Introduction
It is well known that diabetes-induced renal disease
is a common complication for diabetes patients, leading to
end-stage renal failure (1).
Studies in patients with diabetes have demonstrated that podocyte
loss and depletion represent a few of the early pathogenic
mechanisms of diabetic nephropathy (2,3).
Podocytes are highly specialized cells that localize on the outer
surface of the glomerular basement membrane, and are important in
maintaining the function and structure of the glomerular filtration
barrier (4). Thus, podocyte injury
inevitably results in proteinuria (5), as clinically, glomerular proteinuria
is caused by structural and functional abnormalities in the
glomerular filtration barrier (6).
Furthermore, increased levels of oxidative stress are observed in
diabetes in vivo and in vitro (7,8).
Podocyte injury is a major contributor to the pathogenesis of
diabetic nephropathy via the excessive generation of oxidative
stress (9).
Methylglyoxal (MGO), a reactive dicarbonyl
intermediate, is a potent precursor of advanced glycation
end-products (AGEs) and there is increasing evidence demonstrating
that MGO levels in diabetic patients are elevated (10,11).
MGO is highly cytotoxic and forms stable adducts, and in the plasma
of diabetic patients and diabetic animal tissues its concentration
levels have been observed to be markedly higher (12,13).
MGO is hypothesized to cause podocyte damage, and the MGO-induced
generation of reactive oxygen species may elicit apoptosis
(14,15). Our previous study demonstrated that
MGO-induced oxidative stress may be involved in podocyte apoptosis
in diabetic nephropathy (16).
Medicinal herbs have been administered for hundreds
of years as a folk medicine to treat diabetic complications and
diabetes (17,18). Rhizoma Polygonum cuspidatum
(P. cuspidatum) is the dried rhizome of P. cuspidatum
Sieb. et Zucc. (Polygonaceae). This herb has been administered to
control oral diseases in patients in Korea (19), and has been used as a medicinal
herb to treat numerous diseases and disorders in Asian countries,
including inflammatory diseases and Korea, China and Japan
(20). Additionally, P.
cuspidatum has been widely administered as a medicinal herb for
anti-diabetic effects in traditional Chinese medicine (21).
However, P. cuspidatum exerts unknown effects
on diabetic complications. Therefore, the preventative effect of
the ethanol extract from P. cuspidatum on early renal injury
in streptozotocin (STZ)-induced diabetic rats was investigated
Materials and methods
Preparation of P. cuspidatum extract
(PCE)
Rhizoma P. cuspidatum was commercially
obtained from Jung-dong, Daejeon, Korea in November 2008. The dried
and ground plant material (6.8 kg) was extracted with ethanol (108
liters) by maceration at room temperature for three days, and the
extracts were combined and concentrated in vacuo at 40°C to
provide 580 g lyophilized PCE. A voucher specimen was deposited in
a herbarium at the Korea Institute of Oriental Medicine (KIOM;
Daejeon, Korea).
Induction of diabetic rats and
experimental design
All experiments were conducted according to the
National Institutes of Health (NIH) Guide for the Care and Use of
Laboratory Animals and were approved by the KIOM Institutional
Animal Care and Use Committee (22). Seven-week-old male Sprague-Dawley
rats were maintained in an automatically controlled room
(temperature, ~24°C; humidity, 60%) under conventional lighting.
The rats were injected with STZ (60 mg/kg i.p.; Sigma-Aldrich, St.
Louis, MO, USA). Control rats received a vehicle (0.01 M citrate
buffer, pH 4.5). One week after the STZ injection, a blood sample
(100–200 µl) was obtained from the tail vein. The rats
(blood glucose level >300 mg/dl) were randomly assigned to four
groups (n=8). PCE was dissolved in a vehicle (0.5% w/v
carboxymethyl cellulose solution; Sigma-Aldrich) at a concentration
of 50 mg/ml. Two groups of STZ-induced diabetic rats received a
daily gastric gavage of PCE (100 or 350 mg/kg) and the other groups
were administered the same quantity of vehicle gavage for 16 weeks.
Rat body weights and blood glucose levels were monitored one a
week.
Metabolic and physical analysis
When the rats reached 25 weeks of age, fasting blood
glucose was measured using an automated Hitachi912 chemistry
analyzer (Hitachi, Ltd., Tokyo, Japan). A 24-h urine sample (1 ml)
was obtained from the rats using a metabolic cage and the
proteinuria levels were measured using a competitive enzyme-linked
immunosorbent assay (ELISA) kit (UP ELISA kits; NeoScientific,
Ltd., Cambridge, MA, USA).
Quantification of 8-hydroxydeoxyguanosine
(8-OHdG) in the urine and MGO in the renal cortex
To investigate induced oxidative stress by MGO in
the diabetic rats, urine levels of 8-OHdG were measured using a
competitive ELISA kit (JaICA, Shizuoka, Japan). MGO levels in the
renal cortex were analyzed using an MGO adduct (Cosmo Bio Co.,
Tokyo, Japan), as previously described (16).
Immunofluorescent staining and
immunohistochemistry
Immunohistochemistry was performed as previously
described (15). Briefly, at the
end of the experimental period, the rats were anesthetized with
pentobarbital sodium (60 mg/kg, i.p; Hanlim Pharmaceutical Company,
Seoul, Korea) and sacrificed. Following removal of the kidneys,
part was flash frozen in liquid nitrogen and stored at −80°C until
analysis and another part was fixed (4% paraformaldehyde) for
histological analysis. The renal sections (4 µm thick) were
deparaffinized and hydrated by sequential immersion in xylene and
graded alcohol solutions (Sigma-Aldrich) and then treated with
normal serum from the same species that contained the secondary
antibody to block non-specific staining (Abcam, Cambridge, MA,
USA). The slides were incubated overnight at 4°C with the primary
antibody. The primary antibodies were anti-mouse monoclononal MGO
(1:500; cat. no. NOF-N213430-EX; CosmoBio Co, Ltd., Toyko, Japan),
anti-rabbit polyclonal synaptopodin and anti-mouse monoclonal
8-OHdG, (1:500; cat. nos. sc-50459 and sc-393870, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-rabbit polyclonal WT-1
(1:500; cat. no. AF-5729; R&D Systems, Inc., Minneapolis, MN,
USA) and anti-rabbit polyclonal cleaved caspase-3 (1:500; cat. no.
9661; Cell Signaling Technology, Inc., Danvers, MA, USA). To detect
8-OHdG and cleaved caspase-3, sections were incubated with an
Envision™ kit (Dako North America, Inc., Carpinteria, CA, USA) and
visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB;
Vector Laboratories, Inc., Burlingame, CA, USA). The MGO specimens
were visualized using a red alkaline phosphatase substrate kit
(Vector Laboratories, Inc.). To detect Wilms Tumor 1 (WT-1; a known
podocyte marker) (23,24) and synaptopodin, the sections were
incubated with rhodamine-labeled anti-rabbit immunoglobulin (Ig)G
(Santa Cruz Biotechnology, Inc.) and fluorescein
isothiocyanate-labeled anti-mouse IgG (Santa Cruz Biotechnology,
Inc.), respectively, and visualized by fluorescence microscopy
(BX51; Olympus Corporation, Tokyo, Japan). Immunohistochemical
negative controls were performed by incubating the section with rat
normal serum. For morphometric analysis, the positive cell number
and positive staining per individual glomerulus in a total of 50
glomeruli were determined using ImageJ software (v1.46r; NIH,
Bethesda, MD, USA).
