Introduction
As the third leading cause of mortality worldwide,
stroke is a major health issue in the elderly population as it
leads not only to physical impairment, but also to a high risk of
disability and social handicap (1). Evidence from preclinical studies
(2) and randomized control trials
(3) have demonstrated that
combination therapy for ischemic stroke provides a survival
advantage and increases the effectiveness of treatment without
substantial side effects. Chinese herbs or their pharmacodynamic
constituents have been widely used for the treatment of ischemic
cerebral vascular disease through combination therapies (4). However, the complexity of the
chemical ingredients has led to a substantial bottleneck in
determining the mechanism of interaction among the ingredients for
treating ischemic stroke (5).
Microglia, the resident immune cells of the central
nervous system, have been implicated in triggering signaling
cascades that lead to cell death in brain ischemic diseases
(6). In physiological conditions,
resting microglial processes make brief and direct contacts with
neuronal synapses (7). In
addition, microglial cells are restrained by numerous
microenvironmental inhibitory effects, several of which are
produced by neurons (8,9). Neuron-microglial-cell inhibitory
signaling is mediated by interactions, including CD200-CD200
receptor and CD22-CD45, also termed PTPRC (10). In pathological conditions, neuronal
degeneration and microglial activation following transient cerebral
ischemia (11) can effect the
survival of neural cells through several pathways or cause neuronal
injury (12,13). Therefore, the inhibition of
activated microglia may promote the survival of neurons.
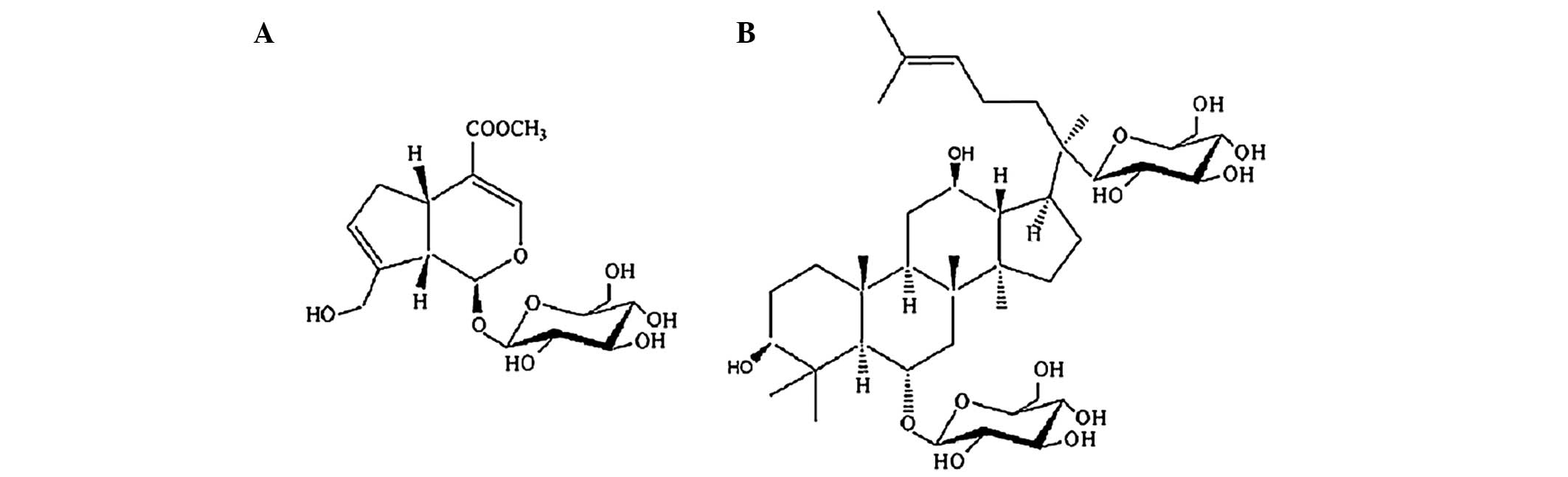
Geniposide and ginsenoside Rg1 (Fig. 1) are bioactive compounds, which are
extracted from Cape Jasmine Fruit (Fructus Gardeniae) and
Sanchi (Radix Notoginseng), respectively (14), and are two Chinese medicines, which
have been used for the treatment of stroke for thousands of years
(15,16). Although the pharmacological
mechanism of the individual use of geniposide or ginsenoside Rg1 on
cerebral ischemia are well understood (17,18),
current understanding of the effect of the combined use of
geniposide and ginsenoside Rg1 on stroke remains limited. Our
previous study demonstrated that the combination of geniposide and
ginsenoside Rg1, prescribed as a Tongluo Jiunao injection, can
reduce the expression of macrophage inflammatory protein (MIP)-1β
and C-C chemokine receptor type 5 (CCR5) in oxygen-glucose
deprivation (OGD)-injured microglial cells (MCs), as well as
inhibit the proliferative activity of microglial cells, suggesting
the therapeutic potential of the combination of geniposide and
ginsenoside Rg1 on ischemic cerebral ischemia (19). However, the synergistic mechanism
of multi-component combined use for complex diseases remains to be
fully elucidated. Synergistic therapeutic effects can be obtained
from combining effective components from Chinese herbs through
cell-cell communication. The present study, based on
microglia-neuron communication, aimed to determine the synergistic
effect of geniposide and ginsenoside Rg1 on hypoxia-injured neurons
through treatment in differently treated microglial
cell-conditioned media (MC-CM).
Materials and methods
BV2 microglia and N2a neuronal cells
culture
The murine BV2 microglia cells and N2a neuronal
cells (obtained from the Cerebrovascular Diseases Research
Institute, Xuanwu Hospital of Capital Medical University, Beijing,
China; third passage) were grown in T-25 tissue culture cell flasks
at 5% CO2 and 37°C humidified atmosphere using
Dulbecco's modified Eagle's medium (DMEM)/F12 (Invitrogen Life
Technologies, Carlsbad, CA, USA) culture media, supplemented with
10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Logan,
UT, USA), 2 mM glutamine and 100 µg/ml
penicillin-streptomycin. The BV2 microglial cells and N2a neuronal
cells were maintained via two to three passages each week.
