Introduction
Diabetes mellitus is the most important risk factor
for cardiovascular disease (CVD) (1). The International Diabetes Federation
estimates there were 366,000,000 individuals with diabetes in 2011,
and this is expected to rise to 552,000,000 by 2030 (2). Despite being aware of its severity,
the underlying mechanism and therapy of diabetes and its
complications remains to be fully elucidated and are underused
(2).
Atherosclerosis (AS), the predominant cause of CVD,
which causes the majority of diabetes-associated mortality and much
of the disability associated with diabetes (3). Macrophages are key in all phases of
atherosclerosis, from the formation of the fatty steak to plaque
rupture (4). Glucosamine is the
product of the hexosamine biosynthetic pathway and, as an effective
nutritional supplement used in human osteoarthritis, glucosamine
supplementation appears to be safe, with no adverse vascular
consequences (4). Orally
administered glucosamine sulfate has been observed to exert an
anti-atherogenic effect (5).
However, in patients with myocardial infarction without diabetes,
higher concentrations of glucosamine were noted, compared with
those with myocardial infarction and diabetes, suggesting that
glucosamine levels may be not directly determined by glucose
concentrations (6). There may be
other mechanisms (6), and data
from animal models and cell culture experiments also support the
suggestion that glucosamine disturb lipid metabolism, increase the
area of atherosclerotic lesions, induce apoptosis-associated
protein expression and accelerate atherosclerosis (7,8).
These findings suggest that glucosamine is not suitable as an
effective preventive strategy for diabetes, however, these changes
were independent of blood glucose concentration, glucose tolerance,
plasma insulin or plasma lipid levels (7,8). In
addition, similar to the above studies, glucosamine has been
recognized as a potent inducer of endoplasmic reticulum (ER) stress
(9). Therefore, the use of agents
against apoptosis, dyslipidemia and ER stress may offer the
possibility for improvement of diabetes.
Quercetin, a natural flavonoid, is reported to
possess several medicinal and therapeutic properties. Epidemiologic
studies have suggested that quercetin may reduce the risk of
cardiovascular disease (10–14).
It can reduce systolic blood pressure, decrease plasma
concentrations of total cholesterol, triglycerides and atherogenic
oxidized-low density lipoprotein (ox-LDL), and increase the
concentration of high density lipoprotein (HDL)-cholesterol
(10,13,14).
In addition, the administration of quercetin to mice (15,16)
and rabbits (17,18) inhibits the development of
atherosclerosis through its anti-oxidant and anti-inflammatory
mechanisms. It is also effective in controlling fasting and
postprandial blood glucose levels in animal models of diabetes
mellitus (19). In vitro
studies have demonstrated that activated macrophages may be a
potential target of dietary flavonoids in the aorta (20), and they may act work on key
atherogenic activities of macrophages, including foam cell
formation and pro-oxidant/pro-inflammatory responses (21). Excess or prolonged ER stress leads
to cell apoptosis (22,23), and the protective property of
quercetin against ER stress induced by ox-LDL, tunicamycin
(24) and Pb (25) have been confirmed. Although
quercetin supplementation has been demonstrated to inhibit high
glucose-induced apoptosis (26),
limited evidence is available regarding the benefits of quercetin
on macrophage apoptosis induced by glucosamine, and the mechanisms
require further elucidation.
In the present study, 15 mM glucosamine and
tunicamycin-treated RAW264.7 macrophages were used to investigate
whether quercetin administration results in the alleviation of
apoptosis and lipid accumulation, and to examine whether this
protective effect of quercetin is, in part, due to the inhibition
of ER stress. This aimed to provide the basis for identifying novel
molecular targets and therapeutic agents for treatment against
diabetes associated atherosclerosis.
Materials and methods
Materials and reagents
The RAW264.7 mouse macrophage cell line was
purchased from the Type Culture Collection of the Chinese Academy
of Medical Sciences (Beijing, China). Dulbecco's modified Eagle's
medium (DMEM) and fetal bovine serum (FBS) were from Gibco Life
Sciences (Carlsbad, CA, USA). Quercetin (≥95%, high performance
liquid chromatography), N-Acetyl-D-glucosamine and
tunicamycin (from Streptomyces sp.) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). An Annexin-V-Fluos staining kit
was purchased from Roche Diagnostics (Mannheim, Germany). A
One-step Terminal-deoxynucleotidyl Transferase Mediated Nick End
Labeling (TUNEL) apoptosis in situ detection kit (cat. no.
KGA7061) and poly-L-lycine (cat. no. KGF026) were obtained from
KeyGEN Biotech Co., Ltd. (Nanjing, China). The tissue/cell
total/free cholesterol assay kits (cat. no. E1015 and E1016) were
from Applygen Technologies, Inc. (Beijing, China). Polyclonal
antibodies against glucose regulated protein 78 (GRP78; cat. no.