Double labeling technique for terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and
synaptopodin expression levels
TUNEL staining was performed using a DeadEnd™
Colorimetric TUNEL system kit (Promega Corporation, Madison, WI,
USA) according to the manufacturer's instructions. Apoptotic cells
were detected with a brown colored solution containing peroxidase
substrate and the stable chromogen, DAB. Labeling with
anti-synaptopodin (Santa Cruz Biotechnnology, Inc.) was performed
on the same section with secondary detection using an alkaline
phosphatase-conjugated anti-mouse IgG and a red alkaline
phosphatase substrate kit (Vector Laboratories, Inc.). To prevent a
cross-reaction with the dual labeling procedure, the slide was
incubated with normal rat serum for 1 h at 37°C following TUNEL
staining. Counterstaining was performed with hematoxylin (Vector
Laboratories, Inc.). For morphometric analysis, the positive signal
for synaptopodin was measured using ImageJ software and the cells
that were dually labeled for TUNEL and synaptopodin were counted
under a microscope (BX51; Olympus Corporation).
Western blot analysis
Renal cortex tissues were homogenized in solutions
containing 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid
(Sigma-Aldrich), 0.1 mM phenylmethylsulfonyl fluoride
(Sigma-Aldrich) and 20 mM potassium phosphate buffer (pH 7.6;
Sigma-Aldrich). Proteins (20 µg) were subjected to
immunoblotting onto polyvinylidene fluoride (PVDF) membranes
(Bio-Rad Laboratories, Inc.) and the PVDF membranes were incubated
with the following primary antibodies: Cleaved caspase-3 (1:1,500)
and β-actin (1:3,000; Sigma-Aldrich) at 4°C overnight. The bound
horseradish peroxidase-conjugated secondary antibody was detected
using an enhanced chemiluminescence detection system (Intron
Biotechnology, Inc., Seoul, Korea). The protein expression levels
were determined by analyzing the signals captured on the PVDF
membranes with an image analyzer (Las-3000; Fuji Photo Film Co.,
Ltd., Tokyo, Japan).
MGO-modified bovine serum albumin (BSA)
formation assay
An MGO-BSA assay was performed with certain
modifications, as previously described (25). BSA (10 mg/ml) was incubated with
MGO (5 mM) in the presence or absence of emodin (Sigma-Aldrich) in
phosphate-buffered saline (100 mM; pH 7.4; Biosesang, Inc.,
Sungnam, Korea) at 37°C for seven days. Emodin was diluted with 1%
dimethyl sulfoxide (Sigma-Aldrich) and aminoguanidine (AG;
Sigma-Aldrich) served as a positive control. After seven days, the
fluorescence of the sample was measured using a Synergy™ HT, Gen5™
fluorescence microplate reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA) at emission and excitation wavelengths of 450
and 360 nm, respectively. All measurements were performed in
triplicate.
MGO scavenging assay
A fluorogenic assay for MGO was performed as
previously described (26). A
potential fluorogenic probe, 4,5-diaminofluorescein (DAF-2), which
reacts with MGO, exhibits a small shift in the fluorescence
wavelength and a marked increase in fluorescence intensity for the
probe-MGO adduct. DAF-2 [100 µl (100 µM);
Sigma-Aldrich) was incubated with 50 µl MGO (2 mM) in 10 mM
phosphate-buffered saline (pH 7.4) at 37°C and the fluorescence
spectra values were obtained at 0, 1 and 2 h. The different
florescence characteristic of the isomeric mixture of the MGO-DAF-2
adduct developed and maximized within 1 h. The excitation and
emission wavelengths were 435 and 509 nm, respectively. Emodin and
AG were serially diluted with the MGO-DAF-2 mixture and each final
concentration was 5 mg/ml in phosphate-buffered saline (pH 7.4). To
accommodate the minimum volume requirement for the microplate used
in the current study, 100 µl DAF-2 solution, 50 µl
MGO solution, and 50 µl emodin and AG solution were used,
respectively. AG served as a positive control. The fluorescence of
the sample was measured using a Gemini XPS Microplate Reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean and analyzed using one-way analysis of variance followed
by Tukey's multiple comparison test or an unpaired Student's
t-test. Prism 6.0 software (Graph pad, San Diego, CA, USA) was used
to perform statistical analyses.
Results
Treatment with PCE reduces
proteinuria
At 25 weeks of age, the body weights were
significantly decreased and the blood glucose levels were
significantly increased in the STZ-induced diabetic rats when
compared with the normal control rats (P<0.01). However, no
statistically significant differences in the body weights and blood
glucose levels between the STZ-induced diabetic rats and the
PCE-treated diabetic rats were observed. The level of proteinuria
was significantly increased in the STZ-induced diabetic rats
compared with the normal control rats (P<0.01). However, PCE
treatment signifi-cantly decreased proteinuria compared with the
vehicle-treated diabetic rats in a dose-dependent manner
(P<0.01; Table I).
 | Table IMetabolic and physical parameters of
the rats. |
Table I
Metabolic and physical parameters of
the rats.
