Introduction
Dry eye is a multifactorial disease involving a
number of different pathological mechanisms, including instability
of the tear film, tear hyperosmolarity and inflammation of the
ocular surface (1,2). Several US and international
population-based studies have demonstrated that dry eye occurs in
between 5 and 35% of the population, and is not confined to the
elderly, but is also present in young individuals (3–5). The
variety of dry eye symptoms commonly include stinging or burning of
the eyes, scratchiness, viscous mucus in or around the eyes,
excessive eye irritation resulting from smoke or wind and excess
tearing, all of which can not only affect the visual quality and
comfort of the patient, but in severe cases can result in corneal
ulcers, loss of vision and other pathologies (6–10).
At present, therapeutic agents including artificial tears are able
to temporarily relieve dry eye, however, treatments that promote
ocular surface healing, and increase mucin secretion and tear film
stability are limited.
A previous in vitro study demonstrated that
fibroblast growth factor 10 (FGF10) is associated with mucin
production and the promotion of the proliferation of cultured rat
conjunctival (Cj) epithelial cells (ECs) (11), which suggested that FGF10 may be
useful in the treatment of dry eye. FGF10, a member of the
fibroblast growth factor family, is also known as keratinocyte
growth factor 2 and promotes the growth, proliferation and
differentiation of epithelial cells (12,13).
It has also been reported to be an important growth factor in the
regulation of corneal epithelial wound healing (14).
Mucins are important structural and functional
components of the tear film and are critical for protection of the
corneal and conjunctival epithelium (15,16).
Corneal damage and inflammation result in an increase in mucin
production by the conjunctiva and induce the upregulation of FGF10.
Although growth factors may promote the proliferation of Cj-ECs and
stimulate mucin production and secretion, previous studies have
reported that the abnormal expression of conjunctival mucin is
implicated in ocular surface disorders, including dry eye (17–19).
In the present in vivo study, the therapeutic
efficacy of FGF10 was examined in an inflammation-induced rabbit
dry eye model. In addition the effect of FGF10 was further
evaluated on corneal epithelial cell healing, apoptosis and the
expression of mucin.
Materials and methods
Animal protocols
A total of 12 New Zealand white female rabbits
(2.0–2.5 kg; Laboratory Animal Center of Shanghai, Shanghai, China)
were treated, according to the Association for Research in Vision
and Ophthalmology Statement for the Use of Animals in Ophthalmic
and Vision Research. The present study was approved by the ethics
committee of the Shanghai General Hospital, Shanghai Jiaotong
University School of Medicine (Shanghai, China). The animals were
maintained in a controlled environment with a 12-h light/dark cycle
at 18±3°C and a minimum of 30% humidity. The rabbits (n=4 per
group) were randomly assigned to one of three groups. In the
phosphate-buffered saline (PBS) control group, rabbit lacrimal
glands were injected with the T-cell mitogen concanavalin A (Con A;
Sigma-Aldrich, St. Louis, MO, USA) and PBS eye drops (Jieshikang
Biotechnology Co., Ltd., Qingdao, China) were topically applied. In
the FGF10 treatment group, rabbit lacrimal glands were injected
with Con A and 25 µg/ml FGF10 eye drops (donated by
Associate Professor Xiaojie Wang of the Wenzhou Eye Research
Institute, Wenzhou, Zhejiang, China) were topically applied. In the
normal control group, rabbit lacrimal glands were injected with
saline and no eye drops were applied. All procedures were performed
under anesthesia by subcutaneous administration of ketamine
hydrochloride (30 mg/kg) and xylazine (6 mg/kg) (Jieshikang
Biotechnology Co., Ltd.). The anesthetized animals were placed in a
conventional holder and received injections into the right lacrimal
glands. Injections were performed by retracting the lower eyelid
and inserting the needle ~1 cm from the nasal canthus into the
suborbital space, to a depth of ~6 mm. A single 30 µl volume
of saline (normal control group) or Con A (PBS control and FGF10
treatment groups) was injected into the lacrimal gland using a
30-gauge needle and a Hamilton Gastight® syringe
(Hamilton, Reno, NV, USA). The experimentally treated eyes (FGF10
treatment group; n=4) received 25 µg/ml FGF10 in PBS, as
reported by Wang et al (14), whereas the control eyes (PBS
control group; n=4) received PBS alone. These solutions were
applied topically four times per day 3 days after the injections.
No eye drops were applied in the normal control group.
Histopathological evaluation
Microscopic examinations were performed on the
paraffin-embedded (Puzheng Biotechnology Co. Ltd,, Shanghai,
China), hematoxylin and eosin (H&E; Gefan Biological Technology
Co., Ltd., Shanghai, China)-stained sections of the excised rabbit
lacrimal glands following animal sacrifice by intravenous injection
of air into the ears of the anesthetized ears of the rabbits. The
lacrimal glands were fixed and preserved in neutral buffered
formalin (Hubei Xingyinhe Chemical Co., Ltd., Hubei, China), and
the fixed gland tissue was dehydrated through ascending ethanol
concentrations, embedded in paraffin, cut into 4 µm sections
and stained with H&E for light microscopy (Digital Biological
Microscope BX53; Olympus Corporation, Tokyo, Japan)
examination.
