Introduction
Hepatocellular carcinoma (HCC) is the most common
type of cancer in Southeast Asia and Southern Africa, and typically
originates from hepatitis B or C virus-associated liver cirrhosis
(1,2). The incidence of HCC in the US and
Europe is also increasing (3,4).
Chemotherapy is one of the most extensively used forms of
anticancer treatment in China at present, alongside surgery and
radiotherapy (5), however, due to
the toxicity and side effects associated with currently available
chemotherapeutic agents, improvements in treatment are required.
Traditional Chinese medicines have long been consumed to prevent
and treat various types of cancer, and several active compounds of
Chinese medicinal herbs have been assessed for their anticancer
effects (6,7).
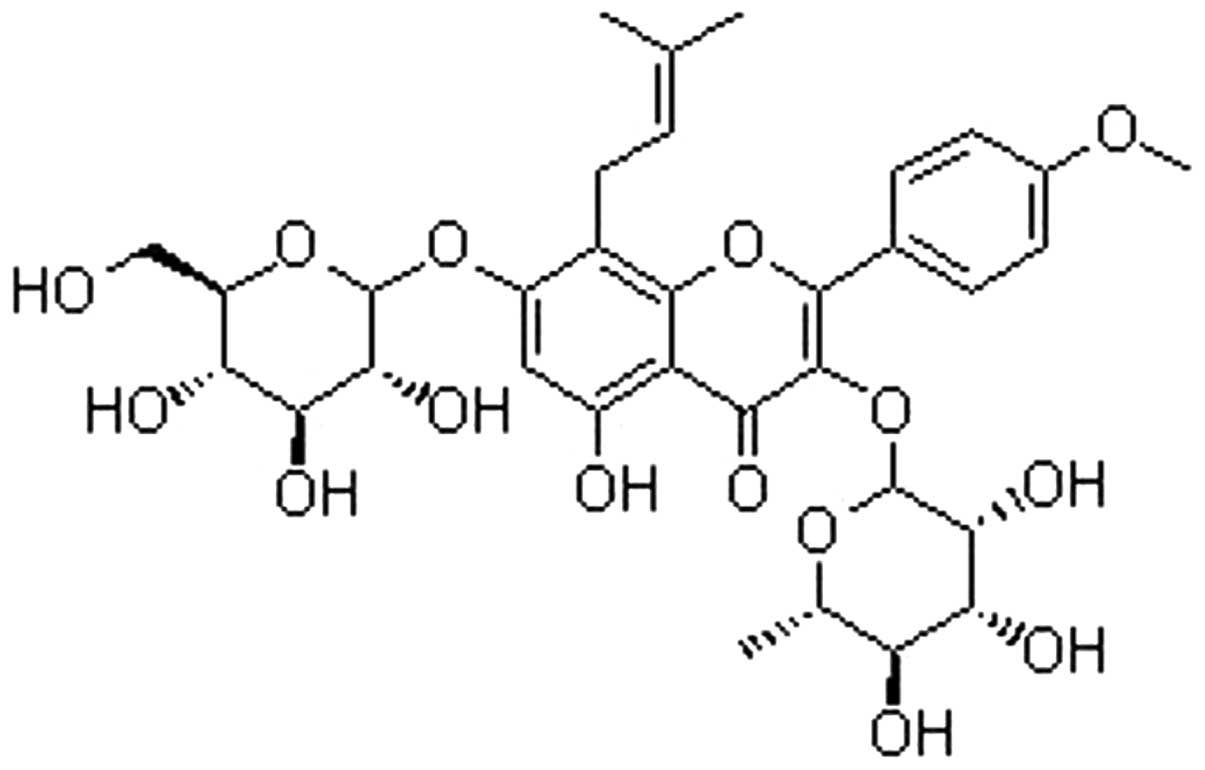
Icariin is a flavonol glycoside, found in
Epimedium spp (Fig. 1). A
number of biological properties of icariin have been identified,
including cardiovascular protection, a therapeutic effect in
erectile dysfunction, and bone-strengthening and anti-hepatotoxic
activities (8–11). Additionally, icariin increases
lymphokine-activated killer cell and natural killer cell activity
in patients with cancer (12).
Malignancy is a disorder involving an imbalance of cell
proliferation, differentiation and apoptosis. A study by Shi et
al (13) reported that icariin
exerts an antiproliferative effect on HepG2-bearing nude mice.
Furthermore, tumor cell invasion and migration are driven by
continuous remodeling of the actin cytoskeleton, which also
provides cellular structure and polarization (14), and is a potential therapeutic
target in tumor cells. Therefore, the present study evaluated the
anticancer effects of icariin on HepG2 cells, focusing on its
effects on proliferation, apoptosis and the actin cytoskeleton.