| Parameter | NOR | DM | PCE-100 | PCE-350 |
|---|
| Blood glucose,
mmol/l | 9.00±0.60 | 32.62±2.41a | 29.46±3.22 | 26.34±4.91 |
| Body weight, g | 475.9±10.70 | 208.5±12.10a | 205.5±23.10 | 206.0±16.40 |
| Proteinuria,
mg/day | 7.42±2.14 | 16.37±3.89a | 13.67±2.56 | 11.54±3.16b |
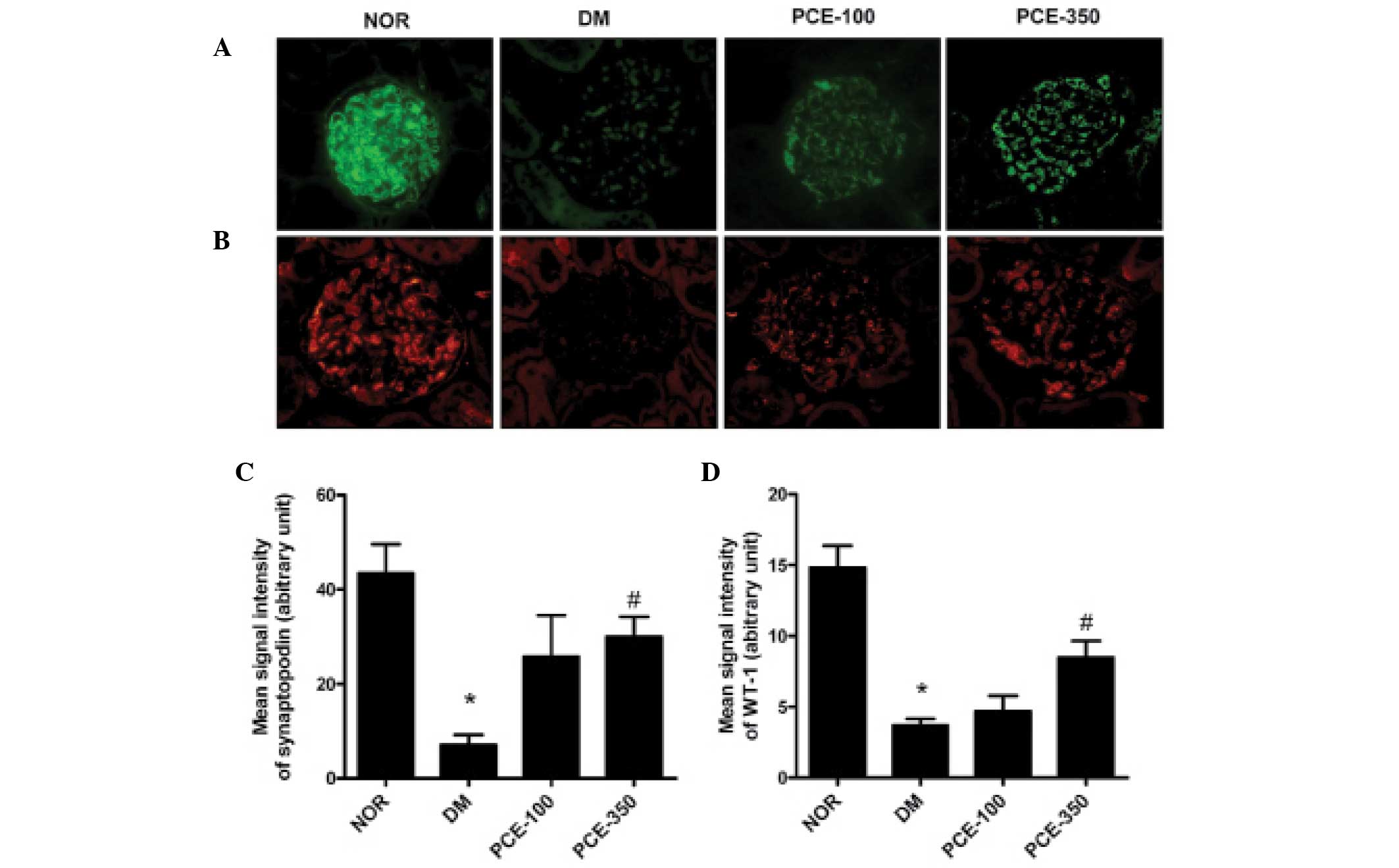
Treatment with PCE ameliorates podocyte
loss in the renal glomerulus
The podocyte content was determined by counting the
cells that were stained with synaptopodin and WT-1, the podocyte
marker (Fig. 1A and B). The number
of synaptopodin- and WT-1-positive cells in the STZ-induced
diabetic rat group was reduced compared with that of the normal
control rats (P<0.01). Treatment with PCE markedly increased the
podocyte content in the renal glomeruli in a dose-dependent manner
(P<0.01; Fig. 1B and C).
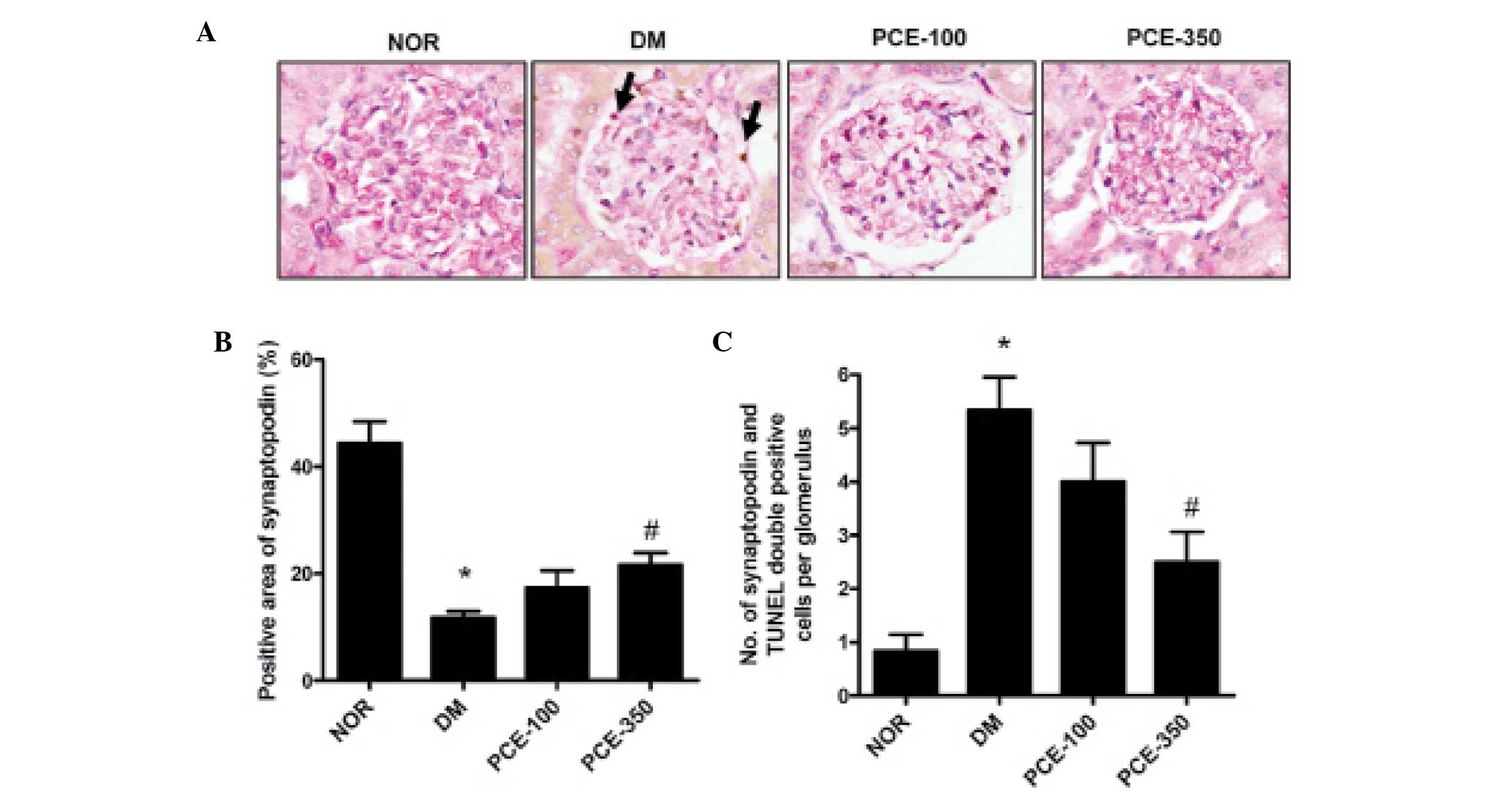
Treatment with PCE inhibits podocyte
apoptosis in the renal glomerulus
To determine the anti-apoptotic effect of PCE, the
double labeling technique for TUNEL and synaptopodin was performed.