Establishment of the OGD model, drug
administration and preparation of different microglial
cell-conditioned media
An OGD model was used to mimic ischemia, as
described in our previous study (20). For the microglial cells or N2a
neuronal cells in OGD, culture medium was replaced with
glucose-free DMEM, and the culture flasks (or plates) were placed
into a sealed tank with a persistent low-flow (1.5 l/min) 95%
N2 and 5% CO2 mixture to expire the oxygen
for 20 min. The inlet and outlet ends of the tubes were then
clipped, and the tank was placed into an incubator for 6 h to mimic
ischemia.
Geniposide (purity>98%; batch. no. 110749-200714)
and ginsenoside Rg1 (purity>95%, batch. no. 110703-201027) were
chemically standardized products obtained from the National
Institutes for Food and Drug Control (Beijing, China), which were
validated using fingerprint chromatographic methodologies,
according to the manufacturer's instructions. The microglial cells
were divided into the following five groups: Control group; model
group; geniposide-treated group (geniposide; 25 µg/ml);
ginsenoside Rg1-treated group (ginsenoside Rg1; 5 µg/ml);
and combination-treated group (geniposide and ginsenoside Rg1 at a
ratio of 1:1). The microglial cells (1×106 cells/ml)
were preconditioned with the different drug treatments for 2 h and
were maintained for another 6 h in hypoxia. The conditioned media
from the five groups were collected and centrifuged at 1,000 x g
for 10 min at 4°C to remove cell debris for the subsequent
experiments.
The N2a neuronal cells were divided into seven
groups: Control group (C), in which N2a neuronal cells were
cultured in normal culture medium; model (M) group, in which N2a
neuronal cells were challenged by OGD; N-MG-CM group, in which N2a
neuronal cells were cultured in normal cultured microglial
cell-conditioned medium;I-MG-CM group: N2a neuronal cells were
OGD-injured and cultured in microglial cell-conditioned medium;
C-MG-CM group, in which N2a neuronal cells were OGD-injured and
cultured in geniposide and ginsenoside Rg1-treated microglial
cell-conditioned medium; G-MG-CM group, in which N2a neuronal cells
were OGD injured and cultured in geniposide-treated microglial
cell-conditioned medium; R-MG-CM: N2a neuronal cells were
OGD-injured and cultured in ginsenoside Rg1-treated microglial
cell-conditioned medium. The proportion of conditioned media in
each group was 100% and the incubation duration in the different
conditioned media was 6 h, according to our previous study
(21).
CCK-8 assay
N2a neuronal cells at 1×103 cells per
well were seeded onto 96-well plates. Following incubation with the
different microglial cell-conditioned media, the media in the
96-well culture plates were replaced with DMEM/F12 to avoid
background interference. Subsequently, 10 µl CCK-8)(Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was added to each
well and incubated for 2 h at 37°C, followed by measurement using a
microplate reader (Safire2; TecanGroup, Ltd., Männedorf,
Switzerland) with a test wavelength of 450 nm and a reference
wavelength of 620 nm.
Lactate dehydrogenase (LDH) assay
N2a neuronal cells at 1×103 cells/well
were seeded onto 96-well plates. A CytoTox assay kit (Promega
Corporation, Madison, WI, USA) was used for the enzymatic
assessment of LDH release in the N2a neuronal cells. Reagents
(substrate mixture and analysis buffer) were added into the
96-wells, according to the manufacturer's instructions. A
fluorescence emission of 590 nm was used for measurement using a
microplate reader. The rate of LDH leakage was calculated,
according to the optical density (OD) values, using the following
equation: LLR = (OD value of the medium supernatant / OD value of
the lysed cell supernatant) × 100%.
Western blot analysis
N2a neuronal cells at 1×105 cells/well
were seeded onto 6-well plates. Western blot analysis was performed
to quantify the protein expression levels of N-methyl-D-aspartate
receptor subunit 1 (NMDAR1) and activated caspase-3 (Abcam,
Cambridge, UK) in the N2a neuronal cells. In brief, the N2a
neuronal cells were washed with ice-cold PBS and scraped in lysis
buffer (Beyotime Institute of Biotechnology, Jiangsu, China)
comprised of 50 mM Tris and 150 mM NaCl (TBS; pH 7.4), containing
1% Triton X-100, 1% Nonidet P-40, 0.5% sodium-deoxycholate, 0.1%
sodium-dodecyl-sulfate, 1 mM phenylmethylsulfonyl fluoride, 15
µg/ml leupeptin, 71 µg/ml phenanthrolyne and 20 U/ml
aprotine. The insoluble material was removed by centrifugation at
9,500 x g for 20 min at 4°C. The protein content was measured,
according to the bicinchoninic acid method (WellBiz Brands, Inc.,
Highlands Ranch, CO, USA). Subsequently, 20 µg protein was
processed using SDS-PAGE separation on 12.5% polyacrylamide gel,
and transferred onto a 0.45 µm nitrocellulose membrane
(Pierce Biotechnology, Inc., Rockford, IL, USA). Non-specific
binding sites were blocked with TBS, comprised of 40 mM Tris, (pH
7.6) and 300 mM NaCl, containing 5% nonfat dry milk, for 1 h at
37°C. The membrane was then incubated with the following
antibodies: Rabbit polyclonal anti-NMDAR1 (1:500; cat. no. ab17345;
Abcam) and rabbit polyclonal anti-activated caspase-3 (1:500; cat.
no. ab2302; Abcam) overnight at 4°C, followed by incubation in a
1:5,000 dilution of horseradish-peroxidase-conjugated goat
anti-rabbit IgG (cat. no. ZB-2301; ZSGB-BIO, Beijing, China) at
37°C for 1 h. Immunoreactive proteins were detected by enhanced
chemiluminescence (Pierce Biotechnology, Inc.), according to the
manufacturer's instructions. The membrane was then incubated with
stripping buffer (Applygen Technologies Inc, Beijing, China) for
0.5 h at room temperature, followed by incubation with rabbit
polyclonal anti-β-actin (1:5,000; cat. no. ab119716; Abcam) and the
corresponding secondary antibody. The experiment was repeated in
triplicate, and three wells were used for each group.