Materials and methods
Antibodies and reagents
Rabbit polyclonal anti-GAPDH immunoglobulin (Ig)G
(sc-25778) and rabbit polyclonal anti-B-cell lymphoma (Bcl)-2 IgG
(sc-492) antibodies, and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit (sc-2004) and goat anti-mouse (sc-2055) secondary
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Icariin (purity≥99.8%) was obtained from the
National Institutes for Food and Drug Control (Beijing, China).
Oxaliplatin (Eloxatin) was purchased from Sanofi-Aventis (Paris,
France). RNase A, RNAiso Plus, the first strand cDNA synthesis kit
(cat. no. DRR047) and the SYBR green kit for reverse
transctiption-polymerase chain reaction (RT-qPCR) were obtained
from Takara Biotechnology Co., Ltd (Dalian, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Fetal bovine serum (FBS), high glucose Dulbecco's
modified Eagle's medium (DMEM-H), penicillin G, streptomycin,
phosphate-buffered saline (PBS), and the 3,3′-diaminoben-zidine
(DAB) HRP Color Development kit were obtained from Thermo Fisher
Scientific (Waltham, MA, USA). Washes consisted of three 5 min
rinses in PBS, unless otherwise specified.
Cell culture
Human HepG2 cells were cultured in DMEM-H
supplemented with 10% FBS, penicillin G (100 U/ml) and streptomycin
(100 µg/ml) in a humidified atmosphere with 5%
CO2 and 95% air at 37°C. When the cells reached 80%
confluence, after 3 days of culture, they were passaged. The
adherent cells were washed and detached using 1 ml 0.25%
trypsin-EDTA solution for 2 min. The resuspended cells were then
placed into three cell culture flasks for incubation under the same
conditions.
Cell proliferation
The effect of icariin on HepG2 cell proliferation
was determined using an MTT assay. Briefly, the HepG2 cells were
seeded in 96-well plates at a density of 1×105 cells per
well. After 24 h incubation to allow attachment, the medium was
replaced with icariin at various concentrations (10−4,
10−5, 10−6, 10−7 or
10−8 mol/l) for periods of 24, 48 and 72 h.
Subsequently, 10 µl MTT (5 mg/ml) was added to each well and
the the plates were incubated at 37°C for an additional 4 h. The
resulting formazan crystals were solubilized by adding 100
µl DMSO to each well for 20 min. When the crystals had fully
dissolved, the plates were read on a micro-plate reader (iMark;
Bio-Rad Laboratories Inc., Hercules, CA, USA) at a wavelength of
490 nm. The concentration of drug required to inhibit 50% of cell
growth (IC50) was calculated.
Cell cycle analysis
Once the optimal conditions of icariin treatment of
HepG2 cells had been established (10−5 M for 72 h), cell
cycle analysis was performed on the three flasks of cells cultured
with either 10% FBS, oxaliplatin (10 mg/l), or icariin
(10−5 mol/l) for 72 h. The cells were digested using
trypsin-EDTA solution, and the resuspended cells were counted
(1×106 per flask). The cells were fixed with 70% ethanol
at −20°C overnight, washed twice with PBS and incubated with RNaseA
(100 mg/ml) in PBS at room temperature for 30 min. The DNA was
labeled in the dark using propidium iodide (50 mg/ml) and then
washed, following which the cells were analyzed using a FACScalibur
flow cytometer (BD Biosciences, San Diego, CA, USA).
Immunocytochemistry
The HepG2 cells were cultured in 12-well plates at a
density of 1×104 cells/well. FBS (10%), oxaliplatin (10
mg/l), or icariin (10−5 mol/l−1) were added
for 72 h, following which the plates were washed and the cells were
fixed with 95% ethanol for 15 min. The cells were then washed
again, and incubated in blocking buffer for 15 min at 37°C to
prevent non-specific antibody binding. The cells were then
incubated in anti-Bcl-2 antibody (1:500) for 1 h at 37°C, and in
secondary antibody (1:2,000) for 20 min at room temperature, prior
to a final washing step. Staining was visualized using DAB, and
nuclei were counterstained with hematoxylin.
Western blot analysis
The cells were seeded in a 2.5 cm2
culture flask. After 24 h, the cells were incubated with FBS (10%),
oxaliplatin (10 mg/l) or icariin (10−5
mol/l−1). After 3 days, the cells were washed with
ice-cold PBS and subsequently lysed using a mammalian tissue
protein extraction kit (RIPA Lysis Buffer kit; Boster Biological
Technology Co. Ltd., Wuhan, China) containing 1 mM
phenylmethanesulfonyl fluoride protease inhibitor. The cells were
centrifuged at 12,000×g for 10 min at 4°C, and the supernatants
were collected. The protein concentration was determined using a
bicinchoninic acid kit (Ding Guo, Beijing, China). Equal quantities
(50 µg) of protein in the cell extracts were separated on
denatured 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels (Ding Guo) and the proteins were transferred
onto polyvinylidene difluoride membranes (Immobilon; EMD Millipore,
Danvers, MA, USA). The membranes were blocked in blocking buffer
[5% (w/v) non-fat dry milk (Yi Li, Inner Mongolia, China) in
Tris-buffered saline containing 0.1% Tween20 (Boster, Wuhan, China)
(TBST)] at 37°C for 60 min and then incubated with anti-Bcl-2
(1:800) or anti-GAPDH antibody (1:1,000) overnight at 4°C.