A reduced number of synaptopodin-labeled cells was observed in the
glomeruli of STZ-induced diabetic rats compared with the normal
control rats (P<0.01). However, in the PCE-treated diabetic
rats, the level of synaptopodin-positive cells had increased
compared with the STZ-induced diabetic rats (P<0.01).
TUNEL-positive cells co-localized to the region of synaptopodin
expression in the double labeling-stained kidney section, which is
consistent with the apoptosis of podocytes in the STZ-induced
diabetic rat glomeruli (Fig. 2A).
In the STZ-induced diabetic rats, the number of TUNEL-positive
cells was markedly increased compared with that observed in the
normal control rats (P<0.01). PCE treatment was effective in
reducing the apoptotic signal in STZ-induced diabetic rats
(Fig. 2B and C).
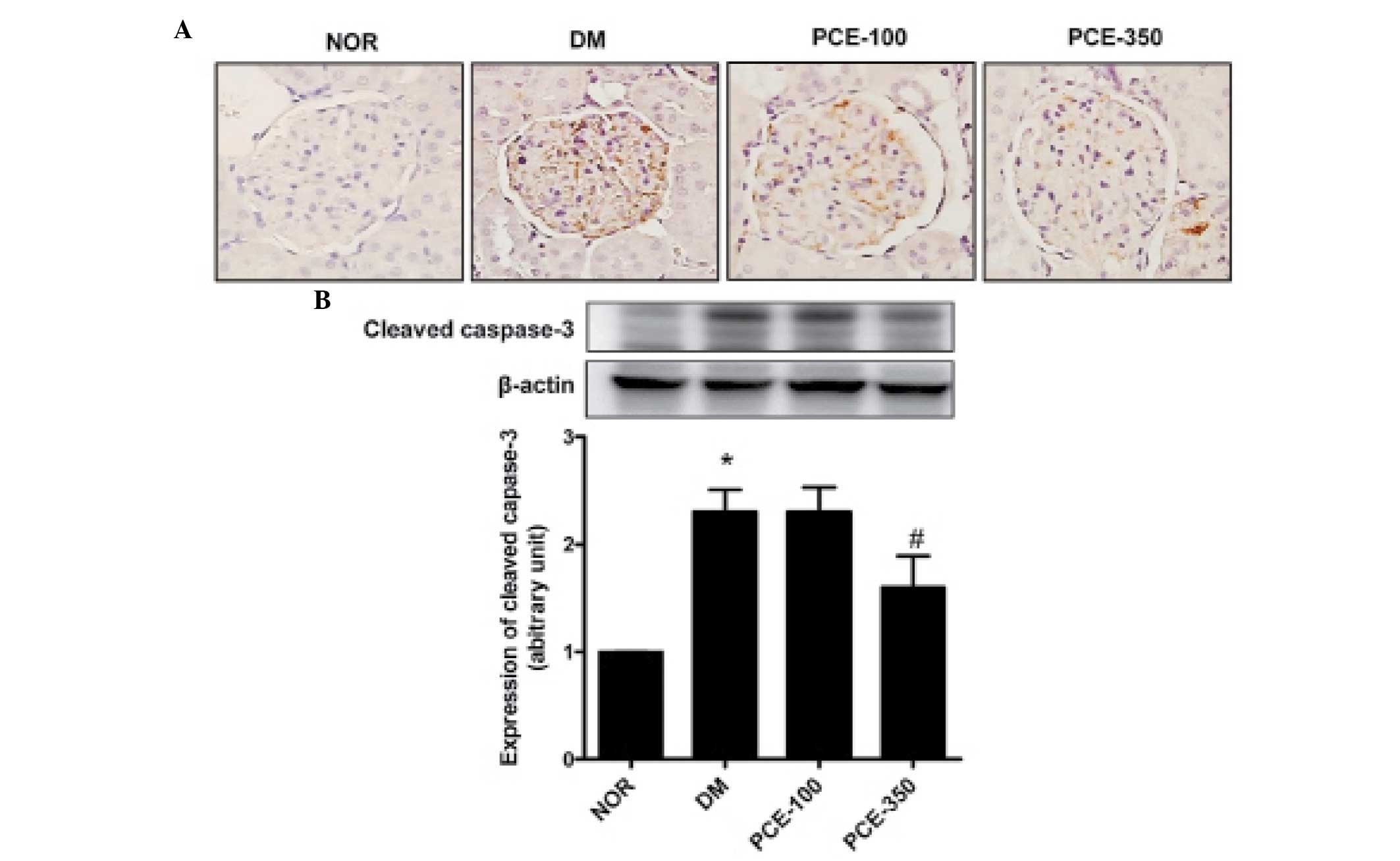
Treatment with PCE decreases the
expression of caspase-3 in renal tissue
To investigate the effect of PCE on cleaved
caspase-3 expression in the renal cortex, an immunoassay was
performed. The immunohistochemical staining for cleaved caspase-3
demonstrated marked cleaved caspase-3 expression levels in the
renal glomeruli of STZ-induced diabetic rats. The expression levels
of cleaved caspase-3 were dose-dependently restored in the
PCE-treated diabetic rats (P<0.01; Fig. 3A). To validate the
immunohistochemistry results, western blot analysis was performed.
STZ-induced diabetic rats demonstrated significantly higher levels
of caspase-3 compared with those of the normal control rats;
furthermore, the increased caspase-3 expression levels in the renal
cortex of STZ-induced diabetic rats were reduced by treatment with
PCE (Fig. 3B). These results
indicate that PCE decreased the cleaved caspase-3 expression levels
in the renal cortex of diabetic nephropathy by exerting an
anti-apoptotic effect.