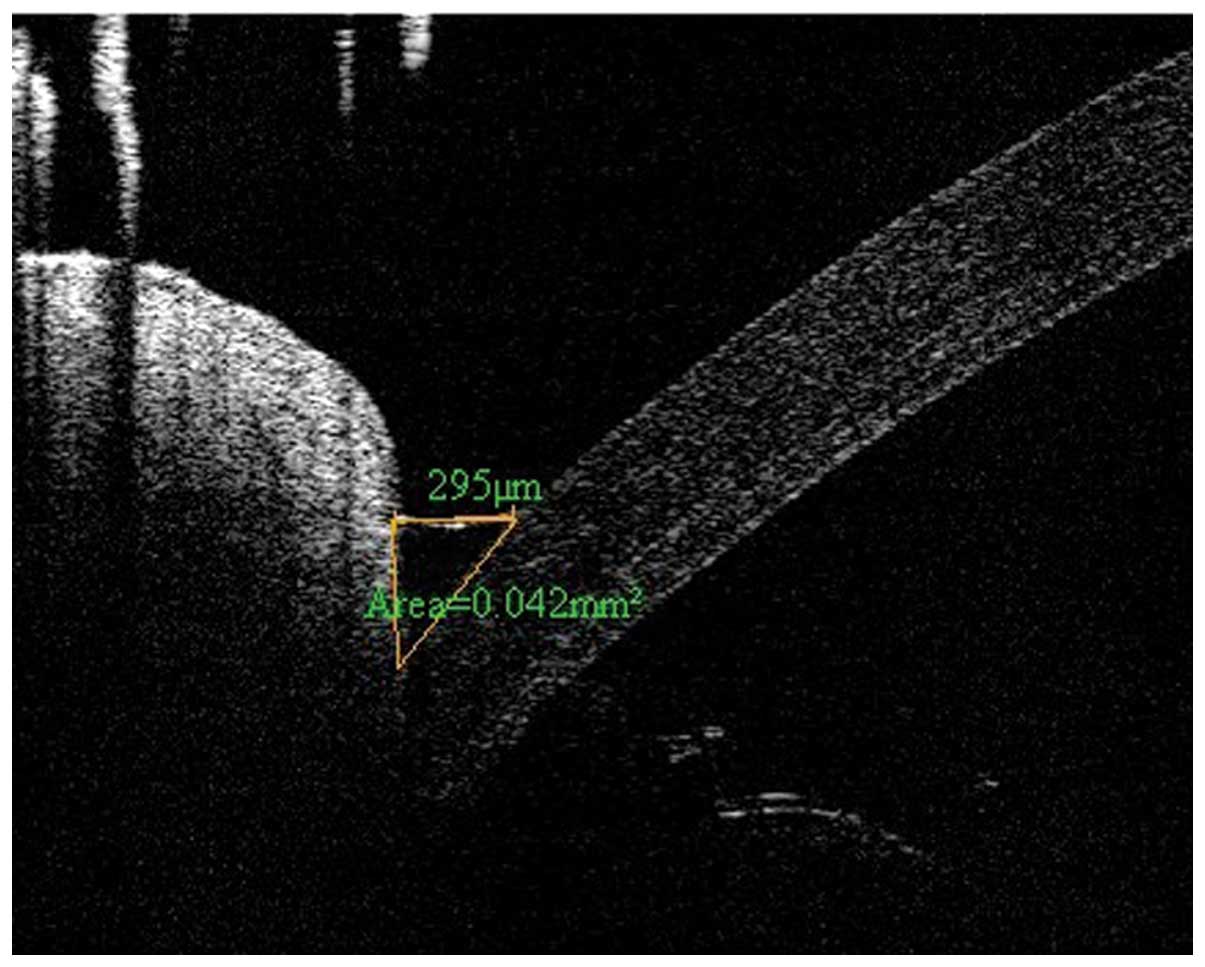
Optical coherence tomography (OCT) lower
tear meniscus parameter measurement
Images of the lower tear meniscus were imaged at the
inferior cornea-lid scan junction, with a 6-mm-vertical ×
2.8-mm-depth scan, using a Fourier Domain-optical coherence
tomography (FD-OCT) system (RTVue; Optovue, Inc., Fremont, CA,
USA), using the measurement techniques described previously
(20). Briefly, the OCT images
were exported for manual computer caliper measurement using ReVue
RTVue software (version 4.0; Optovue, Inc). The lower tear meniscus
height (TMH) was defined as the distance between the
cornea-meniscus junction and the lower eyelid-meniscus junction.
The lower tear meniscus area (TMA) was the area enclosed by the
lower tear meniscus, cornea and lower eyelid. All measurements were
performed by one experienced investigator in a blinded-manner, and
were recorded for data analysis. TMH was classified as follows: +,
<200 µm; ++, <180 µm; +++, <160. A TMH of
+++ was characteristic of dry eye (Fig. 1).
Corneal fluorescein staining and tear
breakup time (TBUT) assessment
TBUT was determined by applying moist sodium
fluorescein test strips (Tianjing Jingming New Technological
Development Co., Ltd., Tianjing, China) onto the rabbit conjunctiva
capsule, following which the eyelids were opened and closed
manually to distribute the fluorescein within the tear film. Under
slit lamp (Topcon Corporation, Tokyo, Japan) observation, the eye
was held open and the time until one or more black spots or streaks
appeared in the precorneal tear film was recorded. TBUT was
classified as follows: +, 10–20 sec; ++, 5–10 sec; +++, <5 sec;
A TBUT of +++ was characteristic of dry eye.
Transmission electron microscopy (TEM)
examination
Samples for TEM were fixed in 2.5% glutaraldehyde
(Hubei Xingyinhe Chemical Co., Ltd.) in 0.1 mol/l phosphate buffer
(pH 7.4) and then post-fixed in 1% osmium acid (Hubei Xingyinhe
Chemical Co., Ltd.). Subsequent to dehydration with an ascending
alcohol series, the samples were embedded in epoxy resin
(Epok812®; Ohkenshoji Co., Ltd., Tokyo, Japan). Small
sections (1 mm3) were cut from the middle area of the
cornea. The sections were subjected to double staining with lead
acetate (Hubei Xingyinhe Chemical Co., Ltd.) and uranyl acetate
(Hubei Xingyinhe Chemical Co., Ltd.) and were observed using a
transmission electron microscope (H-7000; Hitachi, Ltd., Tokyo,
Japan).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining
The TUNEL technique was used to evaluate apoptosis
in the corneal- and Cj-ECs. Each paraffin-embedded section was
deparaffinized and rehydrated. Proteinase K (20 mg/l; Hubei
Xingyinhe Chemical Co., Ltd.) was applied for 30 min and endogenous
peroxidase was quenched using 3% hydrogen peroxide (Hubei Xingyinhe
Chemical Co., Ltd.) for 10 min. An Annexin V-fluorescein
isothiocyanate/propidium iodide Apoptosis Detection kit (Nanjing
Keygen Biotech Co., Ltd., Nanjing, China) was used. The terminal
deoxynucleotidyl transferase reaction was performed for 1 h at 37°C
prior to application of diaminobenzidine chromogen (Hubei Xingyinhe
Chemical Co., Ltd.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from epithelial cells using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), according
to the manufacturer's instructions. The total RNA (1 µg) was
used for cDNA synthesis using a Reverse Transcription System
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
instructions. The cDNA was amplified by qPCR using primers specific
for rabbit Muc1 in a thermal cycler (PCR Sprint; Thermo Hybaid,
Franklin, MA, USA). The primer sequences of Muc1 were forward
5′-GTGCTGTCGTCACCTCTGCCC-3′ and reverse 5′-TGAGACCGACGGGCTGGTGG-3′.
