Introduction
Maladaptive remodeling of arteries following injury,
including balloon angioplasty and endovascular stent implantation,
is characterized by inflammation, neointima formation and media
hypertrophy, which may result in restenosis or re-narrowing of the
affected artery (1). Vascular
injury-induced inflammation involves complex interactions between
multiple vascular cell types, among which vascular endothelial
cells are essential by releasing various types of growth factors,
chemokines and cytokines (2).
Berberine, is one of the main active alkaloids
isolated from plants, including Coptis chinensis and
Hydrastis canadensis, and has long been used to treat
gastrointestinal disorders, including diarrhea (3). Previous studies have demonstrated
that berberine exerts multiple pharmacological effects, including
anti-microbial (4,5), anti-inflammatory (6,7) and
anti-tumor (8,9) activities. Increasing evidence has
indicated that berberine may possess beneficial cardiovascular
effects, due to its anti-hyperglycemic (10,11),
anti-cardiac hypertrophic (12)
and anti-ischemia-reperfusion injury (13) effects. Previous studies have
demonstrated that berberine exerts beneficial vascular effects by
inhibiting vascular smooth muscle cell proliferation, following
various pathogenic conditions, including restenosis (14,15).
Furthermore, a previous study indicated that berberine exerts an
anti-inflammatory effect in patients with acute coronary syndrome
following percutaneous coronary intervention (16), suggesting that the
anti-inflammatory effects of berberine may also contribute to its
vascular protective activity.
Tumor necrosis factor (TNF)-α is a major
proinflammatory factor in the development of vascular inflammation
(17). Due to the inhibitory
effect of berberine on vascular inflammation, the present study
aimed to investigate whether berberine downregulates the expression
of inflammatory molecules. The present study also aimed to
determine how berberine exerts anti-inflammatory effects in
TNF-α-stimulated human aortic endothelial cells (HAECs). The
present study investigated the effects of berberine on the
expression of intercellular adhesion molecule (ICAM)-1, monocyte
chemoattractant protein (MCP)-1, and nuclear factor (NF)-κB in
HAECs. In addition, the underlying molecular mechanism was
explored.
Materials and methods
Reagents
Berberine, dimethyl sulfoxide, and compound C were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
TRIzol® reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The Lactate Dehydrogenase (LDH)
Assay kit was obtained from Beyotime Institute of Biotechnology
(Jiangsu, China). The ImProm-II™ Reverse Transcription system was
purchased from Promega Corporation (Madison, WI, USA). The SYBR
master mix for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was purchased from Applied Biosystems Life
Technologies (Foster City, CA, USA). Antibodies against NF-κB p65,
phosphorylated- and total-adenosine monophosphate-activated protein
kinase (AMPK), phosphorylated- and total-acetyl-CoA carboxylase
(ACC), and histone were obtained from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Anti-GAPDH, AMPKα1/2 specific small
interfering (si)RNA, control scrambled siRNA and the siRNA reagent
system were purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Recombinant human TNF-α, and ELISA kits for ICAM-1 and
MCP-1 were obtained from R&D Systems, Inc. (Minneapolis, MN,
USA).
Cell culture and treatment
The HAECs were obtained from Sciencell Research
Laboratories (Carlsbad, CA, USA) and were maintained in endothelial
cell medium supplemented with growth supplements (cat. no. 1052;
Sciencell Research Laboratories) and fetal bovine serum (5%
vol/vol; Invitrogen Life Technologies) at 37°C in an atmosphere
containing 5% CO2. Once the cells had reached 70–80%
confluence and prior to initiation of the experiments, the cells
were serum starved for 24 h in serum-free medium. Subsequently, the
HAECs were incubated with berberine at various concentrations,
and/or treated with recombinant human TNF-α (10 ng/ml). The cells
were cultured for various time periods, and the supernatants were
subsequently collected and the cells were harvested. To investigate
the potential contribution of the AMPK pathway on the effects of
berberine, the HAECs were pretreated with chemical inhibitor
compound C (10 µM) for 30 min, or were transfected with AMPK
siRNA (100 µM). Briefly, cells were transfected with AMPK
siRNA or control scrambled siRNA (Santa Cruz Biotechnology, Inc.)
using the siRNA reagent system for 6 h; the medium was then
replaced with normal culture medium prior to treatment with
berberine and TNF-α.