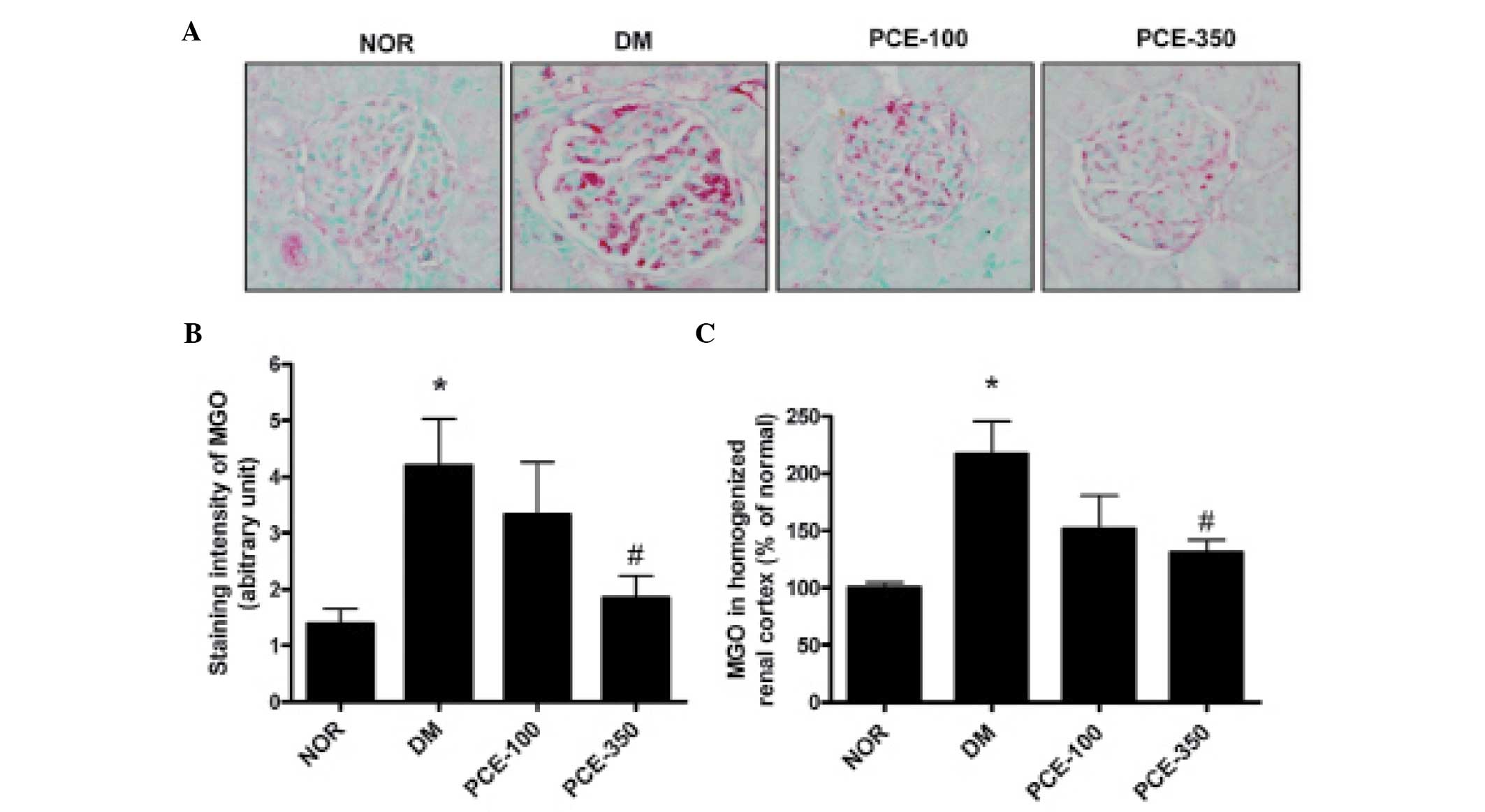
PCE treatment attenuates the expression
levels of MGO in the glomeruli and the renal cortex
The renal glomeruli of STZ-induced diabetic rats
exhibited significant staining for MGO when compared with the
normal control rats (P<0.01). This increase in MGO staining
intensity was reduced in the PCE-treated diabetic rats (Fig. 4A and B). Additionally, in the ELISA
analysis, the MGO levels in the homogenized renal cortex were also
markedly increased in the STZ-induced diabetic rats. PCE-350 was
found to significantly reduce the diabetes-induced increases in MGO
level (Fig. 4C).
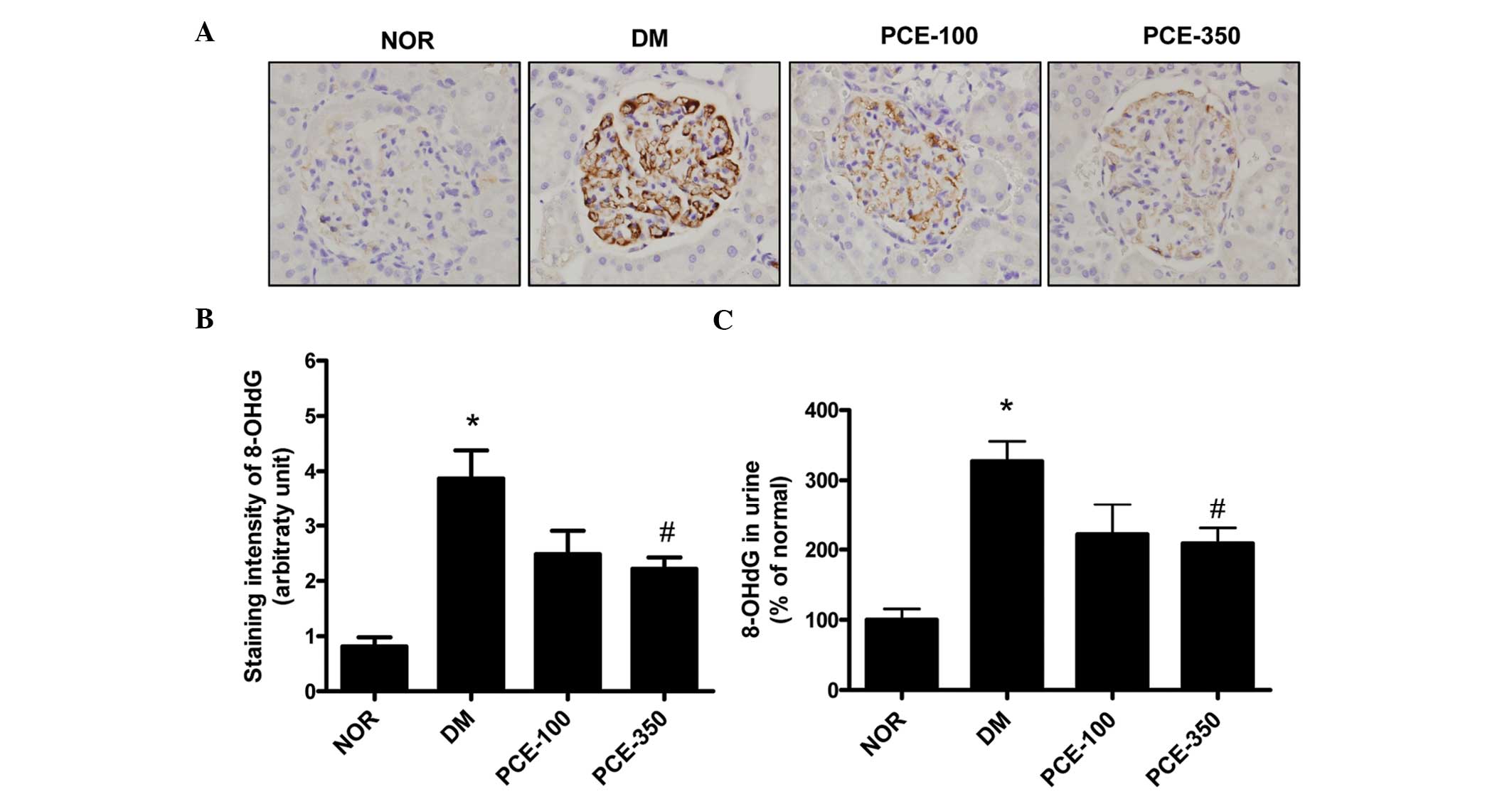
PCE treatment prevents oxidative
stress
Immunohistochemical staining of 8-OHdG expression in
the glomeruli demonstrated a significant increase in the
STZ-induced diabetic rats compared with the normal control rats
(P<0.01). The 8-OHdG expression level was inhibited by PCE-350
treatment (Fig. 5A and B). In
addition, the 8-OHdG levels in the urine were markedly increased in
the STZ-induced diabetic rats (P<0.01). However, PCE attenuated
these diabetes-induced increases in urine 8-OHdG levels (Fig. 5C).