Analysis of mitochondrial transmembrane
potential
N2a neuronal cells at 1×103 cells/well
were seeded onto 24-well plates. The mitochondrial membrane
potential was investigated using a JC-1 probe (Beyotime Institute
of Biotechnology), which exists either as a green fluorescent
monomer at depolar-ized membrane potentials or as a red fluorescent
J-aggregate at hyperpolarized membrane potentials. The JC-1 was
added into the 24-wells, according to the manufacturer's
instructions. Fluorescent images were captured and the ratios of
the mitochondrial aggregates (red) to the monomeric form of JC-1
(green) were analyzed using fluorescence microscopy (Nikon Eclipse
80i; Nikon Corporation, Tokyo, Japan).
Transmission electron microscopy
N2a neuronal cells at 1×103 cells/well
were seeded onto 24-well plates. The mitochondrial changes of the
N2a neuronal cells were observed using transmission electron
microscopy. In brief, the N2a neuronal cells were fixed with 4%
glutaraldehyde and 1% osmic anhydride in sequence, and then
dehydrated with acetone (Sigma-Aldrich, St. Louis, MO, USA).
Subsequently, 50–70 nm ultrathin sections were cut using an
ultramicrotome (LKB, Margate, FL, USA) and stained with 2% uranyl
acetate (Sigma-Aldrich). Transmission electron microscopy (H7650;
Hitachi, Ltd., Tokyo, Japan) was used to observe the autophagosome
in the cells.
Statistical analysis
All results are expressed as the mean ± standard
deviation. SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. One way analysis of variance was
used to determine statistically significant differences among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
N2a neuronal cellular viability and LDH
leakage
The cellular viability and LDH leakage of the N2a
neuronal cells incubated with the different MG-CM were assessed
using a CCK-8 assay and LDH kits. Compared with the control group,
the viability of the N2a neuronal cells in the model group was
reduced significantly by OGD injury (P<0.001; Fig. 2A), while LDH leakage in the N2a
neuronal cells in the model group was increased significantly
following challenge by OGD injury (P<0.001; Fig. 2B), which indicated that the N2a
neuronal cells were injured by OGD. No significant change in
viability or LDH leakage were observed in the N-MG-CM group,
compared with control group, which indicated that the microglial
cells had no effect on the normal cultured N2a neuronal cells. The
viability was reduced and LDH leakage was increased in the N2a
neuronal cells incubated with I-MG-CM, with more severe injury,
compared with the group exposed to OGD injury alone. The viability
and LDH leakage of the N2a neuronal cells incubated with C-MG-CM,
G-MG-CM and R-MG-CM were recovered to different extents. There were
different effects of the MG-CM of geniposide and ginsenoside Rg1 on
the N2a neuronal cells. In terms of cell viability, the effect of
the ginsengoside Rg1-treated MG-CM was more marked than that of
geniposide. For the LDH leakage improvement, the effect of the
geniposide-treated MG-CM was more marked than that of ginsenoside
Rg1. Incubation with MG-CM with the combined use of geniposide and
ginsenoside Rg1 improve cell viability and suppressed LDH
leakage.
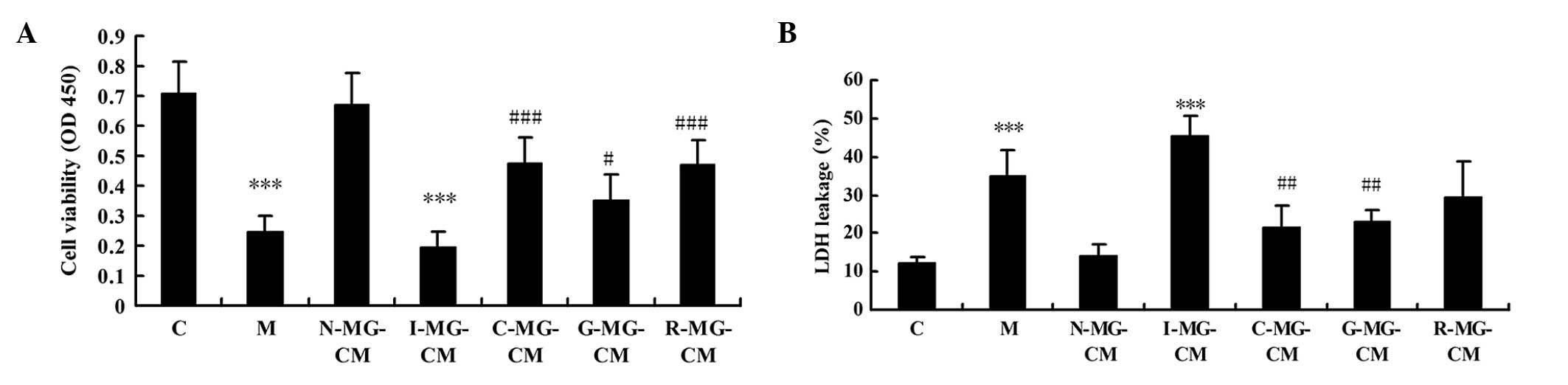 | Figure 2(A) Bar graphs indicating the changes
in neuronal cell viability, determined using a Cell Counting Kit-8
assay. A significant decrease in cell viability was observed in the
neuronal cells in the M or I-MG-CM groups, and this cell viability
was recovered when the neuronal cells were cultured with G-MG-CM,
R-MG-CM or C-MG-CM, with the R-MG-CM and C-MG-CM having the same
effect. (B) Bar graphs indicating the changes of LDH leakage in the
neuronal cell cultural media. The LDH leakage in the M or I-MG-CM
group was significantly increased, compared with the control (C)
group. The LDH leakage in the C-MG-CM and G-MG-CM groups were
suppressed, compared with the M group. No effect was observed in
the R-MG-CM group. ***P<0.001, vs. C group;
#P<0.05, vs. M group; ##P<0.01, vs. M
group; ###P<0.001, vs. M group. Data are expressed as
the mean ± standard deviation. C, control; M, model (ischemia);
MG-CM, microglial cell conditioned medium; C-MG-CM, combined
geniposide and ginsenoside Rg1 MG-CM; G-MG-CM, geniposide MG-CM;
R-MG-CM, ginsenoside Rg1 MG-CM; OD, optical density; LDH, lactate
deydrogenase. |
Expression of NMDAR1 and activated
caspase-3 in N2a neuronal cells
The present study subsequently investigated the
expression levels of NMDAR1 and activated caspase-3 in he N2a
neuronal cells in the different groups. As shown in Fig. 3, NMDAR1 and activated caspase-3 in
the N2a neuronal cells exhibited a significant increase in
expression in the model group (2.89-fold, P<0.05 and 3.73-fold,
P<0.001, respectively) and I-MG-CM group (3.85-fold, P<0.01;
4.34-fold, P<0.001, respectively), compared with the control
group, indicating that injury and apoptosis were induced by OGD and
I-MG-CM. Compared with the model group, the use of MG-CM with
ginsenoside Rg1 alone had no effect on the expression of NMDAR1.
