM in a normal
pregnancy at differing weeks of gestation, in full-term pregnancy
with variable amniotic fluid indexes (oligohydramnios, normal
amniotic fluid volume and polyhydramnios) and in the two subtypes
of severe pre-eclampsia, early onset severe pre-eclampsia (EOSP)
and late-onset severe pre-eclampsia (LOSP) are largely unknown.
The present study assessed differences in the
quantities and distribution of MFBBs and the expression levels of
α-SMA in the FM in normal pregnant females at different weeks of
gestation. These differences were also assessed in patients with
oligohydramnios and polyhydramnios as well as EOSP and LOSP. The
present study also investigated the transformation of chorionic
trophoblasts into MFBBs. The findings of the present study provided
novel insight for improving the understanding of the mechanisms of
FM development under the physiological conditions of normal
gestation and in cases of gestational complication.
Materials and methods
Research subjects and specimen
collection
The present study was approved by the ethics
committee of Jilin University Bethune Second Hospital (Changchun,
China), and performed with informed maternal consent. Samples were
obtained from the Department of Obstetrics, Jilin University
Bethune Second Hospital. A total of 79 pregnant females
hospitalized for delivery between January 2010 and March 2012 were
selected as research subjects. Normal pregnancies (n=30, 16–40
weeks) at different gestational weeks were divided into four groups
according to gestational week: Early (≥16 – <22 weeks, n=7),
early/mid (≥22 – <28 weeks, n=7), mid/late (≥28 – <34 weeks,
n=8) and late (≥34 – ≤40 weeks, n=8), respectively. Full-term
pregnancies (37–40 weeks, n=30) were divided into three groups of
10 according to amniotic-fluid index: Oligohydramnios, (amniotic
fluid indexes <8); normal (amniotic fluid indexes 8–18);
polyhydramnios, (amniotic fluid indexes >18), including six
cases of the previously mentioned full term pregnancies and four
cases of other full-term pregnancies. Based on onset time of
pre-eclampsia, patients (n=25) with severe pre-eclampsia (SP;
diastolic blood pressures ≥110 mmHg and/or systolic blood pressures
≥160 mmHg on at least three occasions, >5 g protein per 24 in
urine) were designated to the EOSP group (n=13, ≥20 – <34 weeks)
with an earlier onset of morbidity (≥14 days) and LOSP (n=12; ≥34 –
≤40 weeks) with a later onset of morbidity (<5 days). Subjects
had no other obstetric or gynecological complications. No
statistical differences were identified in the ages of the
participants (P>0.05).
Following delivery of the placentas, three sections
of FM (3.0 cm × 3.0 cm) in the central and outer regions and were
immediately biopsied under aseptic conditions. Simultaneously,
decidual membranes from half of each section of FM were removed and
subsequently stored at −80°C prior to reverse transcription
polymerase chain reaction (RT-PCR) and western blot analysis.
Additional sections were fixed with 4% formaldehyde for 24 h,
embedded in paraffin and cut into 3-μm sections, dried at
65°C for 7.5 h and subsequently stored at room temperature prior to
immunohistochemical analysis.
Immunohistochemistry
The primary antibody, (α-SMA mouse monoclonal, cat.
no. ZM-0003), secondary antibody kit [poly-horseradish peroxidase
anti-mouse/rabbit immunoglobulin (Ig)G, PV-9000 2-step plus] and
3,3′-diami-nobenzidine (DAB) kit were purchased from Zhongshan
Goldenbridge Biotechnology Co., Ltd. (Beijing, China). Standard
procedures were followed on the 3-μm sections. Antigen
retrieval was performed for 20 min at moderate temperature
(93–97°C) in a microwave. In order to quench the activity of
endogenous peroxidase, slides were placed in 3% hydrogen peroxide
at room temperature for 10 min. The slides were subsequently
incubated with primary antibody (1/200) for 60 min at 37°C in a
humidified chamber. Following this, secondary antibody was added to
the slides for 50 min at 37°C. Subsequently, the DAB kit was used
to detect the staining of α-SMA in the FM sections. The primary
antibody was replaced with phosphate-buffered saline as a negative
control. Placental vessels were used as a positive control. Slides
were assessed by two independent pathologists in 10 random fields
of vision for each slide (BX51; Olympus Corporation, Tokyo,
Japan).
RT-PCR
Total RNA was extracted from the fresh FM samples
with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) and cDNA was reverse transcribed using a Superscript
First-strand Synthesis System (Invitrogen Life Technologies). The
cDNA was used as a template for PCR amplification using the
following primers: α-SMA forward, 5′-GCGTGGCTATTCCTTCGTTAC-3′ and
reverse, 5′-CATAGTGGTGCCCCCTGATAG-3′ (331 bp); and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGT-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′
(226 bp). The primers were designed using Primer Premier software,
version 5.0 (Premier Biosoft, Palo Alto, CA, USA), and were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). GAPDH
was used as an internal control. The amplicon size was 331 base
pairs. The PCR products were analyzed using Image-Pro Plus 6.0
software (National Institutes of Health, Bethesda, MD, USA).