Following four washes with TBST, the membranes were incubated with
HRP-conjugated secondary antibody (1:2,000; Protein Tech Group,
Inc, Chicago, USA) for 1 h at 37°C. The membranes were then washed
five times with TBST. Finally, the immunoblot signals were
visualized using enhanced chemilu-minescence reagent (TransGen,
Beijing, China). Quantification of proteins was performed using an
EC3 Chemi HR 410 imaging system (UVP, Inc., Cambridge, UK).
Confocal microscopic analysis of
F-actin
The cells were incubated in confocal plates at a
density of 1×103 cells per plate. After 3 days, the
cells were washed and fixed with 95% ethanol for 15 min, followed
by another wash. For imaging analysis of the F-actin filaments, the
HepG2 cells were stained with phalloidin-fluorescein isothiocyanate
(FITC; 50 mg/l; Invitrogen Life Technologies, Carlsbad, CA, USA) in
the dark for 20 min, prior to three washes in PBS. Finally, the
samples were analyzed using a confocal laser scanning microscope
(Model FV500; Olympus, Tokyo, Japan). Images were captured and
quantified using FluoView software (Olympus).
Statistical analysis
One-way analysis of variance followed by Tukey's
post-hoc comparison was used to compare groups. All data are
expressed as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was repeated at least three times.
Results
Icariin inhibits HepG2 cell
proliferation
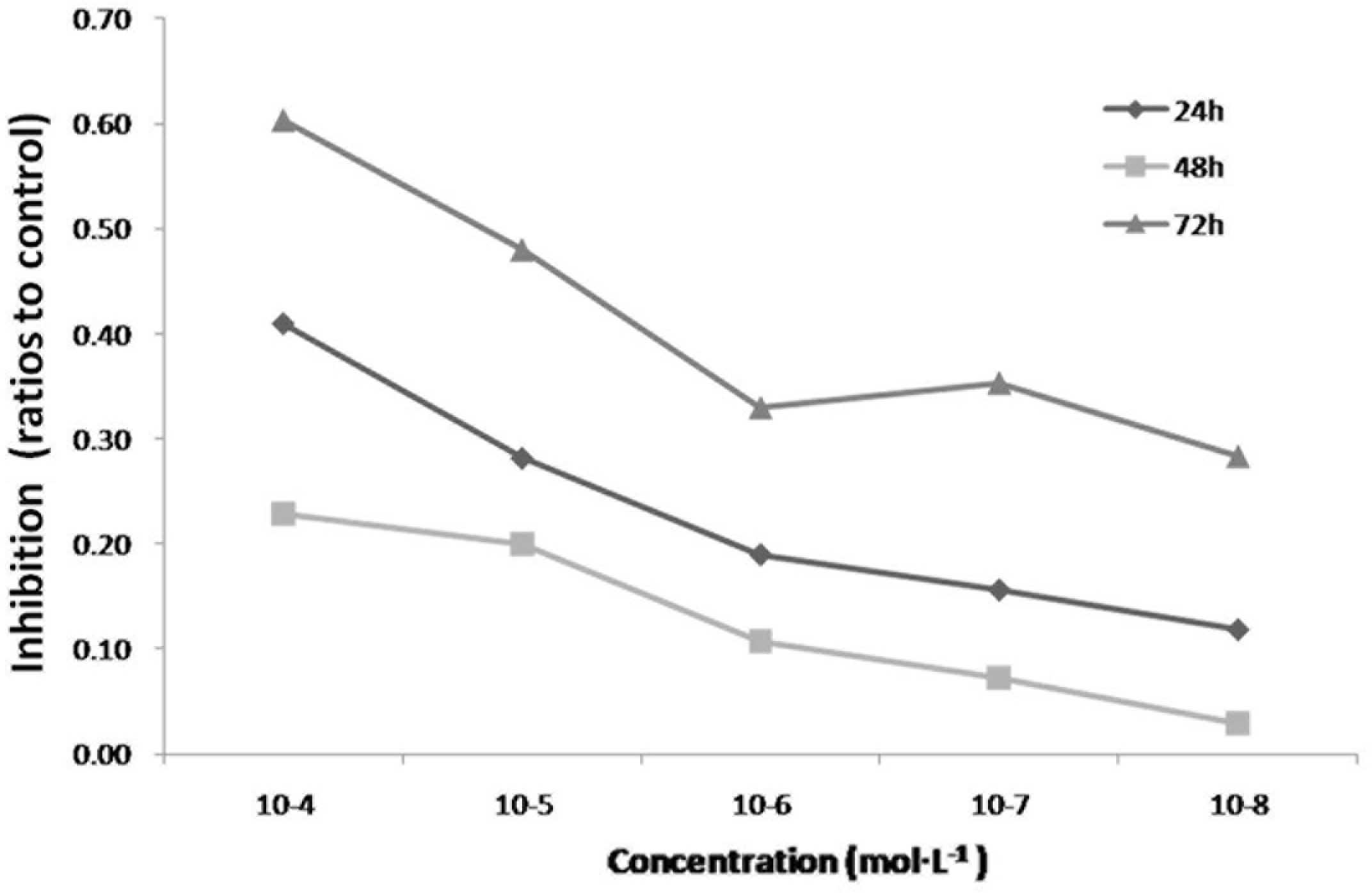
The antiproliferative property of icariin in
cultured HepG2 cells was determined using an MTT assay. Icariin
significantly inhibited the proliferation of the HepG2 cells at
concentrations between 10−4 and 10−8 mol/l
(Fig. 2). The IC50 was
observed at ~10−5 mol/l at 72 h, therefore, this
concentration was selected for use in the subsequent
experiments.
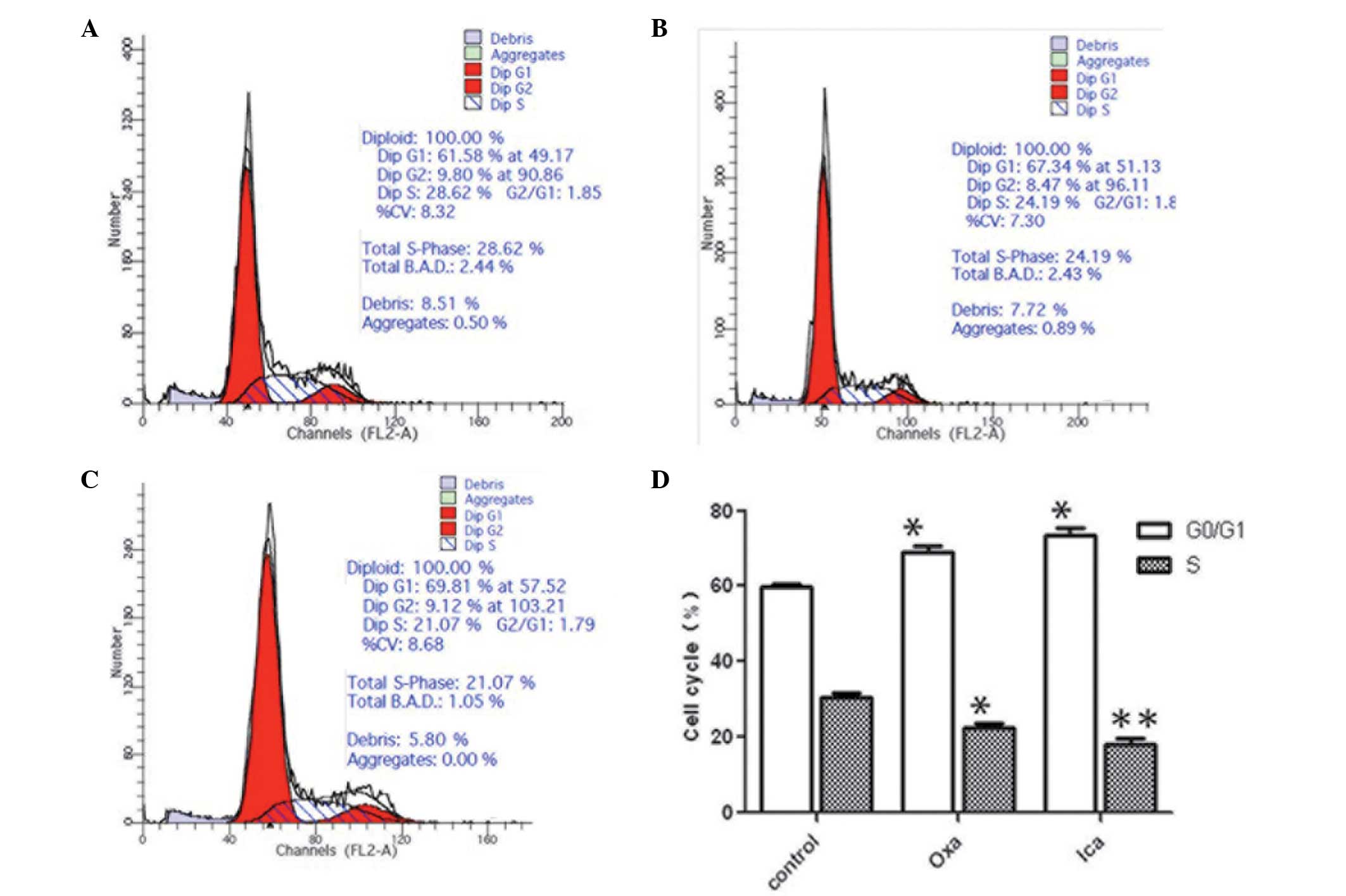
Icariin arrests HepG2 cell cycle at the
G0/G1 phase
The oxaliplatin group and the icariin group
exhibited significantly higher proportions of the cell population
in the G0/G1 phase, compared with the control
group (P<0.05; Fig. 3). The
proportion of HepG2 cells in the oxaliplatin (24.19%) and icariin
(21.07%) groups at the S phase were significantly lower, compared
with that in the control group (28.62%; P<0.05 and P<0.01,
respectively). No significant difference in cell cycle distribution
was observed between the oxaliplatin and icariin groups.