GAPDH served as the internal control and the primer sequences used
were forward 5′-CTGCCGCCTGGAGAAAGC-3′ and reverse
5′-AGACGACCTGGTCCTCGGTG-3′. A standard reaction was performed in a
total volume of 50 µl, consisting of 4 µl cDNA, 2
µl each of the specific forward and reverse primers (both 10
µM), 17 µl dH20 and 25 µl 2X SYBR Green I
PCR mix (Toyobo Co., Ltd., Osaka, Japan). The parameters used were
as follows: 5 min at 94°C, followed by 35 cycles of denaturation
for 30 sec at 94°C, then extension for 1 min at 72°C. The
expression levels were quantified using the 2−ΔΔCT
method (21).
Statistical analysis
All data were statistically analyzed using SAS
Software, version 8.2 (SAS Institute, Inc., Cary, NC, USA). Data
were analyzed using a Student-Newman-Keuls test, following analysis
of variance, which is the most widely used method for pairwise
comparison (22,23), to identify differences between the
groups. The results are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
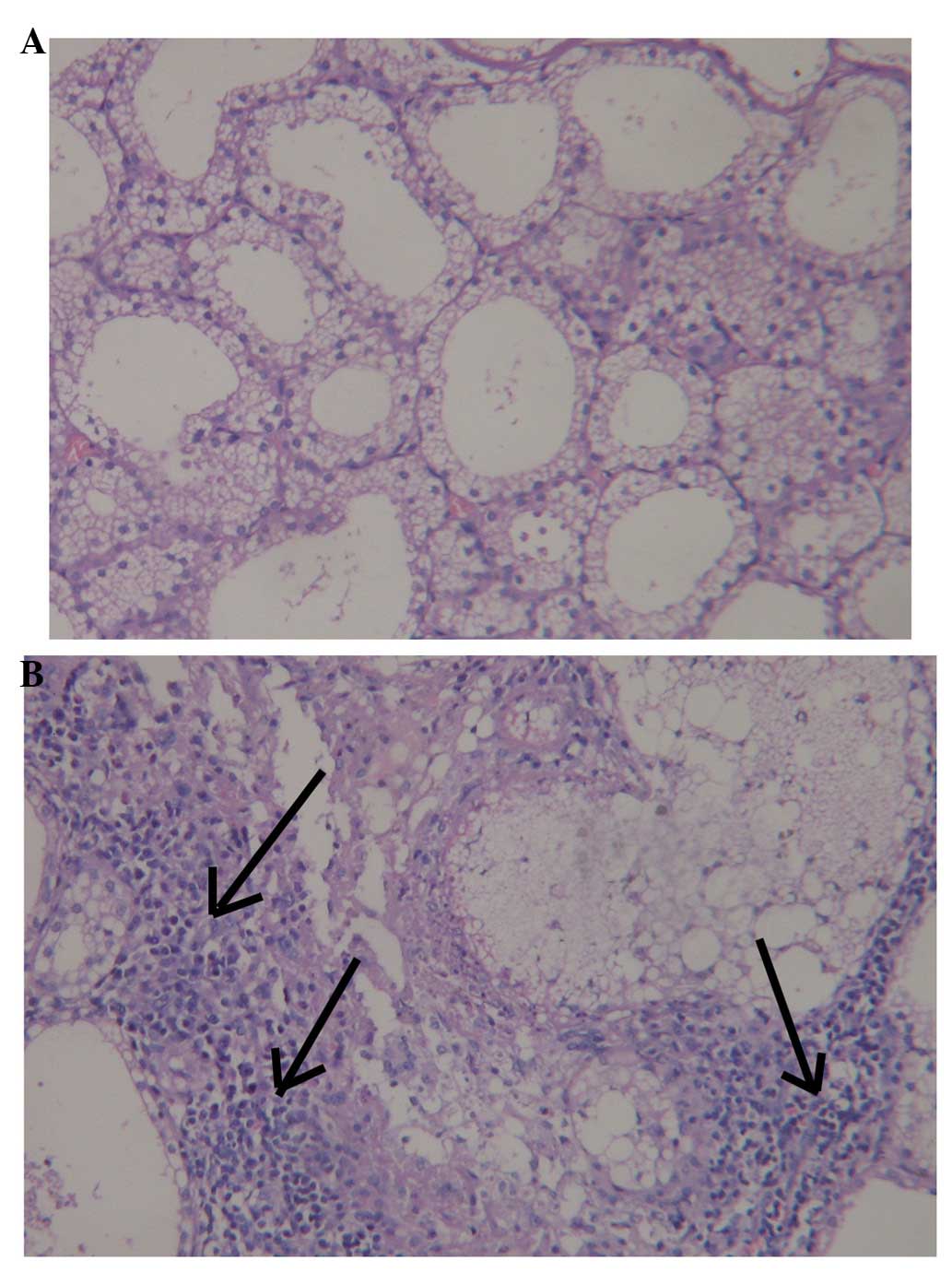
Histopathological evaluation
Histological examination of the lacrimal glands 72 h
following injection with 300 µg Con A revealed moderately
severe necrosis of the epithelial cells and inflammatory cell
infiltration. By contrast, no evidence of inflammation or
abnormality was observed following injection of 30 µl saline
(Fig. 2).