Immunoblotting
The FM specimens were homogenized in lysis buffer
(50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% triton X-100, 0.5% sodium
deoxycholate, 1% NP-40 and 1% SDS) on ice and were centrifuged at
13,800 x g for 8 min at 40°C. The protein was separated by 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF)
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following
blocking with 5% non-fat milk (w/v) and washing with 0.1% Tween 20
Tris-buffered saline, the PVDF membrane was incubated with primary
α-SMA mouse monoclonal antibody (1:1,000; ZM-0003) overnight at
4°C, washed and subsequently incubated with peroxidase conjugated
affinipure goat anti-mouse IgG (1:1,000; ZB-2301; Zhongshan
Goldenbridge Biotechnology Co, Ltd.) and detected using an enhanced
chemiluminescence system (cat. no. WBKLS0100; EMD Millipore,
Billerica, MA, USA).
Statistical analysis
The database was established using Excel 2003
(Microsoft, Redmond, WA, USA) and SPSS 17.0 (SPSS, Inc., Chicago,
IL, USA) statistical software. The data met the criteria of a
normal distribution and were presented as the mean ± standard
deviation. Comparisons between groups were performed using one-way
analysis of variance. A χ2 test was used to establish
whether the prevalence among the groups was significantly
different. P<0.05 was considered to indicate a statistically
significant difference.
Results
Distribution of MFBBs and the thickness
of mesoderm in FMs
In the present study, it was reported for the first
time, to the best of our knowledge, that the majority of MFBBs in
FM mesoderm were interweaved with each other in pregnant females
with and without gestational complications. MFBBs and fibroblasts
were generally distributed parallel to the FM (Fig. 1A a-1-4, Fig. 2A a-1-3 and Fig. 3A a-1-4). Immunohistochemical
results demonstrated that MFBBs were mainly distributed in
chorioamniotic mesoderm at 16–21 weeks and in chorionic mesoderm at
22–40 weeks. Furthermore, it was observed that the fibroblasts were
predominantly positioned in the amniotic mesoderm at 22–40 weeks
(Fig. 1A a-1-4). Of note, MFBBs
stained with antibodies against α-SMA were noted among chorionic