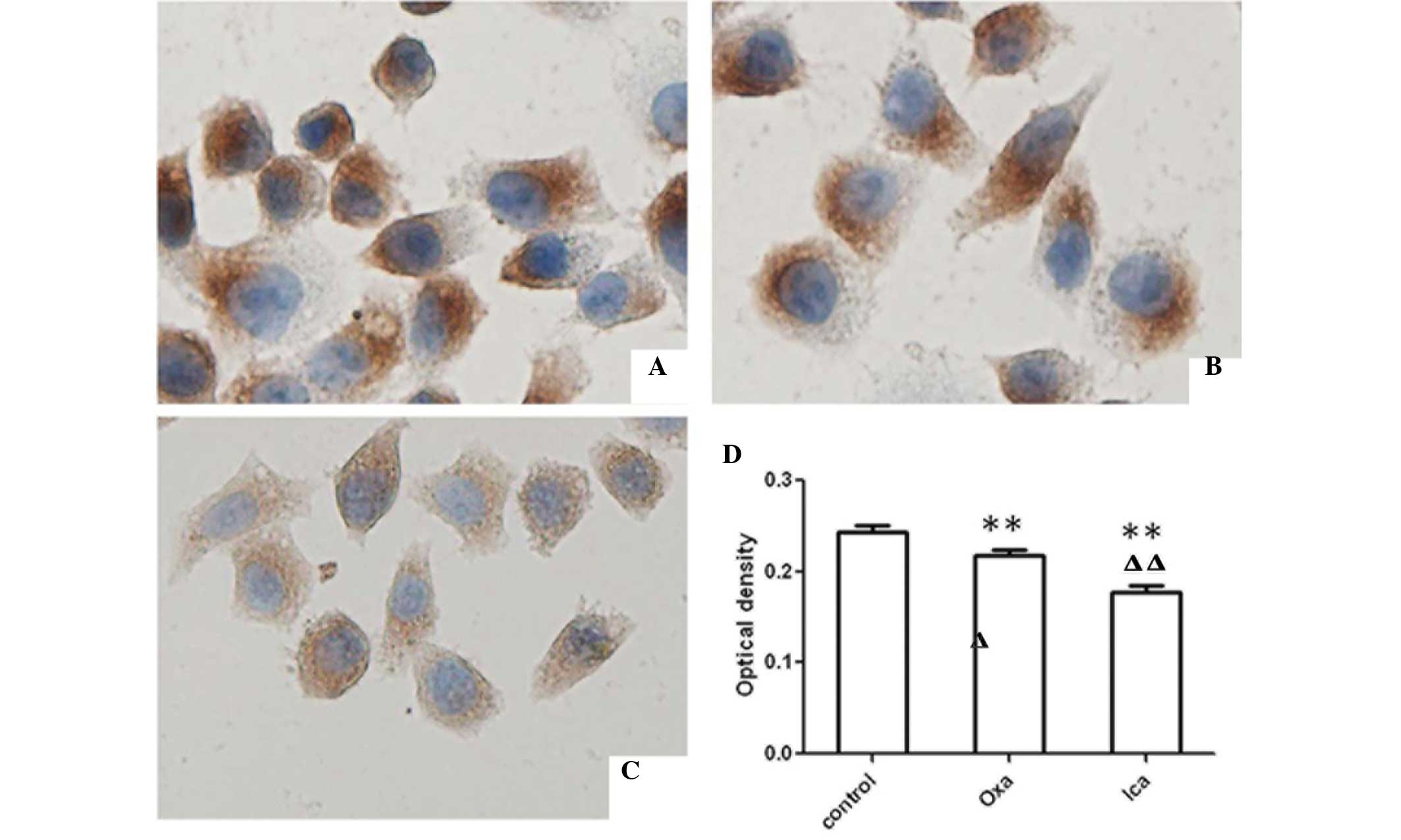
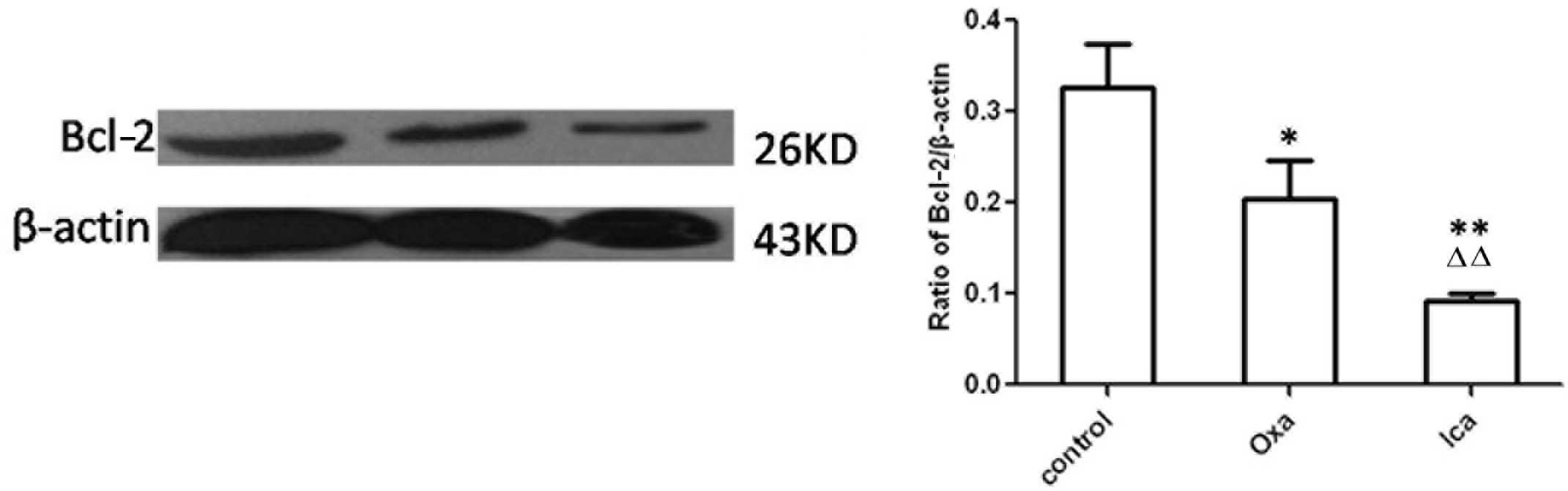
Icariin inhibits the expression of
Bcl-2
Bcl-2, an anti-apoptotic molecule, can be used as a
marker for cell apoptosis. Therefore, the presents study examined
expression of Bcl-2 in HepG2 cells using immunocytochemical and
western blot analyses. The expression levels of Bcl-2 were
significantly lower in the oxaliplatin and icariin groups, compared
with the control group (P<0.01; Fig. 4). Furthermore, the expression of
Bcl-2 in the icariin-treated cells was lower than that in the
oxaliplatin-treated cells (P<0.01). Western blot analysis
confirmed the results of the immunohistochemical analysis, with a
lower expression level of Bcl-2 observed in the oxaliplatin and
icariin groups, compared with the control (P<0.05 and P<0.01,
respectively; Fig. 5). Icariin
treatment led to a sharp decrease in the protein expression of
Bcl-2, compared with oxaliplatin treatment (P<0.01).