Marked suppression of the expression of NMDAR1 was observed in the
N2a neuronal cells incubated in MG-CM with ginsenoside Rg1 alone,
the effect of which was more marked than that of MG-CM with
geniposide and ginsenoside combined (P<0.05). In terms of the
expression of activated caspase-3, the effect of MG-CM with
combined use of geniposide and ginsenoside was the same as that
observed in the R-MG-CM group, with a clear reduction. The protein
level of activated caspase-3 in the R-MG-CM group was unaffected
relative to the model group.
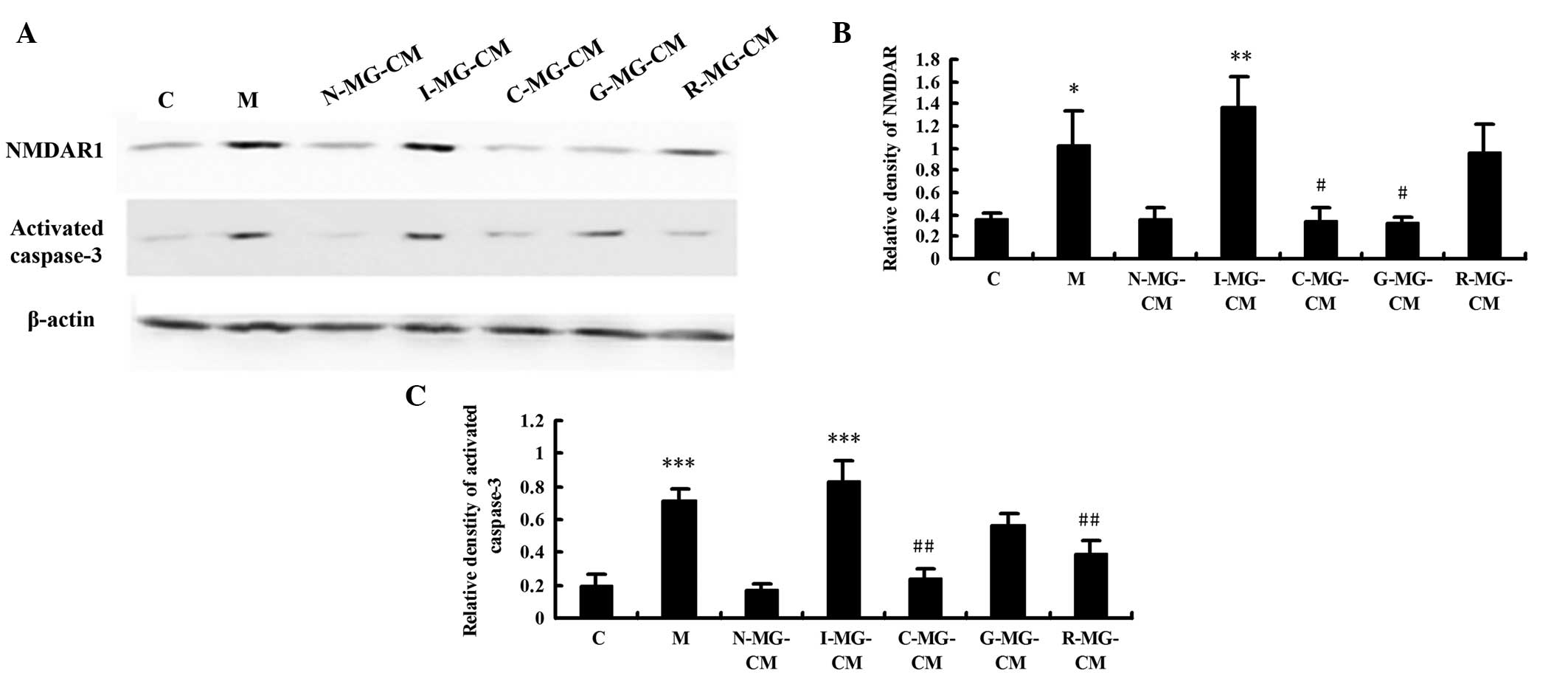 | Figure 3(A) Expression levels of NMDAR1 and
activated caspase-3 in neuronal cells, assessed using western
blotting. Images show representative blots of NMDAR1, activated
caspase-3 and β-actin (loading control). (B and C) Bar graphs
indicate the relative densities of the NMDAR1 and activated
caspase-3 bands, estimated quantitatively using Phoretix 1D image
software. Values represent the mean optical density ratio relative
to the loading control. *P<0.05, vs. C group;
***P<0.001, vs. C group; #P<0.05, vs. M
group; ##P<0.01, vs. M group. Data are expressed as
the mean ± standard deviation. C, control; M, model (ischemia);
MG-CM, microglial cell conditioned medium; C-MG-CM, combined
geniposide and ginsenoside Rg1 MG-CM; G-MG-CM, geniposide MG-CM;
R-MG-CM, ginsenoside Rg1 MG-CM. |
Mitochondrial transmembrane potential of
N2a neuronal cells
Almost all the cells visualized were well-spread and
exhibited red or orange fluorescence in the untreated N2a neuronal
cells (Fig. 4A) and cells in the
N-MG-CM group (Fig. 4B). By
contrast, in the OGD or I-MG-CM groups (Fig. 4C and D), the majority of fluoresced
green exclusively, indicating loss of mitochondrial membrane
potential. The N2a neuronal cells incubated with C-MG-CM, G-MG-CM
or R-MG-CM exhibited red or orange fluorescence, which indicated an
improvement relative to the model group, with C-MG-CM being the
most similar to the control cells (Fig. 4E-G). Ratios of JC-1
aggregates/monomeric forms in the groups (Fig. 4H) indicated that the OGD- or
I-MG-CM-induced loss of N2a neuronal cell mitochondrial membrane
potential was prevented by MG-CM with geniposide and/or
ginsenoside.