epithelial cells with and without gestational complications
(Fig. 1A a-1-4, Fig. 2A a-1-3 and Fig. 3A a-1-4). No significant differences
(P=0.34; P=0.53; P=0.60) were identified in the thicknesses of the
FM mesoderm in normal pregnancies at different gestational weeks,
in full-term pregnancies with different amniotic fluid indexes and
in females with SP irrespective of onset and morbidity time
(Tables I–III).
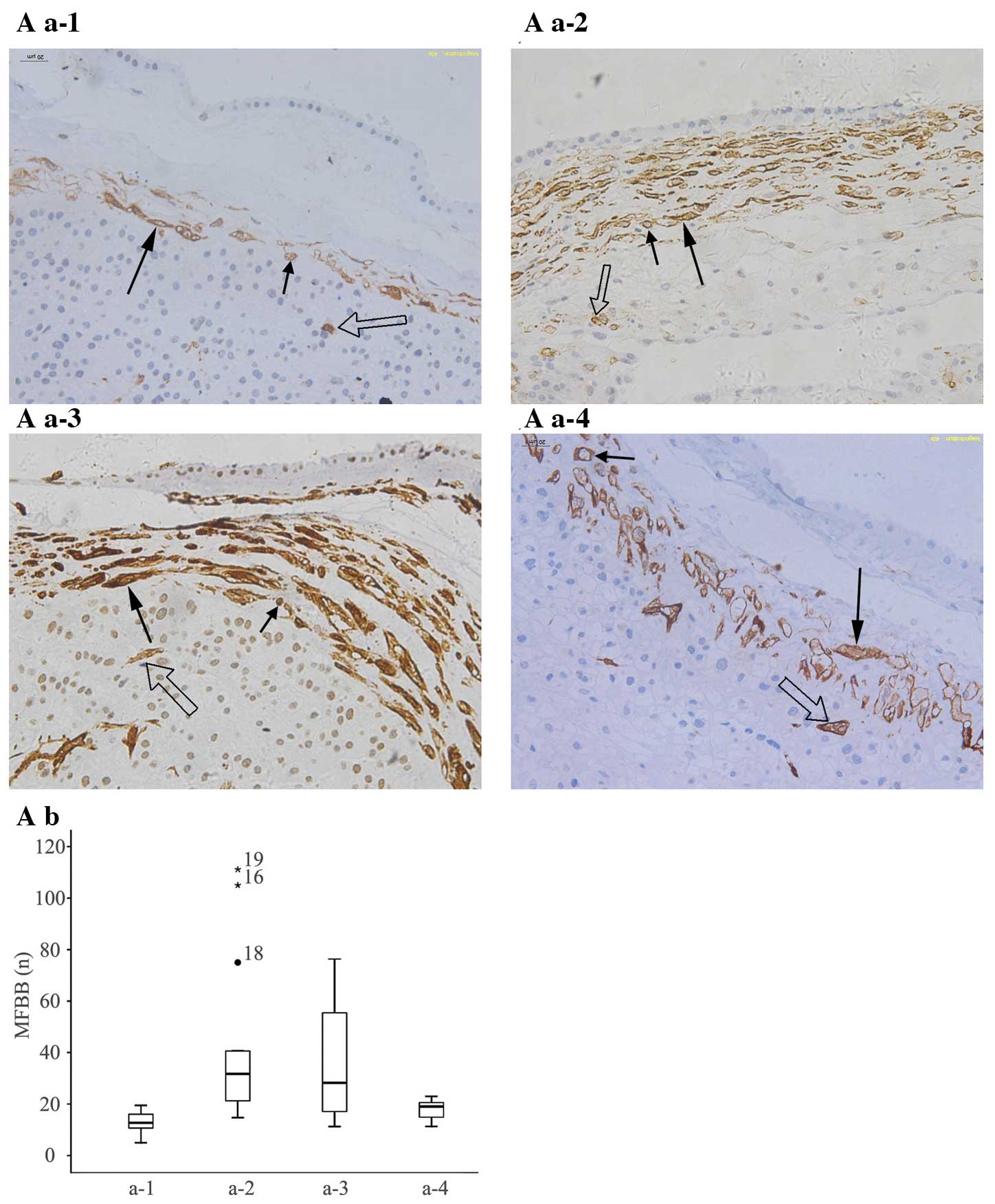
 | Figure 1Number and distribution of MFBBs and
α-SMA expression levels in FMs at different gestational weeks. (A
a-1) Early: ≥16 weeks – <22 weeks. (A a-2) Early/mid: ≥22 weeks
– <28 weeks. (A a-3) Mid/late: ≥28 weeks – <34 weeks. (A a-4)
Late: ≥34 weeks – ≤40 weeks. (Magnification, ×200). Long arrows
indicate MFBBs with vertical section; short arrows indicate MFBBs
with transverse section; hollow arrows indicate MFBBs in chorionic
epithelia. The majority of MFBBs in FM mesoderm was distributed
parallel to FM and interweaved with each other and separated by the
ECM. Fibroblasts were mostly positioned in the amniotic mesoderm at
22 weeks – 40 weeks. There were differences among the MFBB numbers
in the four groups, but they were not statistically significant
(P=0.063). (A b, A c) The numbers of MFBBs were descending along
with elevation of gestational weeks. (B a, C a) Expression of α-SMA
mRNA was detected at 331 bp as a single band, GAPDH served as
internal control and the expression of α-SMA mRNA and α-SMA protein
became weaker with advance of gestation weeks. (B b, C b) α-SMA
mRNA band grey values/GAPDH mRNA band grey values and α-SMA protein
band grey values/GAPDH protein band grey values were decreasing
with advance of gestation weeks. FM, fetal membrane; MFBB,
myofibroblast; SP, severe pre-eclampsia; EOSP, early onset SP;
LOSP, late onset SP; α-SMA, α-smooth muscle actin; ECM,
extracellular matrix. |
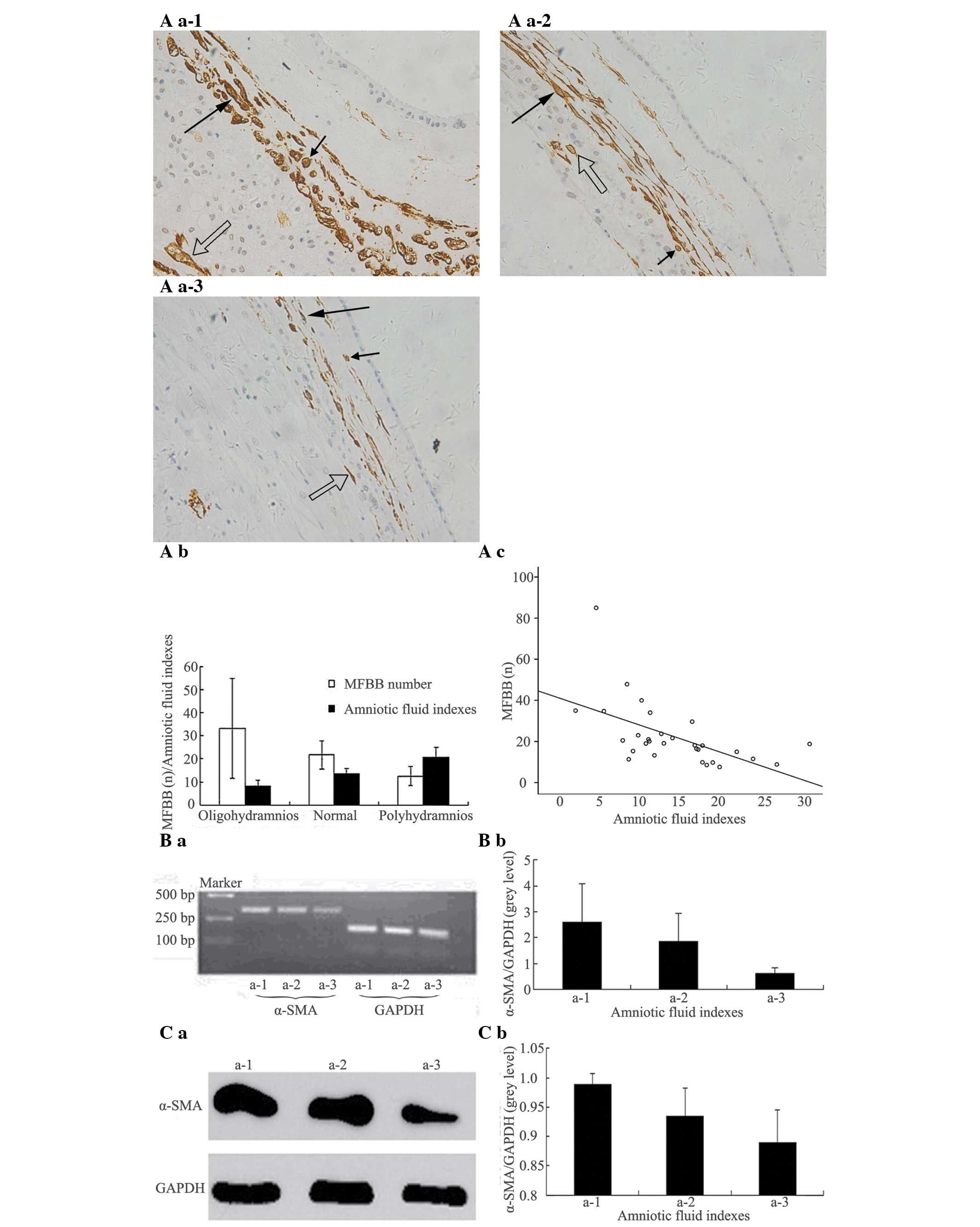 | Figure 2Number and distribution of MFBBs and
α-SMA expression levels in full term FMs with different amniotic
fluid indexes. (A a-1) Oligohydramnios, amniotic fluid indexes
<8. (A a-2) Normal, amniotic fluid indexes 8–18. (A a-3)