Lower tear meniscus measurement
The lower tear meniscus measurements, determined
using FD-OCT are listed in Table
I. The TMH and TMA measurements 3 days following Con A
injection in the PBS and FGF10 groups were significantly reduced,
compared with those of the saline-injected animals. At 3 days
post-treatment, the TMH in the FGF10 group was significantly
increased, compared with the PBS group. At 7 days post-treatment,
the TMA of the FGF10 group was also increased.
 | Table ILower tear meniscus measurement. |
Table I
Lower tear meniscus measurement.
| PBS control group
| FGF10 treatment group
| Normal control group
|
|---|
| Time-point | TMH (µm) | TMA
(mm2) | TMH (µm) | TMA
(mm2) | TMH (µm) | TMA
(mm2) |
|---|
| Day 0 | 231.36±11.59 | 0.025±0.004 | 224.06±7.81 | 0.025±0.006 | 221.75±5.85 | 0.024±0.006 |
| 3 Days after
injection | 155.47±14.57a | 0.011±0.005a | 151.11±37.90a | 0.009±0.005a | 227.00±17.39 | 0.024±0.003 |
| 3 Days after
treatment | 161.00±21.63a | 0.011±0.005a | 229.50±39.53b | 0.019±0.005 | 244.25±28.59 | 0.027±0.005 |
| 7 Days after
treatment | 183.07±10.39a | 0.014±0.002a | 254.00±16.97 | 0.034±0.004b | 230.50±16.26 | 0.025±0.005 |
Corneal fluorescein staining and
TBUT
The baseline tear film stability in rabbits,
measured as the TBUT, was >30 sec when assessed prior to
lacrimal gland injection, and the TBUT of the normal control group
rabbits injected with saline remained unchanged for the duration of
the investigation. By contrast, 300 µg Con A resulted in a
marked reduction in TBUT 3 days after lacrimal gland injection.
However, TBUT increased 7 days after treatment in the FGF10 group
(Table II). Punctate staining of
fluorescein due to corneal epithelial injury significantly reduced
in the FGF10 treatment group, compared with the PBS control group
(Fig. 3).
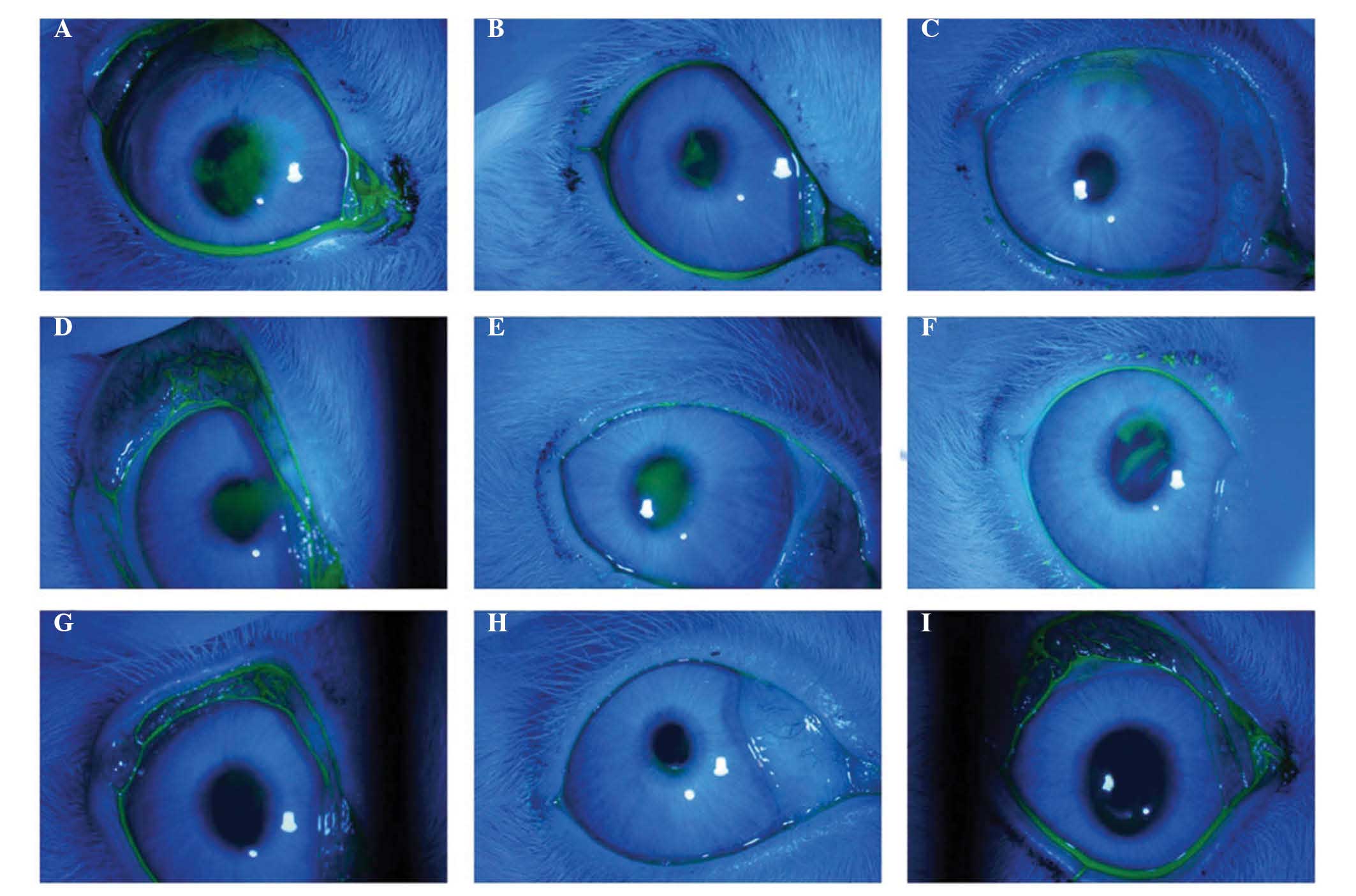 | Figure 3Corneal fluorescein staining of the
three groups. FGF10 treatment group: (A) 3 days following Con A
injection, clear fluorescein staining of the corneal epithelial
injury was observed. (B) 3 days following FGF10 treatment, moderate
fluorescein staining of the corneal epithelial injury remained. (C)
7 days following FGF10 treatment, light, punctate fluorescein
staining of the corneal epithelial injury remained. PBS control
group: (D) 3 days following Con A injection, clear fluorescein
staining of the corneal epithelial injury was observed. (E) 3 days
and (F) 7 days following PBS treatment, fluorescein staining of the
corneal epithelial injury remained. In the normal control group (G)
three days following saline injection, (H) three days following no
eye drop treatment, and (I) seven days followin no eye drop
treatment, no punctuate fluorescein staining of the cornea
epithelium was observed. FGF10, fibroblast growth factor 10; Con A,
concanavalin A. |
 | Table IITear membrane break-up time in the
control and treatment groups. |
Table II
Tear membrane break-up time in the
control and treatment groups.