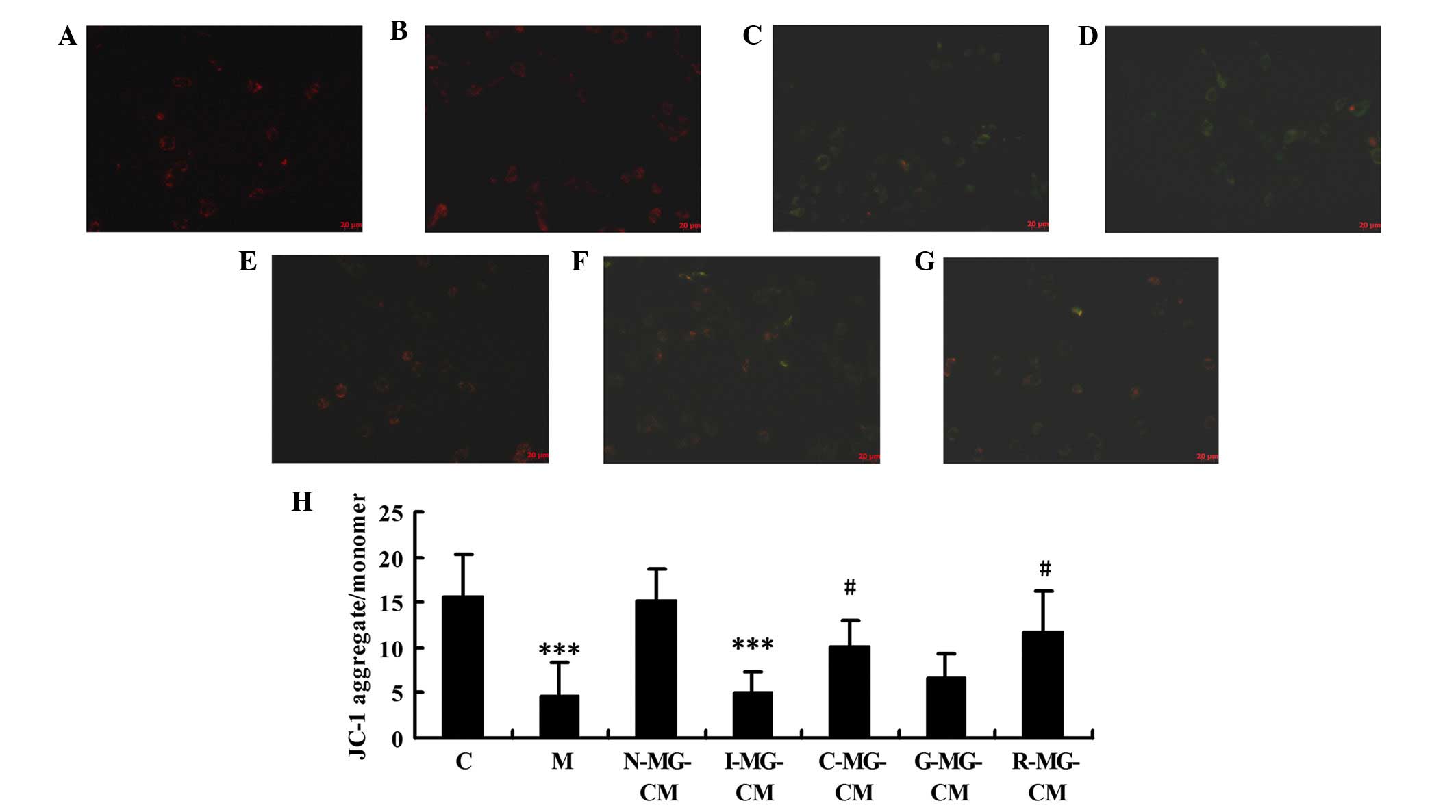 | Figure 4OGD- or OGD-injured MG-CM-induced
mitochondrial membrane potential loss is prevented by ginseonside
Rg1 or the combined use of geniposide and ginsenoside Rg1. A using
a JC-1 probe was used to examine the mitochondrial membrane
potential in neuronal cells, which were (A) untreated or were
treated with (B) N-MG-CM, (C) OGD, (D) I-MG-CM, (E) C-MG-CM, (F)
G-MG-CM or (G) R-MG-CM for 6 h. Magnification, ×400. (H)
Quantitative presentation of the JC-1 assay.
***P<0.001, vs. C group, #P<0.05 vs. M
group. Results are expressed as the mean ± standard deviation of
triplicate determinations. C, control; M, model (ischemia); OGD,
oxygen-glucose deprivation; MG-CM, microglial cell conditioned
medium; N-MG-CM, normal MG-CM; C-MG-CM, combined geniposide and
ginsenoside Rg1 MG-CM; G-MG-CM, geniposide MG-CM; R-MG-CM,
ginsenoside Rg1 MG-CM. |
Mitochondrial changes in the N2a neuronal
cells
The mitochondrial structure of the N2a neuronal
cells in the control group was evident, with an intact
mitochondrial membrane and mitochondrial crista (Fig. 5A). There was no change to the
mitochondrial structure of the cells treated with N-MG-CM (Fig. 5B). By contrast, OGD (Fig. 5C) or I-MG-CM (Fig. 5D) incubation damaged the N2a
neuronal cells mitochondrial structure, characterized by disordered
mitochondrial crista arrangement or vacuolation. Following
incubation with C-MG-CM, G-MG-CM or R-MG-CM, the mitochondrial
structure of the N2a neuronal cells recovered to different extents,
compared with the model group, with the G-MG-CM group and C-MG-CM
group exhibiting more significant effects (Fig. 5E and F).