Cytotoxicity assay
To evaluate the effects of berberine on the
viability of the HAECs, an LDH assay was performed, which is based
on the release of LDH. The HAECs were seeded into 96-well plates at
a density of 5×103 cells/well, and following 3 days
incubation at 37°C the medium was replaced with serum-free medium,
and the HAECs were incubated at 37°C for a further 24 h. Berberine
was then added to the cells at final concentrations of 2, 5, 10 or
25 µM), and cultured for 24 h at 37°C. Subsequently, the
cell culture supernatants were collected and the LDH content was
analyzed, according to the manufacturer's instructions. The
toxicity of berberine on the cells was also investigated using a
trypan blue dye exclusion assay (0.4%; Sigma-Aldrich), as
previously reported (18).
ELISA
Immediately following termination of the experiment
involving treatment with berberine, the cell culture supernatants
were collected and stored at −80°C for subsequent analysis of the
expression of inflammatory molecule proteins. The concentrations of
ICAM-1 and MCP-1 were determined using ELISA kits, according to the
manufacturer's instructions. Absorbance was measured at 450 nm
using an ELISA reader (model 3550; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
RNA extraction and RT-qPCR
Total RNA was extracted from the cells using
TRIzol® reagent, according to the manufacturer's
instructions. The isolated RNA was then reverse transcribed into
cDNA using the reverse transcription system. mRNA expression levels
were analyzed using an ABI Prism 7500 system (Applied Biosystems
Life Technologies) and SYBR Green master mix. The PCR reaction mix
(20 µl) consisted of 1 µl cDNA (50 ng), 10 µl
2X SYBR Green master mix and 5 pmol each of the forward (1
µl) and reverse (1 µl) primer (Beijing Sunbiotech
Co., Ltd., Beijing) made up with sterile water. PCR cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 95°C for 10 sec and 60°C for 40 sec. Data were subsequently
collected and quantitatively analyzed. GAPDH was used as an
endogenous control for sample normalization. The results are
presented as fold increases, compared with the expression of GAPDH.
The primer sequences were as follows: ICAM-1, forward
5′-AATGCCCAGACATCTGTGTCCC-3′ and reverse
5′-GGCAGCGTAGGGTAAGGTTCTT-3′; MCP-1, forward
5′-TTCCATGGACCACCTGGACA-3′ and reverse 5′-TGTCTGGGGAAAGCTAGGGG-3′;
and GAPDH, forward 5′-CTCCCCACACACATGCACTTA-3′ and reverse
5′-CCTAGTCCCAGGGCTTTGATT-3′. Relative quantification was performed
using the 2−ΔΔCt method (19).
Western blot analysis
The HAECs were harvested and lysed with lysis buffer
containing 50 µM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM EDTA and 100 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). The cell lysate was then centrifuged at 14,000 × g
at 4°C for 5 min, and the supernatant was collected. Nuclear
protein extracts were isolated using a Nuclear and Cytoplasmic
Protein Extraction kit (Beyotime Institute of Biotechnology),
according to the manufacturer's instructions. Protein
concentrations were determined using a bicinchoninic acid method
(Beyotime Institute of Biotechnology). Equal quantities of protein
(50 µg) from each sample were separated by 12% SDS-PAGE
(Beyotime Institute of Biotechnology). The proteins were then
transferred onto nitrocellulose membranes (Beyotime Institute of
Biotechnology), blocked with 5% skim milk in tris-buffered saline
supplemented with 0.1% Tween 20 (TBST; Beyotime Institute of
Biotechnology), and incubated overnight at 4°C with the following
polyclonal rabbit anti-human primary antibodies: Anti-NF-κB p65
(1:1,000; cat. no. 3039), anti-phosphorylated-AMPK (1:1,000; cat.
no. 2531), anti-AMPK (1:1,000; cat. no. 2532),
anti-phosphorylated-ACC (1:1,000; cat. no. 3661), anti-ACC
(1:1,000; cat. no. 3662) and anti-histone (1:1,000; cat. no. 2578).