| Time-point | PBS control
group | FGF10 treatment
group | Normal control
group |
|---|
| Day 0 | >30 sec | >30 sec | >30 sec |
| 3 Days after
injection | +++ | +++ | >30 sec |
| 3 Days after
treatment | ++ | ++ | >30 sec |
| 7 Days after
treatment | ++ | + | >30 sec |
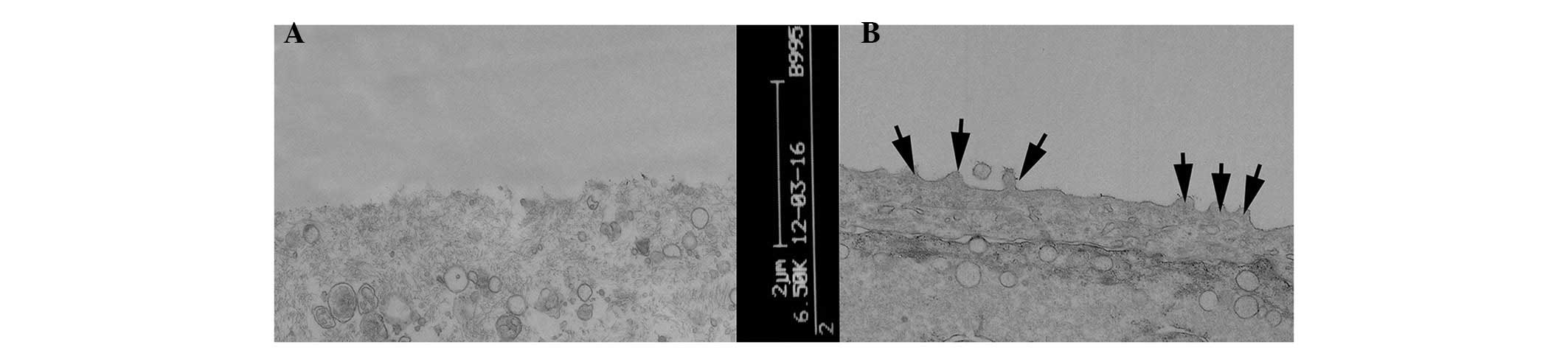
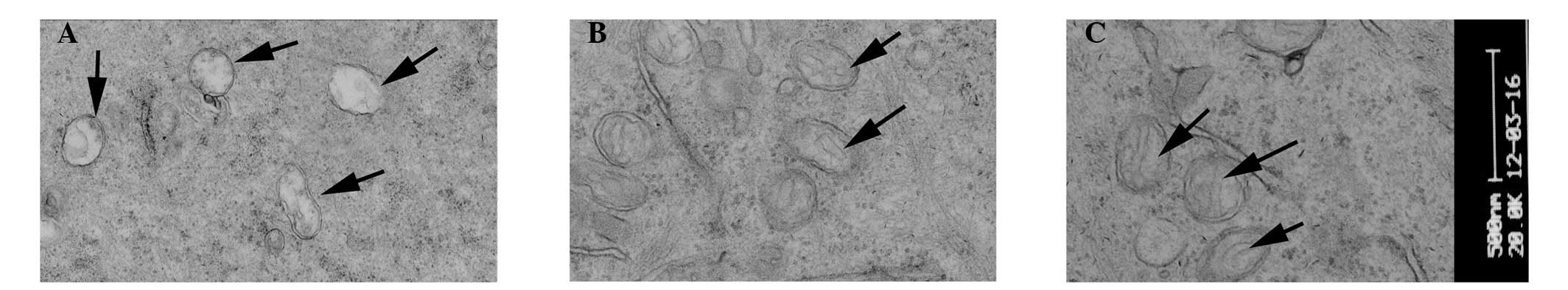
TEM evaluation
TEM evaluation of the corneal epithelial cells
following injection with 30 µl saline, revealed no
abnormalities. By contrast, injection with 300 µg Con A led
to epithelial cells, which exhibited a loss of microvilli
structures and microfolds (Fig.
4), a widening of the intercellular space, shallow cellular
edema, desmosome disintegration, mitochondrial swelling and cristae
disappearance and ballooning degeneration, which is a form of cell
death (Fig. 5A). At 3 days
post-treatment, the FGF10 group exhibited light swelling of
mitochondria and the appearance of cristae (Fig. 5B). At 7 days post-treatment, the
epithelial cells of the FGF10 group exhibited microvilli structures
and microfolds (Fig. 4) and also
the appearance of mitochondrial cristae (Fig. 5C).