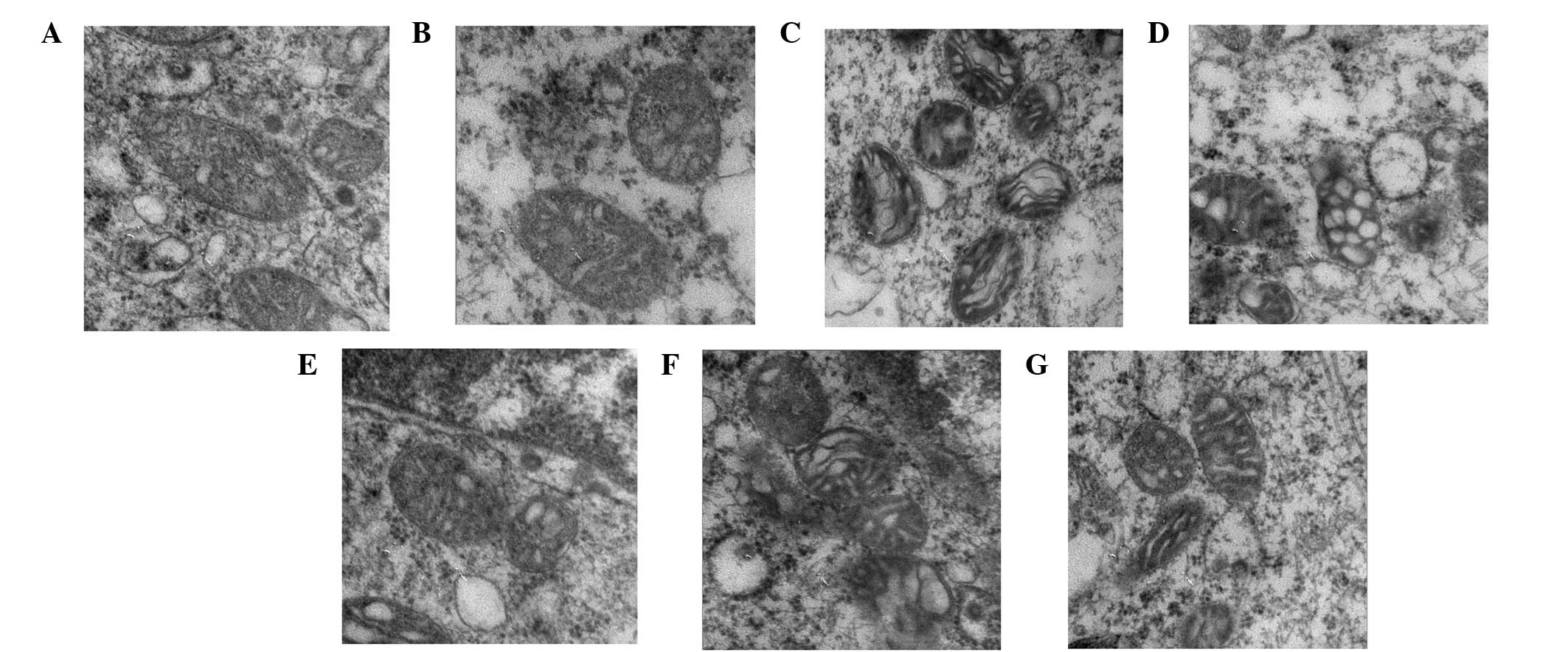 | Figure 5OGD- or OGD-injured MF-CM-induced
mitochondrial abnormalities are prevented by treatment with MG-CM
with individual or combined use of geniposide and ginsenoside Rg1.
Ultrastructural features of mitochondrial change in neuronal cells
in the (A) untreated control, or following treatment with (B)
N-MG-CM, (C) OGD, (D) I-MG-CM, (E) C-MG-CM, (F) G-MG-CM or (G)
R-MG-CM for 6 h. Magnification, ×80,000. OGD, oxygen-glucose
deprivation; C, control; M, model (ischemia); MG-CM, microglial
cell conditioned medium; C-MG-CM, combined geniposide and
ginsenoside Rg1 MG-CM; G-MG-CM, geniposide MG-CM; R-MG-CM,
ginsenoside Rg1 MG-CM. |
Discussion
Pharmacodynamic constituents from natural medicines
have been investigated for the treatment of ischemic stroke.
Multi-component treatments, characterized by two or more agents
interacting with multiple targets simultaneously, are considered to
be a rational and efficient form of therapy that is designed to
control complex diseases (22,23),
including stroke, which is a more complex disease than initially
anticipated. Our previous study and those of others have already
demonstrated that multi-component prescription (Tongluo Jiunao
injection), composed of ginsenoside and ginsenoside Rg1 is
effective for the treatment of stroke due to its anti-inflammatory,
neuronprotecive and neurotrophic roles (19,24).
The present study aimed to elucidate the synergistic effects of
microglial cell-conditioned media treated with geniposide and
ginsenoside Rg1 on hypoxia-injured neurons.
According to the results obtained from the CCK-8
assay and LDH leakage assessment, certain proteins secreted from
the OGD-activated microglial cells were involved in the neuronal
cell damage, which indicated that the OGD-induced microglial cells
generated neurotoxicity. An improvement in the I-MG-CM-induced
increase of LDH was observed by using G-MG-CM, while cell viability
enhancement was not significantly difference. R-MG-CM increased the
neuronal cell viability, however had no effect on LDH leakage,
suggesting that microglial cells treated by ginsenoside Rg1 alone
improved neuron survival by secreting certain cytokines through
pathways other than LDH. MG-CM with the combined use of ginsenoside
and ginsenoside Rg1 increased the cell viability and decreased LDH
leakage, which demonstrated an integral effect of
compatibility.