Polyhydramnios, amniotic fluid indexes >18. Long arrows indicate
MFBBs with vertical section; short arrows indicate MFBBs with
transverse section; hollow arrows indicate MFBBs in chorionic
epithelia. The majority of MFBBs in FM mesoderm was distributed
parallel to FM and interweaved with each other and separated by the
ECM. There were significant differences (P=0.001) among the MFBB
numbers in the three groups (magnification, ×200). (A b, A c) The
number of MFBBs was descending along with increase of amniotic
fluid indexes. (B a, C a) Expression of α-SMA mRNA and α-SMA
protein became weaker with increase of amniotic-fluid indexes. (B
b, C b) α-SMA mRNA band grey values/GAPDH mRNA band grey values and
α-SMA protein band grey values/GAPDH protein band grey values were
decreasing with advance of gestation weeks. FM, fetal membrane;
MFBB: myofibroblast; SP, severe pre-eclampsia; EOSP: early onset
SP; LOSP: late onset SP; α-SMA, α-smooth muscle actin; ECM,
extracellular matrix. |
 | Figure 3Number of FM-mesoderm MFBBs in EOSP
and LOSP. (A a-1) EOSP. (A a-2) EOSP control. (A a-3) LOSP. (A a-4)
LOSP control. (Magnification, ×200). Long arrows indicate MFBBs
with vertical section; short arrows indicate MFBBs with transverse
section; hollow arrows indicate MFBBs in chorionic epithelia. The
MFBBs were distributed parallel to FM and interweaved with each
other and separated by the ECM. A significant decrease (P<0.001;
P=0.004) of MFBB numbers was observed in EOSP compared to EOSP
controls and LOSP, whereas a significant increase (P=0.015) in MFBB
numbers was identified in LOSP compared to LOSP controls. (A b)
Comparison among the numbers of MFBBs in EOSP, EOSP control, LOSP
and LOSP control. FM, fetal membrane; MFBB, myofibroblast; SP,
severe pre-eclampsia; EOSP, early onset SP; LOSP, late onset SP;
α-SMA, α-smooth muscle actin; SP, severe pre-eclampsia; EOSP, early
onset SP; LOSP, late onset SP. |
 | Table IQuantities of MFBBs in the FM and the
thickness of FM mesoderm in normal pregnancies at different
gestational weeks. |
Table I
Quantities of MFBBs in the FM and the
thickness of FM mesoderm in normal pregnancies at different
gestational weeks.