The membranes were then washed three times with TBST, followed by
incubation with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. 7074) for 2 h at room
temperature. The blots were visualized using enhanced
chemiluminescence reagent (Pierce Biotechnology, Inc.). Relative
protein expression levels were determined by densitometric analysis
using ImageJ software, version 1.45 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using GraphPad Prism
5.0 sofrware (GraphPad Software, Inc., La Jolla, CA, USA), and the
statistical significance of differences was determined using
one-way analysis of variance and Student's t-test. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed at least three times.
Results
HAEC toxicity of berberine
The HAECs were cultured with various concentrations
of berberine, in order to evaluate its toxic effects. Treatment
with 2, 5, 10 or 25 µM berberine for 24 h did not lead to
HAEC toxicity, compared with the controls (Fig. 1A). A trypan blue dye exclusion
assay was also used to confirm the effects of berberine on HAEC
toxicity, and similar results were observed (Fig. 1B).
Inhibitory effects of berberine on the
production of ICAM-1 and MCP-1
To investigate the effects of berberine on the
production of inflammatory molecules, the protein expression levels
of ICAM-1 and MCP-1 was detected in the HAECs following
co-treatment with TNF-α for various durations. TNF-α significantly
increased the protein secretion of ICAM-1 and MCP-1 after 24 h
(Fig. 2A and B), and the mRNA
expression levels of ICAM-1 and MCP-1 were also increased 6 h
following TNF-α stimulation (Fig. 2C
and D). By contrast, berberine significantly attenuated TNF-α
stimulated protein secretion and the mRNA expression levels of
ICAM-1 and MCP-1 in HAECs in a concentration-dependent manner
(Fig 2A–D).
Inhibitory effects of berberine on the
activation of NF-κB
Further experiments were performed to examine
whether NF-κB activation was affected by treatment with berberine.
As shown in Fig. 3, the expression
of nuclear NF-κB p65 increased following stimulation of the HAECs
with TNF-α, whereas berberine significantly downregulated the
nuclear expression levels of TNF-α-stimulated NF-κB p65.
Berberine activates AMPK in HAECs
Treatment of the HAECs with berberine induced the
activation of AMPK, which was determined by measuring the
phosphorylation of AMPK. Berberine increased the activation of AMPK
and its downstream molecule, ACC, in a time-dependent manner
(Fig. 4).
Inhibitory effects of berberine on
TNF-α-induced expression of inflammatory moleculed and
NF-κB-activation are mediated by the AMPK pathway
The present study investigated the role of AMPK
activation in the inhibitory effects of berberine on the
TNF-α-induced expression of inflammatory molecules and NF-κB-p65
activation. The inhibitory effects of berberine on TNF-α induced
expression of ICAM-1 and MCP-1 was attenuated when the HAECs were
treated with AMPK inhibitor compound C, or were transfected with
AMPK-specific siRNA (Fig. 5A and
B). In addition, the inhibitory effects of berberine on
TNF-α-induced NF-κB p65 activation were attenuated in the cells,
which were treated with compound C or transfected with AMPK siRNA.
These results indicated that AMPK activation may mediate the
inhibitory effects of berberine on the TNF-α-induced expression of
inflammatory molecules and NF-κB activation (Fig. 5C and D).