Activated-microglia release glutamate, which is the
major neurotoxic factor released into the extracellular space
following neural injury and causes neuronal death at high
concentrations (25). Glutamate
activates ionotropic and metabotropic receptors, and NMDA is a type
of ionotropic receptor. The action of glutamate causes
Na+ and Ca2+ influx, which can lead to
Ca2+ overload and subsequent Ca2+ dependent
neural injury (26,27). Another important mechanism of
OGD-injury in neural cells is apoptosis. Glutamate can also induce
the loss of neurons and activation of caspase-3 (28), a key apoptotic protease-mediated
cascade downstream. Several reports have demonstrated that
cytochrome c-dependent caspase-3 activation is an important
mechanism responsible for ischemia-induced apoptosis (29,30).
Consequently, the present study hypothesized that
the secretion of microglia can be altered due to geniposide and
ginsenoside Rg1 compatibility, to improve the survival of the
neuronal microenvironment. The results demonstratedthat microglial
cells, following treatment with geniposide, downregulated the
expression of the NMDA receptor in neurons, which indicated that
geniposide inhibited the expression of NMDA receptors by reducing
the glutamate secretion of the ischemic microglia. The above effect
was almost absent following treatment with ginsenoside Rg1, however
downregulated of caspased-3 was observed, which indicated an
anti-apoptotic effect. By contrast, the MG-CM with geniposide alone
had no effect on caspase-3, suggesting that other possible
neuroprotective pathways were involved, including anti-inflammatory
or caspase-independent pathways. Treatment with MG-CM with
geniposide and ginsenoside Rg1 in combination suppressed the
expression of NMDA receptor and caspase-3, which demonstrated
integrated and synergistic modulation due to the compatibility of
the effective components.
Mitochondria are one of the important pathways of
neuronal cells apoptosis. Several studies have demonstrated that
OGD induces apoptosis by favoring the release of cytochrome
c and the consequent formation of the apoptotic complex
(31,32). In the present study, when the
neuronal cells were exposed to OGD or I-MG-CM, rapid depolarization
of the mitochondrial membrane potential was observed, which is
indicative of mitochondrial dysfunction. The above mitochondrial
dysfunction was observed in the electron microscopy images,
characterized by mitochondrial swelling and osmotic expansion,
suggestive of typical mitochondrial pathological change (33). The observed mitochondrial
dysfunction was alleviated by treatment with MG-CM with geniposide
and ginsenoside Rg1 in combination, which indicated that the
synergetic use of geniposide and ginsenoside Rg1 suppressed
OGD-induced neuronal apoptosis through inhibition of the
mitochondrial-mediated apoptotic pathway. Of note, it was observed
that, compared with treatment with geniposide alone, ginsenoside
Rg1 exhibited a more significant effect on the improvement of
mitochondrial membrane potential and mitochondrial ultrastructure.
Combined with the results obtained on the expression of caspase-3,
it was hypothesized that, in the synergistic effect of geniposide
and ginsenoside Rg1, ginsenoside Rg1 is the predominant effector in
the inhibition of the mitochondrial-mediated apoptotic pathway.
From the above-mentioned results, it appears that
paracrine signaling in differently treated microglia are involved
in neuroprotection. However, which signal is elicited by geniposide
and/or ginsenoside Rg1, and how the synergistic effect produces the
observed results remains to be fully elucidated. Our previous study
indicated that the synergistic use of geniposide and ginsenoside
Rg1 can balance microglial TNF-α and TGF-β1 following ischemic
injury (34). The balance of
microglial neurotoxic factors and the neuroprotective factor, Rg1,
may explain the neuroprotective effects observed following
geniposide and ginsenoside synergistic use.
In conclusion, the present study demonstrated that
MG-CM with geniposide and ginsenoside Rg1 in combintation exerted a
synergistic effect on the neuronal mitochondrial-mediated apoptotic
pathway triggered by OGD, with geniposide and ginsenoside Rg1
exhibiting different regulatory effects.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. nos. 81102679 and
81473449), the Fundamental Research Funds for the Central Public
Welfare Research Institutes (grant no. ZZ070824), and the National
Basic Research Program of China (973 Program, 2015CB554400).
References
|
1
|
Feigin VL, Lawes CM, Bennett DA,
Barker-Collo SL and Parag V: Worldwide stroke incidence and early
case fatality reported in 56 population-based studies: A systematic
review. Lancet Neurol. 8:355–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faure S, Oudart N, Javellaud J, Fournier
A, Warnock DG and Achard JM: Synergistic protective effects of
erythropoietin and olmesartan on ischemic stroke survival and
post-stroke memory dysfunctions in the gerbil. J Hypertens.
24:2255–2261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smadja D, Chausson N, Joux J, Saint-Vil M,
Signaté A, Edimonana M, Jeannin S, Bartoli B, Aveillan M, Cabre P
and Olindo S: A new therapeutic strategy for acute ischemic stroke:
Sequential combined intravenous tpa-tenecteplase for proximal
middle cerebral artery occlusion based on first results in 13
consecutive patients. Stroke. 42:1644–1647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Li P and Wang Y, Liu J, Zhang Z,
Cheng W and Wang Y: Ameliorative effects of a combination of
baicalin, jasminoidin and cholic acid on ibotenic acid-induced
dementia model in rats. PLoS One. 8:e566582013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Zhou CX, Zhang ZJ, Wang LY, Jing ZW
and Wang Z: Synergistic mechanism of gene expression and pathways
between jasminoidin and ursodeoxycholic acid in treating focal
cerebral ischemia-reperfusion injury. CNS Neurosci Ther.
18:674–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai AY and Todd KG: Microglia in cerebral
ischemia: Molecular actions and interactions. Can J Physiol
Pharmacol. 84:49–59. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wake H, Moorhouse AJ, Jinno S, Kohsaka S
and Nabekura J: Resting microglia directly monitor the functional
state of synapses in vivo and determine the fate of ischemic
terminals. J Neurosci. 29:3974–3980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ransohoff RM and Perry VH: Microglial
physiology: Unique stimuli, specialized responses. Annu Rev
Immunol. 27:119–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanisch UK and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nature Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barclay AN, Wright GJ, Brooke G and Brown
MH: CD200 and membrane protein interactions in the control of
myeloid cells. Trends Immunol. 23:285–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon JB, Lee CH, Park CW, Cho JH, Hwang
IK, Yoo KY, Choi JH, Shin HC and Won MH: Neuronal degeneration and
microglial activation in the ischemic dentate gyrus of the gerbil.