| Group (n) | Age (years) | Gestation time
(weeks) | MFBBs (n) | Thickness (cm) |
|---|
| Early (7) | 29.7±4.9 | 18.2±2.3 | 55.1±41.0 | 2.8±1.5 |
| Early/mid (7) | 28.0±5.9 | 24.2±2.4 | 27.6±8.7 | 2.1±0.6 |
| Mid/late (8) | 32.0±3.4 | 30.3±4.6 | 21.2±7.6 | 2.5±1.5 |
| Late (8) | 28.0±3.6 | 38.0±1.8 | 18.4±3.6 | 2.9±1.4 |
 | Table IIIQuantities of MFBBs in the FM and the
thickness of FM mesoderm in females with SP. |
Table III
Quantities of MFBBs in the FM and the
thickness of FM mesoderm in females with SP.
| Group (n) | Age (years) | Gestation time
(weeks) | MFBBs (n) | Thickness (cm) |
|---|
| EOSP (13) | 27.2±2.7 | 26.8±4.2 | 12.9±4.0 | 2.3±0.7 |
| EOSP Control
(13) | 28.8±2.1 | 25.7±3.5 | 42.6±32.8 | 2.8±1.1 |
| LOSP (12) | 28.3±3.2 | 37.9±1.8 | 37.3±23.5 | 2.4±0.3 |
| LOSP Control
(12) | 29.2±4.0 | 37.8±1.6 | 17.8±23.5 | 2.4±0.8 |
Quantity of MFBBs and the expression
levels of α-SMA in the FM
In the present study, although notable differences
in the quantities of MFBBs were identified in 10 random visual
fields in the same FM slides with and without gestational
complications, correlations of MFBB-quantity changes were observed
in the FM. In normal pregnancies at different gestational weeks
(early, ≥16 weeks – <22 weeks; early/mid, ≥22 weeks – <28
weeks; mid/late, ≥28 weeks – <34 weeks; late, ≥34 weeks – ≤40
weeks), the quantity of MFBBs in FM mesoderm decreased with
advancement of gestation and were negatively correlated with
gestational weeks (r=−0.47, P=0.008; Fig. 1A a-1-4, A b and A c). Variable
quantities of MFBBs were present; however, differences were not
statistically significant (P=0.063) in the four groups at different
gestational weeks (Fig. 1A a-1-4
and Table I). The results
demonstrated that with the increase in the number of gestational
weeks, the expression levels of α-SMA mRNA in the FM were decreased
in normal pregnancies (Fig. 1B a and B
b). Similarly to the expression levels of α-SMA mRNA in FM, the
expression levels of α-SMA protein in FM also significantly and
gradually decreased with progression of gestational weeks in normal
pregnancies (Fig. 1C a and C
b).
In full-term pregnancies with different
amniotic-fluid indexes (oligohydramnios, amniotic-fluid indexes
<8; normal amniotic-fluid indexes, amniotic-fluid indexes 8–18;
polyhydramnios, amniotic-fluid indexes >18), the quantity of
MFBBs in FM mesoderm decreased (r=−0.66, P<0.001) with elevation
of amniotic fluid indexes. Significant differences (P=0.001) were
identified in the cell quantities of MFBBs in the three groups with
different amniotic-fluid indexes (Fig.
2A a-1-4, A b and A c; Table
II). A decrease in the expression levels of α-SMA mRNA and
α-SMA protein in the FM along with elevation of amniotic-fluid
indexes was observed in the three groups (Fig. 2B a and B b; Fig. 2C a and C b).
 | Table IIQuantity of MFBBs in the FM and the
thickness of FM mesoderm in full term pregnancies with different
amniotic fluid indexes. |
Table II
Quantity of MFBBs in the FM and the
thickness of FM mesoderm in full term pregnancies with different
amniotic fluid indexes.
| Group (n) | Age (years) | Gestation time
(weeks) | Amniotic-fluid
indexes | MFBBs (n) | Thickness (cm) |
|---|
| Oligohydramnios
(10) | 29.5±4.5 | 39.0±0.8 | 6.0±1.4 | 33.1±21.6 | 2.8±2.3 |
| Normal (10) | 29.2±5.6 | 39.0±1.1 | 13.5±2.2 | 21.3±5.3 | 2.8±1.7 |
| Polyhydramnios
(10) | 28.1±3.4 | 39.0±0.3 | 21.7±3.6 | 12.4±4.0 | 2.7±1.5 |
Cell quantities of MFBBs in FM mesoderm were
decreased in patients with EOSP compared with those in normal
controls at matched gestational weeks (P<0.001) and those in
LOSP patients. By contrast, LOSP patients exhibited an increase
(P=0.004) in MFBB cell quantities in the FM mesoderm in comparison
to those in normal controls at matched gestational stages (P=0.015;
Fig. 3A a-1-4 and A b; Table III).