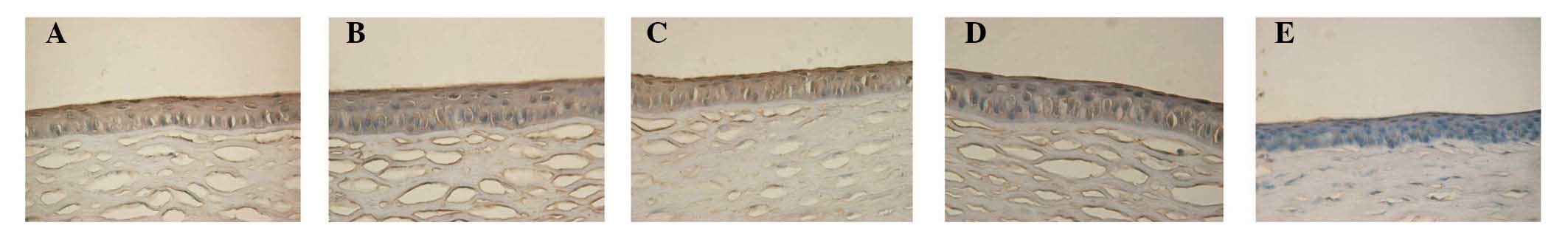
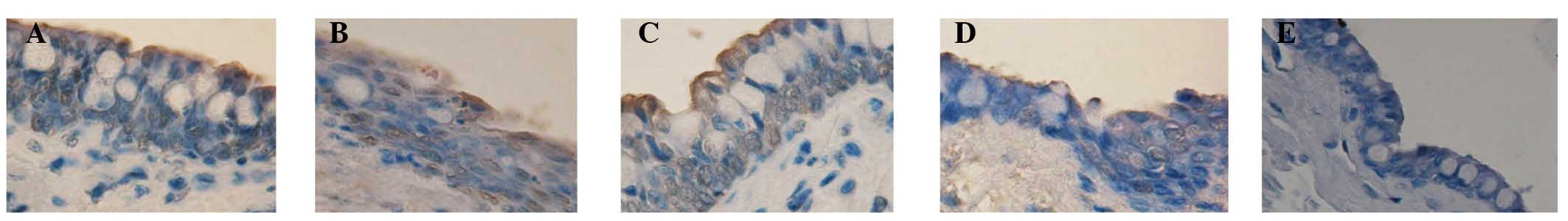
TUNEL staining evaluation
The corneal- and Cj-EC apoptotic rates are listed in
Table III. Compared with the
normal control group, the apoptosis rate of the cells following
injection with 300 µg Con A was significantly higher
(P<0.05). Subsequent to treatment for 3 and 7 days, the
apoptotic rates in the FGF10 group were significantly reduced,
compared with those in the PBS group (P<0.05; Figs. 6 and 7).
 | Table IIICorneal and conjunctival epithelial
cell apoptotic rates. |
Table III
Corneal and conjunctival epithelial
cell apoptotic rates.
| Time-point | Tissue | PBS group (%) | FGF10 group
(%) | Normal control
(%) |
|---|
| 3 Days after
treatment | Corneal | 28.3±1.36a | 12.8±1.15a,b | 3.3±0.56 |
| Conjunctival | 41.7±0.63a | 34.0±1.22a,b | 1.6±0.24 |
| 7 Days after
treatment | Corneal | 61.3±5.29a | 44.9±1.37a,b | 2.7±0.21 |
| Conjunctival | 42.0±2.02a | 29.8±1.06a,b | 2.2±0.32 |
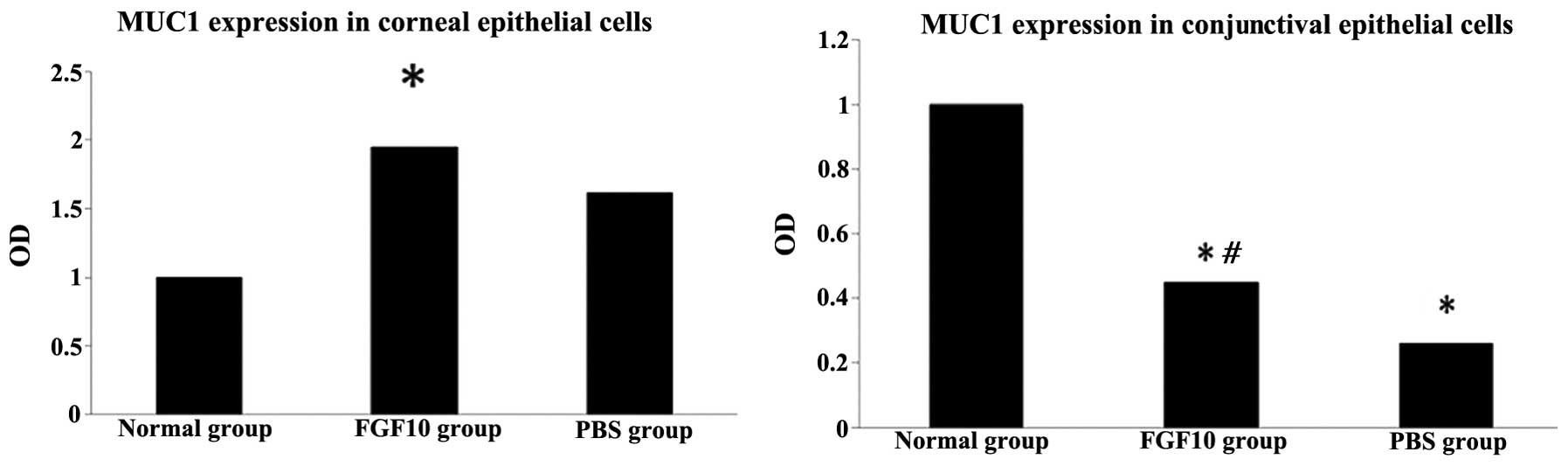
RT-qPCR evaluation
The results of the RT-qPCR analysis demonstrated
that the expression of Muc1 was significantly upregulated by FGF10
treatment in the corneal epithelial cells, compared with the normal
control group (P<0.05). FGF10 significantly upregulated corneal
expression of Muc1, and decreased conjuctival expression of Muc1
(Fig. 8). Additionally, in the
Cj-ECs, Muc1 was significantly upregulated by FGF10, compared with
the PBS control group (P<0.05; Fig.