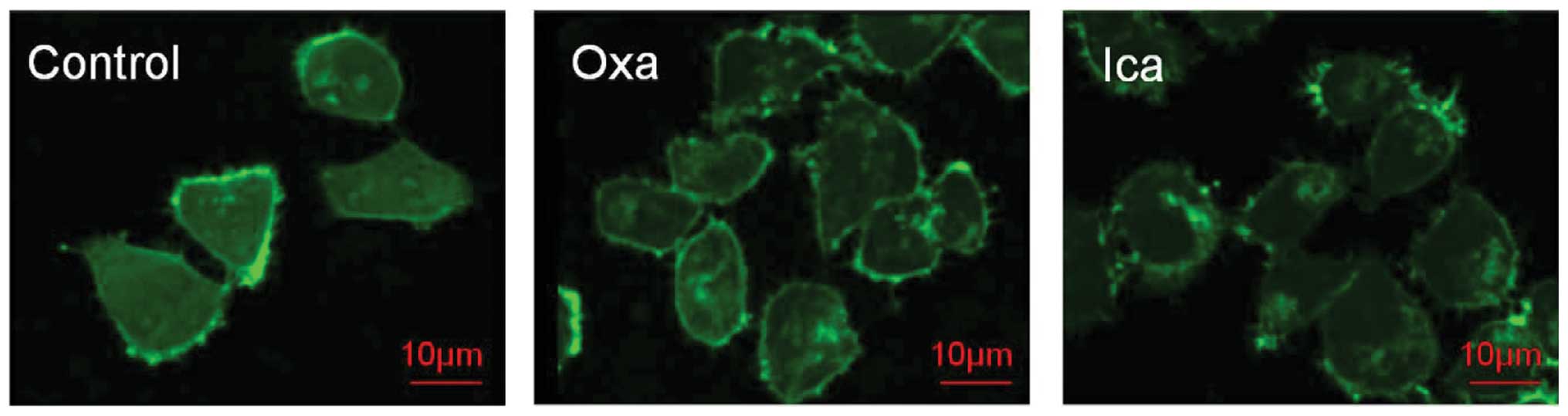
Disruption of F-actin by icariin
Confocal microscopy was used to determine the
effects of icariin on F-actin in the HepG2 cells (Fig. 6). In the untreated HepG2 cell
control group, long-form and regular F-actin filaments were
observed, however, polymerization of F-actin filaments was
noticeably disrupted following 3 days of exposure to either
oxaliplatin or icariin.
Discussion
Icariin, the major component of Herba
epimedii, is reported to exhibit several biological activities
with no clear side effects (15).
The results of the present study demonstrated that, in human HepG2
cells, icariin inhibited the proliferation, affected the cell cycle
and cell apoptosis, and disrupted the F-actin cytoskeleton.
Previous studies have investigated the
antiproliferative efficacy of icariin on HepG2 cells and the
possible underlying mechanism (13,16).
It is well known that DNA damage can induce G1 phase
arrest. Previous studies have demonstrated that cell cycle arrest
at the G0/G1 phase can inhibit the
proliferation of HepG2 cells (17,18).
In the present study, exposure to icariin (10−5 mol/l
for 72 h) significantly increased the proportion of cells in the
G0/G1 phase and decreased the proportion of
cells in the S phase, indicating that icariin inhibited
proliferation and prevented the cells from entering the S
phase.
Apoptosis is regulated by two major pathways: The
death receptor-induced extrinsic pathway and the
mitochondria-apoptosome-mediated intrinsic pathway (19). Bcl-2 family proteins are central in
controlling the mitochondrial pathway, and >20 members of this
family have been identified, including Bcl-2, which is one of the
proteins that suppresses apoptosis (20). The overexpression of Bcl-2 causes
HepG2 cells to become resistant to the induction of apoptosis,
possibly by preventing the release of cytochrome c (21). In the present study, icariin
significantly decreased the protein expression of Bcl-2, indicating
that it may promote the apoptosis of HepG2 cells by suppressing the
protein expression of Bcl-2.
The cytoskeleton is the internal framework of a cell
and is largely composed of actin microfilaments, in addition to
microtubules and intermediate filaments. Actin is important in the
maintenance of cell pattern and tight junctions between cells
(22–24). The balance between microfilament
dissociation and polymerization regulates the movement, adhesion
and fission of cells (25,26). F-actin has been used as a sensitive
index in the assessment of the development of tumor cells in
certain types of early phase cancer (27–29).
In the present study, treatment of the HepG2 cells with icariin
significantly decreased polymerization of the F-actin
cytoskeletons. Thus, it is possible that icariin suppresses the
development of HepG2 cells by acting on the microfilament, and it
is suggested that F-actin may be important in HepG2 cells.
In conclusion, the present study demonstrated that,
at an optimal concentration of 10−5 mol/l, icariin
inhibited the proliferation of HepG2 cells, promoted their
apoptosis by enhancing the protein expression of Bcl-2 and,
importantly, suppressed polymerization of the F-actin cytoskeleton
in the HepG2 cells. Therefore, icariin offers promise as a novel
therapeutic agent in the treatment of HCC.
Acknowledgments
The study was supported by the Science Foundation of
Liaoning University of Traditional Chinese Medicine (grant no
81373527)and the Key Laboratory of Ministry of Education for
Traditional Chinese Medicine Viscera-State Theory and
Applications.
References
|
1
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bell RM: A review of complementary and
alternative medicine practices among cancer survivors. Clin J Oncol
Nurs. 14:365–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang HC, Chen TL and Chen RM:
Cytoskeleton inter-ruption in human hepatoma HepG2 cells induced by
ketamine occurs possibly through suppression of calcium
mobilization and mitochondrial function. Drug Metab Dispos.