Discussion
The present study revealed that MFBBs, which are
capable of generating collagen protein, other ECM proteins
(8) and MMPs (9,17)
are the principal cell components of the FM mesoderm (amniotic
mesoderm and chorionic mesoderm) at 16–22 weeks of gestation. These
findings suggested that MFBBs are critical in regulating the
increased rate of development of FM mesoderm at the earlier phases
of gestation. Between 22–40 weeks, the later and slower
developmental phases of FM mesoderm, the relatively quiescent
fibroblasts were the principal cell components in amniotic
mesoderm. This indicated that fibroblasts are the main contributors
in regulating the basic turnover of the ECM and the tensile force
of the amniotic mesoderm, functioning to maintain amniotic
homeostasis. Simultaneously, the more active MFBBs were present in
chorionic mesoderm, suggesting that the increase in permeability
and tensile force of the FM mesoderm may be associated with the
functions of MFBBs in the chorionic mesoderm. In addition, no
significant differences were identified in the thickness of the FM
mesoderm in normal pregnancies at different gestational stages,
between the patients with different amniotic fluid indexes and the
patients with EOSP and LOSP. Thus, the slight alterations in FM
mesoderm thickness may not be an important factor in the changes in
permeability and tensile force of the FM along with gestational
progression. As the cell quantities of MFBBs in the FM mesoderm and
the expression levels of α-SMA mRNA and α-SMA protein in the FM
decreased with progressive gestational weeks, it was hypothesized
that the changes in MFBB quantities and α-SMA expression levels
were significant factors in regulating the permeability and the
tensile force of the FM mesoderm. Fibroblasts and MFBBs, the latter
in particular, are considered to be capable of producing
actinmyosin interaction-mediated cell traction forces (CTFs)
(18,19), which are relatively slow, sustained
and non-reversible compared with the Ca2+-regulated
rapid and reversible contraction of smooth muscle cells (20,21).
The formation process of CTFs includes the following route:
Intracellular tension by fibroblasts and MFBBs is generated through
adenosine-triphosphate-powered sliding of actin-myosin filaments
and transfer of this tension to the ECM via local adhesion to both
ends of the stress fiber (22,23).
In addition to producing interaction-mediated cell traction, MFBBs
also generate contractile forces through contraction of α-SMA, a
type of cytoskeletal protein of MFBBs that is a potential
mechanosensitive protein (8,9) and
involved in the contraction of collagen protein and other ECM
proteins (24,25). The present study also demonstrated
that the MFBBs and the fibroblasts in the FM mesoderm were
distributed parallel to the FM and the MFBBs were interweaved with
each other. The distribution characteristics of MFBBs and
fibroblasts in the FM mesoderm may be beneficial to tensile force,
intracellular tension and contraction. These observations may
partially explain the direct or indirect roles of MFBBs in
regulating the permeability, interaction-mediated cell traction and
contraction of FM mesoderm.
Previous studies demonstrated that no AQP and no
water transport channels are present in FM mesoderm, while FM
permeability has been established to increase with gestational
progression as well as the tensile force of the FM. However, the
permeability and the tensile force of FM, a pair of conflicting FM
properties, have been hypothesized to be dependent on the turnover
of collagen protein and ECM proteins. The present study
demonstrated that in full-term pregnancies, no significant changes
were present in the thickness of FM mesoderm accompanied by
polyhydramnios, oligoamnios and normal volume amniotic fluid.
Finally, these findings endorsed the basic function of MFBBs and
fibroblasts in the maintenance of connective tissue thickness of FM
mesoderm in order to co-ordinate these conflicting FM properties.
Simultaneously, the numbers of MFBBs in FM mesoderm and the
expression trends of α-SMA mRNA and α-SMA protein in the FM were
significantly and negatively correlated (r=−0.66, P<0.001) with
amniotic fluid indexes. The greater quantities of MFBBs and the
higher expression levels of α-SMA mRNA and α-SMA protein were
present in the FM accompanied by oligoamnios, whereas the FM
accompanied by polyhydramnios was characterized by a lesser
quantity of MFBBs and lower expression levels of α-SMA mRNA and
protein. These results indicated that the quantity differences and
distribution states of MFBBs in FM mesoderm and the expression
levels of α-SMA in the FM may be important in affecting the
permeability of FM mesoderm, inducing changes to amniotic fluid
volume. There are a number of conflicting studies regarding the
effect of hypoxia on MFBB cell numbers and α-SMA expression levels.