8).
Discussion
The present in vivo study was performed using
a short-term model of rabbit dry eye, which was strategically
developed to advance the discovery of drugs for dry eye (24). In the present study, the
inflammatory response, characterized by necrosis of the epithelial
cells of the lacrimal gland, was observed to be pronounced 3 days
following Con A injection. Lacrimal gland inflammation
significantly reduced the TMH, TMA, TBUT values, and increased
corneal damage.
The measurement of lower tear meniscus using FD-OCT
is an objective and noninvasive method of assessment (25,26).
Several previous studies have reported the use of FD-OCT in
measuring human TMH and TMA, which correlate well with the symptoms
of dry eye disease and Schirmer's test (24,27).
However, to the best of our knowledge, no previous studies have
used this method in the rabbit dry eye model. In the present study,
FD-OCT was found to be accurate for the quantification of tear
volume.
The present study demonstrated that FGF10 promoted
TMH and TMA, and increased TBUT length in the rabbit dry eye. These
data suggested that FGF10 provided effective treatment in the
rabbit model of dry eye. FGF10 is a member of the FGF family, which
is involved in a wide variety of physiological and pathological
processes, including inflammation, repair and regeneration
(13,28). Our previous in vitro study
(11) demonstrated that FGF10
promoted the proliferation of rat Cj-ECs. The present study,
demonstrated for the first time, to the best of our knowledge, that
FGF10 can increase TMH and maintain tear film stability.
Wang et al (14) reported that FGF10 is important in
successful corneal wound healing, which is consistent with the
observations of the present study that FGF10 repaired corneal
damage in the dry eye model. In the present study, TEM revealed
that FGF10 induced the repair of the structure of microvilli,
microfolds and mitochondria of epithelial cells, which may affect
their apoptosis. Previous studies have reported that FGF10
attenuates DNA damage and apoptosis of epithelial cells, in part,
by mitogen-activated protein kinase/extracellular signal-related
kinase-dependent signaling, which affects the
mitochondria-regulated death pathway (29,30).
Therefore, the apoptosis of corneal- and Cj-ECs was further
investigated in the present study. The TUNEL staining assay
demonstrated that the percentage of apoptotic cells in the FGF10
treatment group was significantly lower than that of the PBS
control group. This suggested that FGF10 may act to protect
corneal- and Cj-ECs from apoptosis in this rabbit dry eye
model.
Mucins are high-molecular weight glycoproteins,
which are involved in the protection of the corneal and
conjunctival epithelia. Our previous study (11) revealed that FGF10 stimulates the
expression and production of mucin in rat Cj-ECs. In the present
study, it was observed in corneal epithelial cells that the
expression of Muc1 was significantly upregulated by FGF10
treatment. In Cj-ECs, the normal control group exhibited the
highest expression level of Muc1, which was higher than that of the
FGF10 treatment group. This indicated that, in this short-term
animal model of dry eye, conjunctival injury may be so severe in
the Con A injection animals that the Cj-ECs were unable compensate
for the lack of moisture. Caffery et al (31) reported that higher expression
levels of Muc1 in patients with Sjogren's syndrome may represent
compensatory or protective responses to chronic insult to the
ocular surface. The expression levels of Muc1 in the conjunctiva
may be complex and exhibit different expression levels over the
course of the progression of dry eye. As Muc1 was significantly
upregulated by FGF10 treatment in the present study, it was
hypothesized that FGF10 promotes the expression of Muc1. A
long-term follow-up investigation is, however, required in order to
confirm this conclusion.
In conclusion, the data reported in the present
study indicated that the rabbit inflammation-induced dry eye model
may be successfully established within 3 days following Con A
injection, and that FGF10 may exert the following effects in the
rabbit dry eye model: Increased TMH and TMA, corneal epithelial
healing, reduced apoptosis of the corneal- and Cj-ECs and increased
Muc1 production. As the present study is the first, to the best of
our knowledge, to examine the effect of FGF10 in the rabbit dry eye
model, the biological function data associated with FGF10 suggested
that FGF10 may be a promising candidate for the treatment of dry
eye.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (grant no. 81200713), the Natural
Science Foundation of Shanghai, China (grant no. 09ZR1425400) and
the Foundation of Shanghai Jiaotong University, Shanghai, China
(grant no. YG2011MS62).
References
|
1
|
Lemp MA: Advances in understanding and
managing dry eye disease. Am J Ophthalmol. 146:350–356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The definition and classification of dry
eye disease: Report of the definition and classification
subcommittee of the international dry eye WorkShop 2007. Ocul Surf.
5:75–92. 2007. View Article : Google Scholar
|
|
3
|
Friedman NJ: Impact of dry eye disease and
treatment on quality of life. Curr Opin Ophthalmol. 21:310–316.