PCE has an effect on MGO scavenging and
inhibition of MGO-BSA formation
PCE contains various flavones as its major chemical
constituents, including resveratrol and emodin (27,28).
To identify the biological activity of these compounds in PCE
against MGO-mediated glycation, MGO scavenging and MGO-BSA
formation assays were performed. As shown in Table II, emodin significantly inhibited
the probe-MGO adduct when compared with AG, a known positive
control. The half maximal inhibitory concentrations
(IC50) for emodin and AG were 0.41±1.25 and 1.05±2.07
mM, respectively. In addition, emodin significantly inhibited the
formation of MGO-derived AGEs and was demonstrated to be more
effective than AG, with IC50 values of 0.08±0.01 and
7.12±0.73 mM, for emodin and AG, respectively (Table III).
 | Table IIMethylglyoxal scavenging activity of
emodin. |
Table II
Methylglyoxal scavenging activity of
emodin.
| Compound | % inhibition |
IC50 |
|---|
| Emodin,
µg/ml | | |
| 50 | 83.29±12.23 | 112.54±6.01
µg/ml (0.41±2.07 mM) |
| 100 | 52.08±3.71 |
| 250 | 45.82±2.09 |
| Aminoguanidine,
µg/ml | | |
| 100 | 71.52±9.64 | 116.52±8.49
µg/ml (1.05±1.25 mM) |
| 250 | 38.23±9.85 |
| 500 | 27.49±5.99 |
 | Table IIIInhibitory effect of emodin on
methylglyoxal-derived advanced glycation end-product formation. |
Table III
Inhibitory effect of emodin on
methylglyoxal-derived advanced glycation end-product formation.
| Compound | % inhibition |
IC50 |
|---|
| Emodin,
µg/ml | | 23.81±2.28
µg/ml |
| 5 | 93.22±2.01 | 0.08±0.01 mM |
| 10 | 73.49±1.90 | |
| 25 | 48.44±2.84 | |
| Aminoguanidine,
µg/ml | | 787.65±81.76
µg/ml |
| 250 | 97.6±9.53 | 7.12±0.73 mM |
| 500 | 70.6±5.73 | |
| 1,000 | 35.0±1.36 | |
Discussion
The current study demonstrates that PCE treatment
significantly improved certain biochemical markers of renal
function, including proteinuria in STZ-induced diabetic rats. The
present study revealed that PCE exerts a preventative effect on
podocyte loss and apoptosis in the early stages of injury in
STZ-induced diabetic rats. In STZ-induced diabetic rats, podocyte
apoptosis was associated with the upregulation of cleaved caspase-3
overexpression, which was partially restored to normal levels by
PCE treatment. In addition, PCE ameliorated the MGO level,
resulting in inhibition of the cytotoxic formation of stable
adducts and 8-OHdG expression (a known oxidative damage marker) in
STZ-induced diabetic rats.
Severe hyperglycemia and podocyte loss is present at
an early stage in STZ-induced diabetic animal models (29). Furthermore, podocyte damage
correlates with worsening proteinuria and albuminuria (3). Previous studies have demonstrated
that podocyte apoptosis is significant in the progression of
glomerulosclerosis and proteinuria in diabetic nephropathy
(30,31). Therefore, there is an urgent
requirement for the development of therapeutic agents for
proteinuria and renal diseases, which act via direct podocyte
targeting (32). Experimental and
clinical studies have demonstrated podocyte loss, due to apoptosis,
and that depletion leads to proteinuria in diabetic nephropathy
(5,33). Based on these results, it was
hypothesized in the current study that PCE may preserve the
podocyte cell number, ameliorate podocyte loss and reduce
proteinuria. These protective effects were partly attributed to the
anti-apoptotic action of PCE.
The effect of PCE on the levels of a key downstream
effector of apoptosis, cleaved caspase-3 was investigated in the
present study. Caspase-3 is frequently activated by cell death
processes, such as apoptosis and initiates apoptotic DNA
fragmentation by proteolytic processing (34). In the current study, cleaved
capase-3 expression levels were increased in the kidneys of
STZ-induced diabetic rats. However, PCE treatment markedly reduced
cleaved caspase-3 overexpression. The inhibitory effect of PCE on
podocyte loss and apoptosis correlated with the reduction in
caspase-3 overexpression in the renal glomerular of STZ-induced
diabetic rats. Thus, PCE may inhibit podocyte apoptosis in the
kidneys of STZ-induced diabetic rats, in part, by reducing cleavage
of caspase-3.
In addition, significantly increased MGO and 8-OHdG
levels were observed in STZ-i nduced diabetic rats. The formation
of MGO is enhanced under diabetic conditions (11,35).
MGO is a known precursor of AGEs, and MGO-derived AGE levels have
been found to be of AGEs, and MGO-derived AGE levels have been
found to be increased in diabetic renal tissue (13,16).
Furthermore, loss and depletion of glomerular cells as a result of
apoptosis occurs in STZ-induced diabetic rats (36). Additionally, our previous study
demonstrated that MGO is involved in podocyte loss and the
expression of 8-OHdG in podocytes of the diabetic kidney (16). In addition, the formation of
8-OHdG-adducted bases is associated with oxidative stress (37). When DNA is damaged by oxidative
stress, cells elicit either apoptosis or cell cycle delay with
apoptosis (38). MGO increases the
intracellular oxidative stress level and enhances MGO accumulation,
which is responsible for apoptotic damage (39). Thus, the results of the present
study demonstrated that MGO and 8-OHdG levels were increased in the
kidney of STZ-induced diabetic rats, which was subsequently
attenuated by PCE treatment.