Rogers et al (26)
demonstrated that the structure of intercellular actin, a
cytoskeletal protein, is disrupted under hypoxic conditions. A
study by Modarressi et al (27) revealed that hypoxia impairs the
differentiation and function of skin MFBB, elucidated through
quantifying α-SMA expression and cell contraction in collagen gels
and on wrinkling silicone substrates. Misra et al (11) reported that hypoxia induces a
phenotypic switch of fibroblasts to MFBBs via an MMP-2/tissue
inhibitors of metalloproteinase-mediated pathway. Pre-eclampsia is
a common and serious complication of gestation involving hypoxia.
Based on the onset time of pre-eclampsia, it may be grouped as
early-onset pre-eclampsia (onset time, <34 weeks) and late-onset
pre-eclampsia (onset time, >34 weeks). Depending on the severity
of pre-eclampsia, the disorder is also classified into mild
pre-eclampsia and severe pre-eclampsia (28). Generally, early onset pre-eclampsia
is the more severe subtype of pre-eclampsia. Emerging evidence from
animal studies, including, human trials, has implicated that
placental ischemia and hypoxia may be a central causative factor in
the onset and development of pre-eclampsia (29). Of note, in the present study,
significant differences were noted among the MFBB numbers in FM
mesoderm between EOSP and EOSP controls with matched gestational
stages and between LOSP and LOSP controls with matched gestational
stages as well as between EOSP and LOSP. The quantity of MFBBs in
FM mesoderm from EOSP patients with a longer time period of
morbidity was significantly lower than that in EOSP controls and
LOSP patients with a shorter time period of morbidity. Furthermore,
the number of MFBBs in FM mesoderm from LOSP patients was
significantly higher than that in the LOSP controls. The results
indicated that a different onset time and different duration of
hypoxia may lead to the variable effects of MFBBs on proliferation
or apoptosis and the expression levels of α-SMA in MFBBs.
Chorionic trophoblasts are considered to be
well-differentiated epithelial cells; however, their function
remains to be elucidated, while it is likely that they act as a
protective barrier. In the present study, it was noted that there
were a number of MFBBs labeled with α-SMA antibody in the
trophoblast layers of chorionic membranes from normal pregnancies
and those with gestational complications. This was consistent with
previous studies (14,15), suggesting that certain epithelial
cells may differentiate into mesenchymal cells. The epithelial
cells of certain types of cancer can transform into mesenchymal
cells of the cancer tissue and human retinal pigment epithelial
cells may transform into cells similar to MFBBs (30). The results of the present study
suggested that chorionic trophoblasts have the potential to
differentiate into MFBBs.
In conclusion, the present study revealed that the
differences in quantity and the distribution states of MFBBs in FM
mesoderm as well as the α-SMA expression levels in the FM may be
the main contributors to the permeability, tensile force and
intracellular tension of the FM by affecting the turnover of
collagen protein and other ECM proteins and also the contractility,
proliferation and apoptosis of MFBBs. In addition, the onset time
and persistence of hypoxia in MFBBs may induce differential impacts
on MFBB numbers and α-SMA expression levels. Ultimately, chorionic
trophoblasts may have the potential to differentiate into
MFBBs.
Acknowledgments
The authors would like to thank Professors Hongwen
Gao and Mei Sun (Department of Pathology, Jilin University Bethune
Second Hospital, Changchun, China) for helpful discussions during
the study of histology and immunohistochemistry in the placental
and fetal membrane samples. This study was supported by the Science
and Technology Department of Jilin Province, China (grant no.
20090464) and the Science and Technology Agency of Changchun, China
(grant no. 08SF44).
References
|
1
|
Minh HN, Douvin D, Smadja A and Orcel L:
Fetal membrane morphology and circulation of the liquor amnii. Eur
J Obstet Gynecol Reprod Biol. 10:213–223. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sha XY, Xiong ZF, Liu HS, Di XD and Ma TH:
Maternal-fetal fluid balance and aquaporins: from molecule to
physiology. Acta Pharmacol Sin. 32:716–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujisato T, Tomihata K, Tabata Y, Iwamoto
Y, Burczak K and Ikada Y: Cross-linking of amniotic membranes. J
Biomater Sci Polym Ed. 10:1171–1181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jabareen M, Mallik AS, Bilic G, Zisch AH
and Mazza E: Relation between mechanical properties and
microstructure of human fetal membranes: an attempt towards a
quantitative analysis. Eur J Obstet Gynecol Reprod Biol. 144(Suppl
1): S134–S141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McParland PC, Taylor DJ and Bell SC:
Myofibroblast differentiation in the connective tissues of the
amnion and chorion of term human fetal membranes-implications for
fetal membrane rupture and labour. Placenta. 21:44–53. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T and Schneider J: Fine structure of
human chorionic membrane. Ultrastructural and histochemical
examinations. Arch Gynecol. 233:187–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruidenier L, MacDonald TT, Collins JE,
Pender SL and Sanderson IR: Myofibroblast matrix metalloproteinases
activate the neutrophil chemoattractant CXCL7 from intestinal
epithelial cells. Gastroenterology. 130:127–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li B and Wang JH: Fibroblasts and
myofibroblasts in wound healing: force generation and measurement.