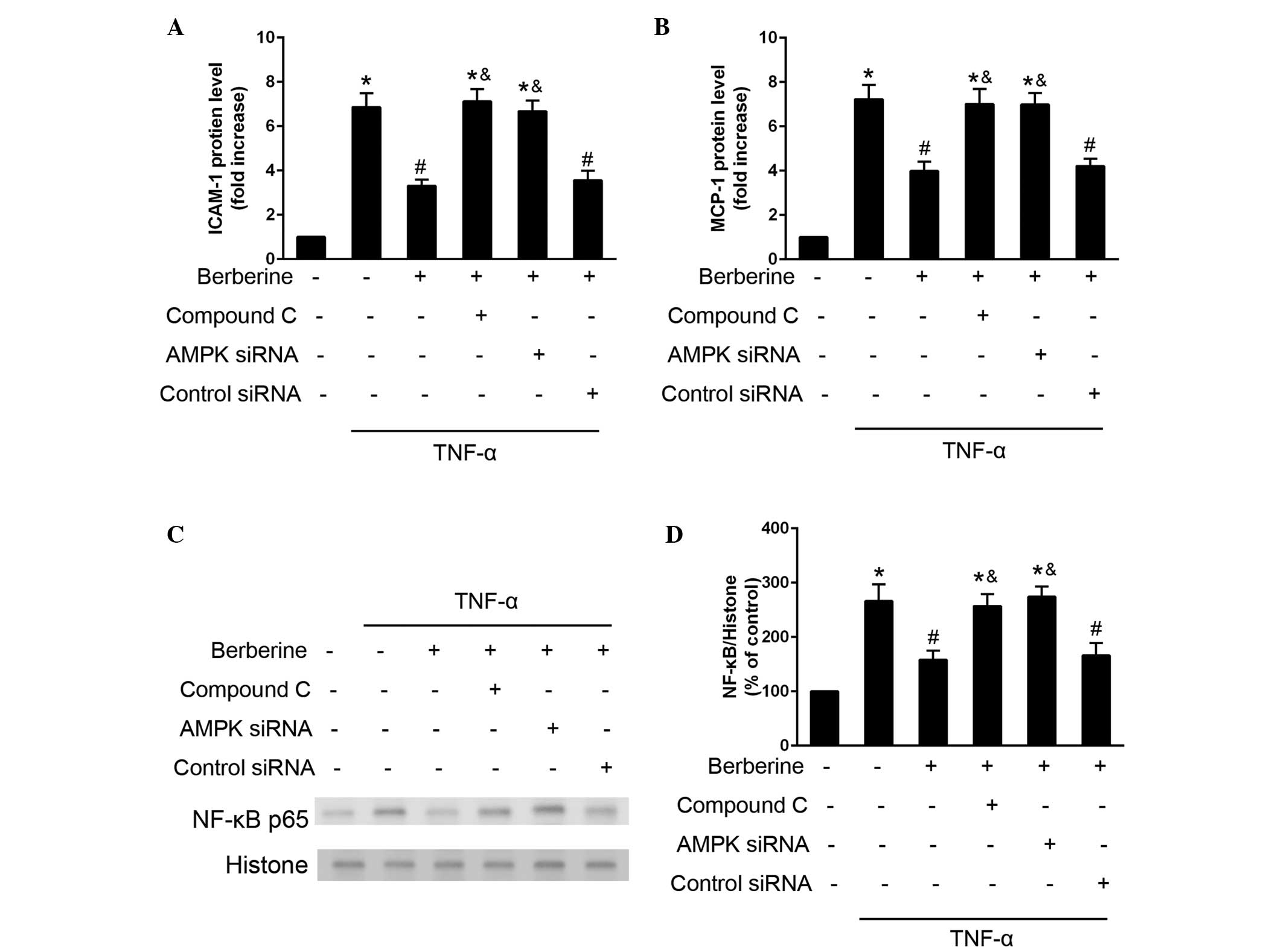 | Figure 5Berberine inhibits TNF-α induced
inflammatory molecule expression and NF-κB expression via the AMPK
pathway. HAECs were treated with berberine (25 µM), compound
C (10 µM), or were transfected with AMPK siRNA (100
µM) or control scrambled siRNA (100 µM) 30 min prior
to stimulation with TNF-α. (A and B) Protein expression levels of
ICAM-1 and MCP-1 were determined using an ELISA assay after 24 h.