P. cuspidatum contains flavones, such as
resveratrol and emodin as the major chemical constituents (27,28).
Resveratrol is a natural antioxidant polyphenol that is present in
P. cuspidatum, which exerts various biological functions,
including anti-inflammatory and antioxidant activities (40,41).
In addition to antioxidant effects, resveratrol possesses
additional properties that ameliorate diabetes or high
glucose-induced kidney injury by activating 5′AMP-activated protein
kinase or Sirtuin 1. Ding et al (42) reported that resveratrol treatment
attenuates renal hypertrophy and urinary albumin excretion in the
early stages of diabetes in STZ-induced diabetic rats, without
affecting the blood glucose levels. In addition, resveratrol
scavenges MGO and exerts inhibitory effects on AGE formation
(25). Emodin performs various
biological activities, such as antioxidant, anti-diabetic and
anti-inflammatory activities (43–45).
In particular, emodin exerts the protective effect of suppressing
fibronectin and transforming growth factor-β1 overexpression by
inhibiting nuclear factor-κB activation in diabetic nephropathy
(46). Recently, emodin was
demonstrated to affect antioxidant capacity and anti-apoptosis
activity in hepatic cells, by directly affecting the mitochondria
and acting against cytotoxicity (45). Notably, the present study revealed
that emodin is a potent MGO scavenger and inhibited MGO-derived AGE
formation, and its efficacy was markedly stronger than that of AG,
a particularly well-known AGE inhibitor. These findings indicate
that emodin and resveratrol are biologically active compounds of
PCE, and the biological activities of PCE were attributed to the
antioxidant and carbonyl scavenging capacity of these active
ingredients. Although resveratrol and emodin are two major active
chemicals of PCE, the primary active compound of PCE remains
unclear. However, it is likely that treatment with PCE is effective
against diabetic nephropathy, due to inhibition of apoptotic
activity, which would promote renal podocyte loss, to the
scavenging of the cytotoxic levels of MGO and oxidative stress for
example.
In conclusion, these findings indicate that podocyte
loss and proteinuria in STZ-induced diabetic rats were ameliorated
by treatment with PCE, which was in part achieved by inhibiting
podocyte apoptosis and cleaved caspase-3 expression, and by
restoring the balance of MGO and oxidative stress. Therefore, PCE
may serve as a valuable therapeutic strategy for the treatment of
early diabetic nephropathy.
Acknowledgments
The present study was supported by grants from the
KIOM (Daejeon, Korea: grant no. K14040).
References
|
1
|
White KE and Bilous RW: Type 2 diabetic
patients with nephropathy show structural-functional relationships
that are similar to type 1 disease. J Am Soc Nephrol. 11:1667–1673.
2000.PubMed/NCBI
|
|
2
|
Steffes MW, Schmidt D, McCrery R and
Basgen JM; International Diabetic Nephropathy Study Group:
Glomerular cell number in normal subjects and in type 1 diabetic
patients. Kidney Int. 59:2104–2113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar
|
|
4
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PloS one. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Chen B, Hou XH, Guan GJ, Liu G,
Liu HY and Li XG: Effects of mycophenolate mofetil, valsartan and
their combined therapy on preventing podocyte loss in early stage
of diabetic nephropathy in rats. Chin med J. 120:988–995.
2007.PubMed/NCBI
|
|
6
|
Estacio RO and Schrier RW: Diabetic
nephropathy: Pathogenesis, diagnosis and prevention of progression.
Adv Intern Med. 46:359–408. 2001.
|
|
7
|
Ha H, Kim C, Son Y, Chung MH and Kim KH:
DNA damage in the kidneys of diabetic rats exhibiting
microalbuminuria. Free Radic Biol Med. 16:271–274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakimoto M, Inoguchi T, Sonta T, Yu HY,
Imamura M, Etoh T, Hashimoto T and Nawata H: Accumulation of
8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in
kidney of diabetic rats. Diabetes. 51:1588–1595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marshall CB, Pippin JW, Krofft RD and
Shankland SJ: Puromycin aminonucleoside induces oxidant-dependent
DNA damage in podocytes in vitro and in vivo. Kidney Int.
70:1962–1973. 2006.PubMed/NCBI
|
|
10
|
Yamada H, Miyata S, Igaki N, Yatabe H,
Miyauchi Y, Ohara T, Sakai M, Shoda H, Oimomi M and Kasuga M:
Increase in 3-deoxyglucosone levels in diabetic rat plasma.
Specific in vivo determination of intermediate in advanced Maillard
reaction. J Biol Chem. 269:20275–20280. 1994.PubMed/NCBI
|
|
11
|
McLellan AC, Thornalley PJ, Benn J and
Sonksen PH: Glyoxalase system in clinical diabetes mellitus and
correlation with diabetic complications. Clin Sci (Lond). 87:21–29.
1994. View Article : Google Scholar
|
|
12
|
Lapolla A, Flamini R, Dalla Vedova A,
Senesi A, Reitano R, Fedele D, Basso E, Seraglia R and Traldi P:
Glyoxal and methylglyoxal levels in diabetic patients: Quantitative
determination by a new GC/MS method. Clin Chem Lab Med.
41:1166–1173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Randell EW, Vasdev S and Gill V:
Measurement of methylg-lyoxal in rat tissues by electrospray
ionization mass spectrometry and liquid chromatography. J Pharmacol
Toxicol Methods. 51:153–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amicarelli F, Colafarina S, Cattani F,
Cimini A, Di Ilio C, Ceru MP and Miranda M: Scavenging system
efficiency is crucial for cell resistance to ROS-mediated
methylglyoxal injury. Free Radic Biol Med. 35:856–871. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedchenko VK, Chetyrkin SV, Chuang P, Ham
AJ, Saleem MA, Mathieson PW, Hudson BG and Voziyan PA: Mechanism of
perturbation of integrin-mediated cell-matrix interactions by
reactive carbonyl compounds and its implication for pathogenesis of
diabetic nephropathy. Diabetes. 54:2952–2960. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim J, Sohn E, Kim CS and Kim JS: Renal
podocyte apoptosis in Zucker diabetic fatty rats: Involvement of
methylglyoxal-induced oxidative DNA damage. J Comp Pathol.
144:41–47. 2011. View Article : Google Scholar
|
|
17
|
Alarcon-Aguilara FJ, Roman-Ramos R,
Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC and
Flores-Saenz JL: Study of the anti-hyperglycemic effect of plants
used as antidiabetics. J Ethnopharmacol. 61:101–110. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li WL, Zheng HC, Bukuru J and De Kimpe N:
Natural medicines used in the traditional Chinese medical system
for therapy of diabetes mellitus. J Ethnopharmacol. 92:1–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ban SH, Kwon YR, Pandit S, Lee YS, Yi HK
and Jeon JG: Effects of a bio-assay guided fraction from Polygonum
cuspidatum root on the viability, acid production and
glucosyltranferase of mutans streptococci. Fitoterapia. 81:30–34.