J Tissue Viability. 20:108–120. 2011. View Article : Google Scholar
|
|
9
|
Powell DW, Mifflin RC, Valentich JD, Crowe
SE, Saada JI and West AB: Myofibroblasts. I Paracrine cells
important in health and disease. Am J Physiol. 277:C1–C9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoyes AD: Ultrastructure of the
mesenchymal layers of the human chorion laeve. J Anat. 109:17–30.
1971.PubMed/NCBI
|
|
11
|
Misra S, Fu AA, Misra KD, Shergill UM,
Leof EB and Mukhopadhyay D: Hypoxia-induced phenotypic switch of
fibroblasts to myofibroblasts through a matrix metalloproteinase
2/tissue inhibitor of metalloproteinase-mediated pathway:
implications for venous neointimal hyperplasia in hemodialysis
access. J Vasc Interv Radiol. 21:896–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szczodry M, Zhang J, Lim C, Davitt HL,
Yeager T, Fu FH and Wang JH: Treadmill running exercise results in
the presence of numerous myofibroblasts in mouse patellar tendons.
J Orthop Res. 27:1373–1378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross R, Everett NB and Tyler R: Wound
healing and collagen formation. VI The origin of the wound
fibroblast studied in parabiosis. J Cell Biol. 44:645–654. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J and Liu Y: Dissection of key events
in tubular epithelial to myofibroblast transition and its
implications in renal interstitial fibrosis. Am J Pathol.
159:1465–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carlson MA, Longaker MT and Thompson JS:
Wound splinting regulates granulation tissue survival. J Surg Res.
110:304–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sankaralingam S, Arenas IA, Lalu MM and
Davidge ST: Preeclampsia: current understanding of the molecular
basis of vascular dysfunction. Expert Rev Mol Med. 8:1–20. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: one
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanger JW, Sanger JM and Jockusch BM:
Differences in the stress fibers between fibroblasts and epithelial
cells. J Cell Biol. 96:961–969. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hinz B and Gabbiani G: Mechanisms of force
generation and transmission by myofibroblasts. Curr Opin
Biotechnol. 14:538–546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katoh K, Kano Y, Amano M, Onishi H,
Kaibuchi K and Fujiwara K: Rhokinase – mediated contraction of
isolated stress fibers. J Cell Biol. 153:569–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balaban NQ, Schwarz US, Riveline D,
Goichberg P, Tzur G, Sabanay I, et al: Force and focal adhesion
assembly: a close relationship studied using elastic micropatterned
substrates. Nat Cell Biol. 3:466–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Beurden HE, Von den Hoff JW, Torensma
R, Maltha JC and Kuijpers-Jagtman AM: Myofibroblasts in palatal
wound healing: prospects for the reduction of wound contraction
after cleft palate repair. J Dent Res. 84:871–880. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bogatkevich GS, Tourkina E, Abrams CS,
Harley RA, Silver RM and Ludwicka-Bradley A: Contractile activity
and smooth muscle alpha-actin organization in thrombin-induced
human lung myofibroblasts. Am J Physiol. 285:L334–L343. 2003.
|
|
25
|
Liu T and Hu XD: Transdifferentiation of
fibroblasts into myofibroblasts in the skin lesion of systemic
sclerosis: role of transforming growth factor beta1 and its signal
transduction. Nan Fang Yi Ke Da Xue Xue Bao. 31:1840–1845. 2011.In
Chinese. PubMed/NCBI
|
|
26
|
Rogers KR, Morris CJ and Blake DR: The
cytoskeleton and its importance as a mediator of inflammation. Ann
Rheum Dis. 51:565–571. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Modarressi A, Pietramaggiori G, Godbout C,
Vigato E, Pittet B and Hinz B: Hypoxia impairs skin myofibroblast
differentiation and function. J Invest Dermatol. 130:2818–2827.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
von Dadelszen P, Magee LA and Roberts JM:
Subclassification of preeclampsia. Hypertens Pregnancy. 22:143–148.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
George EM and Granger JP: Recent insights
into the pathophysiology of preeclampsia. Expert Rev Obstet
Gynecol. 5:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Zhang Q, Moe MC and Zhu T:
Regulation of cell-mediated collagen gel contraction in human
retinal pigment epithelium cells by vascular endothelial growth
factor compared with transforming growth factor-beta. Clin
Experiment Ophthalmol. 40:e76–e86. 2012. View Article : Google Scholar
|

