(C and D) Expression of NF-κB was analyzed using western blotting
30 min after TNF-α stimulation. Data are presented as the mean ±
standard error of the mean. *P<0.05, compared with
the control; #P<0.05, compared with the cells treated
with TNF-α alone; &P<0.05, compared with the
TNF-α and berberine co-treatment group. NF, nuclear factor; TNF,
tumor necrosis factor; AMPK, adenosine monophosphate-activated
protein kinase; HAECs, human aortic endothelial cells; siRNA, small
interfering RNA; ICAM, intercellular adhesion molecule; MCP,
monocyte chemoattractant protein. |
Discussion
The present study demonstrated that treatment with
berberine significantly inhibited TNF-α-induced expression of
inflammatory molecules, without affecting the viability of the
HAECs. Berberine effectively reduced the expression levels of
ICAM-1 and MCP-1, and the activation of NF-κB, possibly via an
AMPK-dependent pathway.
Vascular remodeling following injury is associated
with inflammation, neointima formation and media hypertrophy
(1). Previous studies have
demonstrated that berberine exerts vascular protective effects via
various pathways, including the inhibition of vascular smooth
muscular cell activation (15) and
the reduction of inflammatory molecules (16). Vascular endothelial cells are
important in vascular inflammation by releasing diverse types of
growth factors, chemokines and cytokines. In our previous study,
berberine was observed to suppress the migration of human aortic
smooth muscle cells by reducing the expression levels of matrix
metalloproteinase (MMP)-2, MMP-9, and urokinase-type plasminogen
activator (20), whereas the
effects of berberine on vascular endothelial cells and the
underlying molecular mechanisms remained to be elucidated. Previous
studies have demonstrated that berberine exerts an inhibitory
effect on inflammation in several cell lines, and the expression
levels of proinflammatory genes, including interleukin (IL)-1β,
IL-6, TNF-α and MCP-1, are downregulated following berberine
administration (21–23). The adhesion molecule, ICAM-1, and
chemokine, MCP-1, are important in the inflammatory process,
predominantly via the promotion of monocyte-endothelial adhesion
and transendothelial migration (24–27).
The results of the present study demonstrated that berberine
inhibited the mRNA and protein expression levels of ICAM-1 and
MCP-1. These results indicated that the mechanism underlying the
anti-inflammatory effects of berberine was, at least partially,
associated with downregulation of these genes.
NF-κB is a well-known transcription factor, which is
critical in vascular injury-associated proinflammatory gene
regulation and is rapidly activated by various agents, including
the inflammatory cytokine, TNF-α (28). NF-κB translocates into the nuclear
compartment upon stimulation with various stimuli (29). Inflammatory molecules, including
ICAM-1, MCP-1 and other pro-inflammatory molecules, including IL-6
and E-selectin, have previously been reported to promote vascular
remodeling via NF-κB-dependent coordinated induction (30–32),
Concordantly, the results of the present study indicated that
berberine inhibited NF-κB activation, suggesting that the NF-κB
pathway is associated with the anti-inflammatory effects of
berberine.
AMPK is a heterotrimeric serine/threonine protein
kinase, which is generally referred to as a 'metabolite-sensing
kinaseʼ. Evidence has demonstrated that AMPK is key in
cardiovascular functioning, metabolism, insulin signaling, reactive
oxygen species regulation and inflammatory processes (33,34).
However, compared with the well-accepted roles of AMPK in
metabolite sensing, the involvement of AMPK in inflammatory
processes remains to be fully elucidated. Previous studies have
demonstrated that attenuation of the activation of NF-κB is
mediated via AMPK activation in various cell lines, including
endothelial cells (35–38), suggesting that AMPK may be the
upstream signaling molecule through which berberine exerts its
anti-inflammatory effects. Concordant with the results of these
previous studies, the findings of the present study indicated that
AMPK activation was associated with the inhibitory effects of
berberine on TNF-α-induced NF-κB activation and the expression of
inflammatory molecules in HAECs.
In conclusion, the results of the present study
suggested that berberine may attenuate the TNF-α-induced expression
of inflammatory molecules by inhibiting NF-κB following AMPK
activation in the HAECs. These results indicate a novel molecular
mechanism underlying the anti-inflammatory effects of berberine in
vascular remodeling processes.