2010.PubMed/NCBI
|
|
4
|
The epidemiology of dry eye disease:
Report of the epidemiology subcommittee of the international dry
eye WorkShop 2007. Ocul Surf. 5:93–107. 2007. View Article : Google Scholar
|
|
5
|
Uchino M, Dogru M, Uchino Y, Fukagawa K,
Shimmura S, Takebayashi T, Schaumberg DA and Tsubota K: Japan
Ministry of Health study on prevalence of dry eye disease among
Japanese high school students. Am J Ophthalmol. 146:925–929. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo B, Lu P, Chen X, Zhang W and Chen R:
Prevalence of dry eye disease in Mongolians at high altitutde in
China: The Henan eye study. Ophthalmic Epidemiol. 17:234–241. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schein OD, Muñoz B, Tielsch JM,
Bandeen-Roche K and West S: Prevalence of dry eye among the
elderly. Am J Opthalmol. 124:723–728. 1997. View Article : Google Scholar
|
|
8
|
Brewitt H and Sistani F: Dry eye disease:
The scale of the problem. Surv Ophthalmol. 45(Suppl2): S199–S202.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baudouin C: The pathology of dry eye: Surv
Ophthalmol. 45(Suppl2): S211–S220. 2001.
|
|
10
|
Rolando M and Zierhut M: The ocular
surface and tear film and their dysfunctionin dry eye disease. Surv
Ophthalmol. 45(Supp12): S207–S210. 2001.
|
|
11
|
Ma MM, Zhang ZW, Niu W, Kelimu J, Ke B and
Zhang Z: Fibroblast growth factor 10 upregulates the expression of
mucins in rat conjunctival epithelial cells. Mol Vis. 17:2789–2797.
2011.PubMed/NCBI
|
|
12
|
Marchese C, Felici A, Visco V, Lucania G,
Igarashi M, Picardo M, Frati L and Torrisi MR: Fibroblast growth
factor 10 induces proliferation and differentiation of human
primary cultured keratinocytes. J Invest Dermatol. 116:623–628.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jurjus A, Atiyeh BS, Abdallah IM, Jurjus
RA, Hayek SN, Jaoude MA, Gerges A and Tohme RA: Pharmacological
modulation of wound healing in experimental burns. Burns.
33:892–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Zhou X, Ma J, Jiao Y, Zhang R,
Huang Z, Xiao J, Zhao B, Qian H, Li X and Tian H: Effects of
keratinocyte growth factor-2 on corneal epithelial wound healing in
a rabbit model of carbon dioxide laser injury. Biol Pharm Bull.
33:971–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gipson IK: The ocular surface: The
challenge to enable and protect vision-the friendenwald lecture.
Invest Ophthalmol Vis Sci. 48:4391–4398. 2007. View Article : Google Scholar
|
|
16
|
Gipson IK and Argüeso P: Role of mucins in
the function of the corneal and conjunctival epithelia. Int Rev
Cytol. 231:1–49. 2003. View Article : Google Scholar
|
|
17
|
Dartt DA: Control of mucin production by
ocular surface epithelial cells. Exp Eye Res. 78:173–185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Micera A, Stampachiacchiere B, Normando
EM, Lambiase A and Bonini S and Bonini S: Nerve growth factor
modulates toll-like receptor (TLR) 4 and 9 expression in cultured
primary VKC conjunctival epithelial cells. Mol Vis. 15:2037–2044.
2009.PubMed/NCBI
|
|
19
|
Gu J, Chen L, Shatos MA, Rios JD, Gulati
A, Hodges RR and Dartt DA: Presence of EGF growth factor ligands
and their effects on cultured rat conjunctival goblet cell
proliferation. Exp Eye Res. 86:322–334. 2008. View Article : Google Scholar
|
|
20
|
Savini G, Barboni P and Zanini M: Tear
meniscus evaluation by optical coherence tonmgraphy. Ophthalmic
Surg Lasers Imaging. 37:112–118. 2006.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2010.
View Article : Google Scholar
|
|
22
|
Feinstein AR: Principle of Medical
Statistics. Chapman and Hall/CRC; London: 2005
|
|
23
|
Rosner B: Fundamentals of Biostatistics
6th Edition. Brooks Cole; Pacific Grove, CA: 2005
|
|
24
|
Nagelhout TJ, Gamache DA, Roberts L, Brady
MT and Yanni JM: Preservation of tear film integrity and inhibition
of corneal injury by dexamethasone in a rabbit model of lacrimal
gland inflammation-induced dry eye. J Ocular Pharmacol Ther.
21:139–148. 2005. View Article : Google Scholar
|
|
25
|
Baikoff G, Lutun E, Ferraz C and Wei J:
Analysis of the eye's anterior segment with optical coherence
tomography. Static and dynamic study. J Fr Ophtalmol. 28:343–352.
2005.In French. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baikoff G, Lutun E, Ferraz C and Wei J:
Static and dynamic analysis of the anterior segment with optical
coherence tomography. J Cataract Refract Surg. 9:1843–1850. 2004.
View Article : Google Scholar
|
|
27
|
Nguyen P, Huang D, Li Y, Sadda SR, Ramos
S, Pappuru RR and Yiu SC: Correlation between optical coherence
tomography-derived assessments of lower tear meniscus parameters
and clinical features of dry eye disease. Cornea. 31:680–685. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohuchi H, Hori Y, Yamasaki M, Harada H,
Sekine K, Kato S and Itoh N: FGF10 acts a major ligand for FGF
receptor 2 IIIb in mouse multi-organ development. Biochem Biophys
Res Commum. 277:643–649. 2000. View Article : Google Scholar
|
|
29
|
Upadhyay D, Panduri V and Kamp DW:
Fibroblast growth factor-10 prevents asbestos-induced alveolar
epithelial cell apoptosis by a mitogen-activated protein
kinase-dependent mechanism. Am J Respir Cell Mol Biol. 32:232–238.
2005. View Article : Google Scholar
|
|
30
|
Tai CC, Curtis JL, Sala FG, Del Moral PM,
Chokshi N, Kanard RJ, Al Alam D, Wang J, Burns RC and Ford HR:
Induction of fibroblast growth factor 10 (FGF10) in the ileal crypt
epithelium after massive small bowel resection suggests a role for
FGF10 in gut adaptation. Deve Dyn. 238:294–301. 2009. View Article : Google Scholar
|
|
31
|
Caffery B, Heynen ML, Joyce E, Jones L,
Ritter R III and Senchyna M: MUC1 expression in Sjogren's syndrome,
KCS and control subjects. Mol Vis. 16:1720–1727. 2010.PubMed/NCBI
|