2010. View Article : Google Scholar
|
|
20
|
Bralley EE, Greenspan P, Hargrove JL,
Wicker L and Hartle DK: Topical anti-inflammatory activity of
Polygonum cuspidatum extract in the TPA model of mouse ear
inflammation. J Inflamm (Lond). 5:12008. View Article : Google Scholar
|
|
21
|
Zhang H, Li C, Kwok ST, Zhang QW and Chan
SW: A review of the pharmacological effects of the dried root of
(hu zhang) and its constituents. Evid Based Complement Alternat
Med. 2013(208349)2013.
|
|
22
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. The National
Academies Press; Washington, DC, USA: 2011
|
|
23
|
Mundel P, Reiser J and Kriz W: Induction
of differentiation in cultured rat and human podocytes. J Am Soc
Nephrol. 8:697–705. 1997.PubMed/NCBI
|
|
24
|
Schiffer M, Mundel P, Shaw AS and
Böttinger EP: A novel role for the adaptor molecule CD2-associated
protein in transforming growth factor-beta-induced apoptosis. J
Biol Chem. 279:37004–37012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang D, Zhu JX, Wu AG, Xu YH, Duan TT,
Zheng ZG, Wang RS, Li D and Zhu Q: Pre-column incubation followed
by fast liquid chromatography analysis for rapid screening of
natural methylglyoxal scavengers directly from herbal medicines:
Case study of Polygonum cuspidatum. J chromatogr A. 1286:102–110.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shaheen F, Shmygol A, Rabbani N and
Thornalley PJ: A fluorogenic assay for methylglyoxal. Bioche Soc
Trans. 42:548–555. 2014. View Article : Google Scholar
|
|
27
|
Pan M and Wang X: Chemical constituents
and pharmacological action of Polygonum cuspidatum Sieb. Et Zucc
Zhong Yao Cai. 23:56–58. 2000.
|
|
28
|
Qian G, Leung SY, Lu G and Leung KS:
Differentiation of rhizoma et radix polygoni cuspidati from closely
related herbs by HPLC fingerprinting. Chem Pharm Bull (Tokyo).
54:1179–1186. 2006. View Article : Google Scholar
|
|
29
|
Siu B, Saha J, Smoyer WE, Sullivan KA and
Brosius FC III: Reduction in podocyte density as a pathologic
feature in early diabetic nephropathy in rodents: Prevention by
lipoic acid treatment. BMC nephrol. 7:62006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riedl E, Pfister F, Braunagel M,
Brinkkötter P, Sternik P, Deinzer M, Bakker SJ, Henning RH, van den
Born J, Krämer BK, et al: Carnosine prevents apoptosis of
glomerular cells and podocyte loss in STZ diabetic rats. Cell
Physiol Biochem. 28:279–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sohn E, Kim J, Kim CS, Kim YS, Jang DS and
Kim JS: Extract of the aerial parts of Aster koraiensis reduced
development of diabetic nephropathy via anti-apoptosis of podocytes
in streptozotocin-induced diabetic rats. Biochem Biophys Res
Commun. 391:733–738. 2010. View Article : Google Scholar
|
|
32
|
Reiser J, Gupta V and Kistler AD: Toward
the development of podocyte-specific drugs. Kidney Int. 77:662–668.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meyer TW, Bennett PH and Nelson RG:
Podocyte number predicts long-term urinary albumin excretion in
Pima Indians with Type II diabetes and microalbuminuria.
Diabetologia. 42:1341–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar
|
|
35
|
Oya T, Hattori N, Mizuno Y, Miyata S,
Maeda S, Osawa T and Uchida K: Methylglyoxal modification of
protein. Chemical and immunochemical characterization of
methylglyoxal-arginine adducts. J Biol Chem. 274:18492–18502. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pesce C, Menini S, Pricci F, Favre A, Leto
G, DiMario U and Pugliese G: Glomerular cell replication and cell
loss through apoptosis in experimental diabetes mellitus. Nephron.
90:484–488. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li CS, Wu KY, Chang-Chien GP and Chou CC:
Analysis of oxidative DNA damage 8-hydroxy-2′-deoxyguanosine as a
biomarker of exposures to persistent pollutants for marine mammals.
Environ Sci Technol. 39:2455–2460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zgheib O, Huyen Y, DiTullio RA Jr, Snyder
A, Venere M, Stavridi ES and Halazonetis TD: ATM signaling and
53BP1. Radiother Oncol. 76:119–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim OS, Kim J, Kim CS, Kim NH and Kim JS:
KIOM-79 prevents methyglyoxal-induced retinal pericyte apoptosis in
vitro and in vivo. J Ethnophamacol. 129:285–292. 2010. View Article : Google Scholar
|
|
40
|
Ikizler M, Ovali C, Dernek S, Erkasap N,
Sevin B, Kaygisiz Z and Kural T: Protective effects of resveratrol
in ischemia-reperfusion injury of skeletal muscle: A clinically
relevant animal model for lower extremity ischemia. Chin J Physiol.
49:204–209. 2006.PubMed/NCBI
|
|
41
|
Kong LD, Yang C and Qiu X: Effects of
processing on antioxidation of radix et rhizoma rhei and rhizoma
polygoni cuspidati. Zhongguo Zhong Yao Za Zhi. 26:388–391. 2001.In
Chinese.
|
|
42
|
Ding DF, You N, Wu XM, Xu JR, Hu AP, Ye
XL, Zhu Q, Jiang XQ, Miao H, Liu C and Lu YB: Resveratrol
attenuates renal hypertrophy in early-stage diabetes by activating
AMPK. Am J Nephrol. 31:363–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Y, Jeong YT, Li X, Kim MJ, Park PH,
Hwang SL, Son JK and Chang HW: Emodin isolated from polygoni
cuspidati radix inhibits tnf-alpha and il-6 release by blockading
nf-kappab and map kinase pathways in mast cells stimulated with PMA
Plus A23187. Biomol Thera. 21:435–441. 2013. View Article : Google Scholar
|
|
44
|
Yang J, Zeng Z, Wu T, Yang Z, Liu B and
Lan T: Emodin attenuates high glucose-induced TGF-β1 and
fibronectin expression in mesangial cells through inhibition of
NF-κB pathway. Exp Cell Res. 319:3182–3189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui YT, Liu B, Xie J, Xu P, Habte-Tsion HM
and Zhang YY: The effect of emodin on cytotoxicity, apoptosis and
antioxidant capacity in the hepatic cells of grass carp
(Ctenopharyngodon idellus). Fish Shellfish Immunol. 38:74–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Liu W, Wang Q, Liu P, Deng Y, Lan T,
Zhang X, Qiu B, Ning H and Huang H: Emodin suppresses cell
proliferation and fibronectin expression via p38MAPK pathway in rat
mesangial cells cultured under high glucose. Mol Cell Endocrinol.
307:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|