Abbreviations:
|
HAECs
|
human aortic endothelial cells
|
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
MCP-1
|
monocyte chemotactic protein-1
|
References
|
1
|
Chaabane C, Otsuka F, Virmani R and
Bochaton-Piallat ML: Biological responses in stented arteries.
Cardiovasc Res. 99:353–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabbani GH, Butler T, Knight J, Sanyal SC
and Alam K: Randomized controlled trial of berberine sulfate
therapy for diarrhea due to enterotoxigenic Escherichia coli and
Vibrio cholerae. J Infect Dis. 155:979–984. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saha P, Bhattacharjee S, Sarkar A, Manna
A, Majumder S and Chatterjee M: Berberine chloride mediates its
anti-leishmanial activity via differential regulation of the
mitogen activated protein kinase pathway in macrophages. PLoS One.
6:e184672011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE,
Choi NY and You YO: Antimicrobial activity of berberine alone and
in combination with ampicillin or oxacillin against
methicillin-resistant Staphylococcus aureus. J Med Food. 8:454–461.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choo BK and Roh SS: Berberine protects
against esophageal mucosal damage in reflux esophagitis by
suppressing proinflam-matory cytokines. Exp Ther Med. 6:663–670.
2013.PubMed/NCBI
|
|
7
|
Kuo CL, Chi CW and Liu TY: The
anti-inflammatory potential of berberine in vitro and in vivo.
Cancer Lett. 203:127–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahata S, Bharti AC, Shukla S, Tyagi A,
Husain SA and Das BC: Berberine modulates AP-1 activity to suppress
HPV transcription and downstream signaling to induce growth arrest
and apoptosis in cervical cancer cells. Mol Cancer. 10:392011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katiyar SK, Meeran SM, Katiyar N and
Akhtar S: p53 cooperates berberine-induced growth inhibition and
apoptosis of non-small cell human lung cancer cells in vitro and
tumor xenograft growth in vivo. Mol Carcinog. 48:24–37. 2009.
View Article : Google Scholar
|
|
10
|
Leng SH, Lu FE and Xu LJ: Therapeutic
effects of berberine in impaired glucose tolerance rats and its
influence on insulin secretion. Acta Pharmacol Sin. 25:496–502.
2004.PubMed/NCBI
|
|
11
|
Yin J, Hu R, Chen M, Tang J, Li F, Yang Y
and Chen J: Effects of berberine on glucose metabolism in vitro.
Metabolism. 51:1439–1443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong Y, Hui SS, Chan BT and Hou J: Effect
of berberine on catecholamine levels in rats with experimental
cardiac hypertrophy. Life Sci. 72:2499–2507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng XH, Zeng XJ and Li YY: Efficacy and
safety of berberine for congestive heart failure secondary to
ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol.
92:173–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang KW, Ting CT, Yin SC, Chen YT, Lin
SJ, Liao JK and Hsu SL: Berberine suppresses MEK/ERK-dependent
Egr-1 signaling pathway and inhibits vascular smooth muscle cell
regrowth after in vitro mechanical injury. Biochem Pharmacol.
71:806–817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee S, Lim HJ, Park HY, Lee KS, Park JH
and Jang Y: Berberine inhibits rat vascular smooth muscle cell
proliferation and migration in vitro and improves neointima
formation after balloon injury in vivo. Berberine improves
neointima formation in a rat model. Atherosclerosis. 186:29–37.
2006. View Article : Google Scholar
|
|
16
|
Meng S, Wang LS, Huang ZQ, Zhou Q, Sun YG,
Cao JT, Li YG and Wang CQ: Berberine ameliorates inflammation in
patients with acute coronary syndrome following percutaneous
coronary intervention. Clin Exp Pharmacol Physiol. 39:406–411.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone F and Montecucco F: Inflammation
in arterial diseases. IUBMB Life. 67:18–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Korcum AF, Sanlioglu S, Aksu G, Tuncel N
and Erin N: Radiotherapy-induced decreases in substance P levels
may potentiate melanoma growth. Mol Med Rep. 2:319–326.