J Vet Med Sci. 71:1381–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor DL, Pirianov G, Holland S,
McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D and
Mehmet H: Attenuation of proliferation in oligodendrocyte precursor
cells by activated microglia. J Neurosci Res. 88:1632–1644.
2010.PubMed/NCBI
|
|
13
|
Hur J, Lee P, Kim MJ, Kim Y and Cho YW:
Ischemia-activated microglia induces neuronal injury via activation
of gp91phox NADPH oxidase. Biochem Biophys Res Commun.
391:1526–1530. 2010. View Article : Google Scholar
|
|
14
|
Liu Y, Hua Q, Lei H and Li P: Effect of
Tong Luo Jiu Nao on Aβ-degrading enzymes in AD rat brains. J
Ethnopharmacol. 137:1035–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Pan Y, Jia X, Li K, Liu C, Wang X
and Li P: Effects of Huanqin (dried root of Scutellaria
baicalensis) and Zhizi (dried fruit of Gardenia jasminoides) used
in combination on ischemic cascade reaction in the rat model of
focal cerebral ischemia and reperfusion. Beijing Zhong Yi Yao Da
Xue Xue Bao. 25:31–33. 2002.In Chinese.
|
|
16
|
Li K: Systematic review of Sanqi agents
for acute ischaemic stroke. Lin Chuang Hui Cui. 22:1–5. 2007.In
Chinese.
|
|
17
|
Chen J, Sun M, Wang X, Lu J, Wei Y, Tan Y,
Liu Y, Götz J, He R and Hua Q: The herbal compound geniposide
rescues formaldehyde-induced apoptosis in N2a neuroblastoma cells.
Sci China Life Sci. 57:412–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie CL, Wang WW, Xue XD, Zhang SF, Gan J
and Liu ZG: A systematic review and meta-analysis of
Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep.
5:77902015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Li PT, Du H, Hou JC, Li WH, Pan YS
and Chen HC: Tong Luo Jiu Nao injection, a traditional Chinese
medicinal preparation, inhibits MIP-1β expression in brain
microvascular endothelial cells injured by oxygen-glucose
deprivation. J Ethnopharmacol. 141:151–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Hou J, Zhang P, Li D, Zhang C and
Liu J: Geniposide reduces inflammatory responses of oxygen-glucose
deprived rat microglial cells via inhibition of the TLR4 signaling
pathway. Neurochem Res. 37:2235–2248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Li PT, Du H, Hou JC, Li WH, Pan
YS, Hua Q and Chen HC: Impact of paracrine signals from brain
microvascular endothelial cells on microglial proliferation and
migration. Brain Res Bull. 86:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carey KM, Comee MR, Donovan JL and Kanaan
AO: A polypill for all? Critical review of the polypill literature
for primary prevention of cardiovascular disease and stroke. Ann
Pharmacother. 46:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zimmermann GR, Lehár J and Keith CT:
Multi-target therapeutics: When the whole is greater than the sum
of the parts. Drug Discov Today. 12:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Xu J, Wang X and Yuan G: Protective
effect of ginsenoside Rg1 on lidocaine-induced apoptosis. Mol Med
Rep. 9:395–400. 2014.
|
|
25
|
Umebayashi D, Natsume A, Takeuchi H, Hara
M, Nishimura Y, Fukuyama R, Sumiyoshi N and Wakabayashi T: Blockade
of gap junction hemichannel protects secondary spinal cord injury
from activated microglia-mediated glutamate exitoneurotoxicity. J
Neurotrauma. 31:1967–1974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawasaki Y, Kohno T, Zhuang ZY, Brenner
GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ and Ji RR:
Ionotropic and metabotropic receptors, protein kinase A, protein
kinase C, and Src contribute to C-fiber-induced ERK activation and
cAMP response element-binding protein phosphorylation in dorsal
horn neurons, leading to central sensitization. J Neurosci.
24:8310–8321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Novitskaya YA, Dravolina OA, Zvartau EE,
Danysz W and Bespalov AY: Interaction of blockers of ionotropic
NMDA receptors and metabotropic glutamate receptors in a working
memory test in rats. Neurosci Behav Phsyiol. 40:807–811. 2010.
View Article : Google Scholar
|
|
28
|
Xu GY, Liu S, Hughes MG and McAdoo DJ:
Glutamate-induced losses of oligodendrocytes and neurons and
activation of caspase-3 in the rat spinal cord. Neuroscience.
153:1034–1047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin J, Li H, Feng C and Zuo Z: Inhibition
of brain ischemia-caused notch activation in microglia may
contribute to isoflurane postconditioning-induced neuroprotection
in male rats. CNS Neurol Disord Drug Targets. 13:718–732. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X and Huang N: Berberine induces
selective apoptosis through the AMPK-mediated mitochondrial/caspase
pathway in hepatocellular carcinoma. Mol Med Rep. 8:505–510.
2013.PubMed/NCBI
|
|
31
|
Wang L, Chen M, Yuan L, Xiang Y, Zheng R
and Zhu S: 14,15-EET promotes mitochondrial biogenesis and protects
cortical neurons against oxygen/glucose deprivation-induced
apoptosis. Biochem Biophys Res Commun. 450:604–609. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Wang P, Li S, Wang S, Li Y, Liang
N and Wang M: Mdivi-1 prevents apoptosis induced by
ischemia-reperfusion injury in primary hippocampal cells via
inhibition of reactive oxygen species-activated mitochondrial
pathway. J Stroke Cerebrovasc Dis. 23:1491–1499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee WK and Thévenod F: A role for
mitochondrial aquaporins in cellular life-and-death decisions? Am J
Physiol Cell Physiol. 291:C195–C202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Hou JC, Xiang LH, Zhang J and Ju
DH: Compatibility of geniposide and ginsenoside rg1: Their
regulating roles in secretion of anoxia induction injured microglia
inflammatory cytokines. Zhongguo Zhong Xi Yi Jie He Za Zhi.
34:91–95. 2014.In Chinese. PubMed/NCBI
|