37:24–31. 2009. View Article : Google Scholar
|
|
4
|
Colombo E, Marine J-C, Danovi D, Falini B
and Pelicci PG: Nucleophosmin regulates the stability and
transcriptional activity of p53. Nat Cell Biol. 4:529–533. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He W, Sun H, Yang B, Zhang D and Kabelitz
D: Immunoregulatory effects of the herba Epimediia glycoside
icariin. Arzneimittelforschung. 45:910–913. 1995.PubMed/NCBI
|
|
7
|
Huo X, Xu XJ, Chen YW, Yang HW and Piao
ZX: Filamentous-actins in human hepatocarcinoma cells with CLSM.
World J Gastroenterol. 10:1666–1668. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lau WY and Lai EC: Hepatocellular
carcinoma: Current management and recent advances. Hepatobiliary
Pancreat Dis Int. 7:237–257. 2008.PubMed/NCBI
|
|
10
|
Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L and
Guo YL: Effects of icariin on erectile function and expression of
nitric oxide synthase isoforms in castrated rats. Asian J Androl.
7:381–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiume L, Manerba M, Vettraino M and Di
Stefano G: Effect of sorafenib on the energy metabolism of
hepatocellular carcinoma cells. Eur J Pharmacol. 670:39–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okuda K: Hepatocellular carcinoma: Recent
progress. Hepatology. 15:948–963. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shumilina EV, Negulyaev YA, Morachevskaya
EA, Hinssen H and Khaitlina SY: Regulation of sodium channel
activity by capping of actin filaments. Mol Biol Cell.
14:1709–1716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun L, Chen W, Qu L, Wu J and Si J:
Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma
cells via downregulation of MDR1 and P-glycoprotein expression. Mol
Med Rep. 8:1883–1887. 2013.PubMed/NCBI
|
|
16
|
Tong JS, Zhang QH, Huang X, et al:
Icaritin causes sustained ERK1/2 activation and induces apoptosis
in human endometrial cancer cells. PLoS One. 6:e167812011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang XH, Zou ZQ, Xu CW, Shen YZ and Li D:
Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting
the activity of the cyclin B/Cdc2 complex. Mol Med Rep. 4:273–277.
2011.PubMed/NCBI
|
|
18
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anti-cancer natural products
isolated from chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang QQ, Zhang ZY, Xiao JY, Yi C, Li LZ,
Huang Y and Yun JP: Knockdown of nucleophosmin induces S-phase
arrest in HepG2 cells. Chin J Cancer. 30:853–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Dong H, Zhu M, Ou Y, Zhang J, Luo
H, Luo R, Wu J, Mao M, Liu X, et al: Icariin exterts negative
effects on human gastric cancer cell invasion and migration by
vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J
Pharmacol. 635:40–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2008.
View Article : Google Scholar
|
|
22
|
Williams JI, Weitman S, Gonzalez CM, Jundt
CH, Marty J, Stringer SD, Holroyd KJ, Mclane MP, Chen Q, Zasloff M
and Von Hoff DD: Squalamine treatment of human tumors in nu/nu mice
enhances platinum-based chemotherapies. Clin Cancer Res. 7:724–733.
2001.PubMed/NCBI
|
|
23
|
Skillman KM, Diraviyam K, Khan A, et al:
Evolutionarily divergent, unstable filamentous actin is essential
for gliding motility in apicomplexan parasites. PLoS Pathog.
7:e10022802011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozyamak E, Kollman JM and Komeili A:
Bacterial actins and their diversity. Biochemistry. 52:6928–6939.
2015. View Article : Google Scholar :
|
|
25
|
Xu HB and Huang ZQ: Icariin enhances
endothelial nitric-oxide synthase expression on human endothelial
cells in vitro. Vascul Pharmacol. 47:18–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan MX, Yang J, Sun Q, Liu CH, Wang YG and
Wang WQ: Hepatocellular carcinoma that arose from primary Sjögren's
syndrome. Ann Hepatol. 12:824–829. 2013.PubMed/NCBI
|
|
27
|
Yang JX, Fichtner I, Becker M, Lemm M and
Wang XM: Anti-proliferative efficacy of icariin on HepG2 hepatoma
and its possible mechanism of action. Am J Chin Med. 37:1153–1165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang YL: Polymerization of actins. Biology
(Basel). 18:13–14. 1995.
|
|
29
|
Zhang DW, Cheng Y, Wang NL, Zhang JC, Yang
MS and Yao XS: Effects of total flavonoids and flavonol glycosides
from Epimedium koreanum Nakai on the proliferation and
differentiation of primary osteoblasts. Phytomedicine. 15:55–61.
2008. View Article : Google Scholar
|