2009.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Liu SJ, Yin CX, Ding MC, Xia SY, Shen QM
and Wu JD: Berberine suppresses in vitro migration of human aortic
smooth muscle cells through the inhibitions of MMP-2/9, u-PA, AP-1,
and NF-κB. BMB Rep. 47:388–392. 2014. View Article : Google Scholar :
|
|
21
|
Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC
and Ho TY: Berberine suppresses inflammatory agents-induced
interleukin-1beta and tumor necrosis factor-alpha productions via
the inhibition of IkappaB degradation in human lung cells.
Pharmacol Res. 56:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi BH, Ahn IS, Kim YH, Park JW, Lee SY,
Hyun CK and Do MS: Berberine reduces the expression of adipogenic
enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol
Med. 38:599–605. 2006. View Article : Google Scholar
|
|
23
|
Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY,
Shin HJ, Kim WS and Kim JB: Berberine suppresses proinflammatory
responses through AMPK activation in macrophages. Am J Physiol
Endocrinol Metab. 296:E955–E964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charo IF and Taubman MB: Chemokines in the
pathogenesis of vascular disease. Circ Res. 95:858–866. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaneko H, Anzai T, Morisawa M, Kohno T,
Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, et
al: Resveratrol prevents the development of abdominal aortic
aneurysm through attenuation of inflammation, oxidative stress, and
neovascularization. Atherosclerosis. 217:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melgarejo E, Medina MA, Sánchez-Jiménez F
and Urdiales JL: Monocyte chemoattractant protein-1: A key mediator
in inflammatory processes. Int J Biochem Cell Biol. 41:998–1001.
2009. View Article : Google Scholar
|
|
27
|
Egashira K: Molecular mechanisms mediating
inflammation in vascular disease: Special reference to monocyte
chemoattractant protein-1. Hypertension. 41:834–841. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Martin R, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-kappaB and the regulation of
vascular cell function. Aterioscler Thromb Vasc Biol. 20:E83–E88.
2000. View Article : Google Scholar
|
|
29
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tham DM, Martin-McNulty B, Wang YX, Wilson
DW, Vergona R, Sullivan ME, Dole W and Rutledge JC: Angiotensin II
is associated with activation of NF-kappaB-mediated genes and
downregulation of PPARs. Physiol Genomics. 11:21–30. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brasier AR: The nuclear
factor-kappaB-interleukin-6 signalling pathway mediating vascular
inflammation. Cardiovasc Res. 86:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aljada A, Ghanim H, Saadeh R and Dandona
P: Insulin inhibits NFkappaB and MCP-1 expression in human aortic
endothelial cells. J Clin Endocrinol Metab. 86:450–453.
2001.PubMed/NCBI
|
|
33
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fisslthaler B and Fleming I: Activation
and signaling by the AMP-activated protein kinase in endothelial
cells. Circ Res. 105:114–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cacicedo JM, Yagihashi N, Keaney JF Jr,
Ruderman NB and Ido Y: AMPK inhibits fatty acid-induced increases
in NF-kappaB transactivation in cultured human umbilical vein
endothelial cells. Biochem Biophys Res Commun. 324:1204–1209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okayasu T, Tomizawa A, Suzuki K, Manaka K
and Hattori Y: PPARalpha activators upregulate eNOS activity and
inhibit cytokine-induced NF-kappaB activation through AMP-activated
protein kinase activation. Life Sci. 82:884–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hattori Y, Nakano Y, Hattori S, Tomizawa
A, Inukai K and Kasai K: High molecular weight adiponectin
activates AMPK and suppresses cytokine-induced NF-kappaB activation
in vascular endothelial cells. FEBS Lett. 582:1719–1724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hattori Y, Suzuki K, Hattori S and Kasai
K: Metformin inhibits cytokine-induced nuclear factor kappaB
activation via AMP-activated protein kinase activation in vascular
endothelial cells. Hypertension. 47:1183–1188. 2006. View Article : Google Scholar : PubMed/NCBI
|



