3183), C/EBP homologous protein (CHOP; cat. no. 2895) and β-actin
(cat. no. 4970) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA); those against c-Jun N-terminal kinase (JNK)
(sc-7345), phosphorylated (p-)JNK (cat. no. sc-6254) and caspase-12
(cat. no. sc-5627) were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, US), and antibody against activating
transcriptional factor 6α (cat. no. ATF6α; full length) was
obtained from Acris Antibodies GmbH (Herford, Germany). Goat
anti-mouse IgG (H+L)-horeseradish peroxidase (HRP; cat. no.
ZB-2305) and goat anti-rabbit IgG (H+L)-HRP (cat. no. ZB-2301) were
obtained from Beijing Zhongshan Golden Bridge Biotchnology Co.,
Ltd. (Beijing, China).
Cell culture and treatment
The RAW264.7 cells were cultured in DMEM with 10%
FBS at 37°C in a humidified, 5% CO2 atmosphere. The
cells were subcultured in culture flasks (Corning Incorporated,
Corning, NY, USA) and passaged every 3–4 days. The cells were
cultured in serum-free medium for 12 h prior to the subsequent
intervention once grown to 70% confluence. The cells were treated
with 15 mM glucos-amine, with or without different doses of
quercetin (5, 10 and 20 µM) for 24 h. In another group of
experiments, the cells were pretreated with 5 µg/ml
tunicamycin for 4 h followed by treatment with glucosamine and
quercetin. Cells incubated in normal medium (DMEM with 10% FBS) for
24 h were used as a vehicle group, and cells treated with 15 mM
mannitol, instead of glucosamine, for 24 h were used as an
osmolarity control. All the above cells were incubated at 37°C in a
humidified, 5% CO2 atmosphere.
Flow cytometric analysis
Following treatment in 60 mm culture dishes, the
cells (1×106) were digested using the trypsin
enzymwithout EDTA, following which were harvested, washed with
phosphate-buffered saline (PBS) and centrifuged at 200 × g for 5
min. The cell pellet was then resuspended in 100 µl of
Annexin-V-Fluos labeling solution, and incubated for 15 min at
25°C. Finally, the cells were analyzed on a flow cytometer
(FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) at 488 nm
excitation, with a 515 nm bandpass filter for fluorescein detection
and a filter >600 nm for propidium iodide (PI) detection.
TUNEL staining
DNA fragmentation provides markers of apoptosis,
which was assessed in the present study using a TUNEL assay. The
cells (2.5×106) were treated on cover slips with
poly-L-lycine in 6-well plates, and were fixed in 4%
paraformaldehyde for 30 min at room temperature. The cells were
then washed three times with 1X PBS, and 1% Triton-X-100 was added
for 5 min to promote permeability, followed by washing with 1X PBS.
The cells were then blocked with 3% H2O2, and
incubated with 50 µl TdT enzyme reaction solution,
containing 45 µl equilibration buffer, 1.0 µl
TRITC-5-dUTP (cat. no. KGA7061; KeyGEN Biotech Co., Ltd.) and 4.0
µl TdT enzyme, for 60 min at 37°C in the dark. Using 543 nm
excitation and 571 nm detection wavelengths, the fluorescence
intensity was analyzed using a fluorescence microscope (Nikon
Eclipse TE2000-S; Nikon Corporation, Tokyo, Japan), with apoptotic
cells exhibiting intense red fluorescence.
Intracellular total cholesterol and free
cholesterol assay
The accumulation of cholesterol in the cells is
closely associated with the formation of foam cells. Following
starvation for 12 h, the cells were treated with glucosamine and
quercetin for 24 h, and then co-incubated with 50 mg/l ox-LDL for
12 h. Following the treatments described above, the cells were
washed three times with 1X PBS, and 1×106 cells were
lysed in 0.1 ml lysis buffer provided in the tissue/cell total/free
cholesterol assay kits (cat. nos. E1015 and E1016; Applygen
Technologies, Inc.). The total lysates were centrifuged at 2,000 g
for 5 min, and the supernatant was harvested and divided into three
equal volumes to be prepared for the measurement of protein
concentration, total cholesterol (TC) and free cholesterol (FC)
content. The samples (10 µl) were then assayed for TC and FC
using 190 µl enzyme regent of the GPO Trinder enzymatic
reaction in a 96-plate well. The absorbtion was measured at 550 nm
on a BioTek EON™ plate reader (BioTek Instruments, Inc., Winooski,
VT, USA). Protein content was determined using a Bicinchoninic Acid
(BCA) Protein Assay kit (Beyotime Institute of Biotechnology,
Haimen, China), with the concentrations of TC and FC expressed as
nmol/mg total cellular protein.
Western blot analysis
Following treatment for 24 h in 10 mm culture
dishes, the cells (1×107)were washed three times with
ice-cold 1X PBS and lysed for 30 min in lysis buffer with 1 mM
phenylmethylsulfonyl fluoride (cat. no. 78830; Sigma-Aldrich). The
total protein lysates were centrifuged at 12,000 g for 5 min at
4°C, and the protein content in the supernatants were quantified
using a BCA Protein Assay kit. The protein samples (20 µg)
from each group were mixed with 5X sample loading buffer and were
used for western blotting analysis through 8, 10 or 12% SDS-PAGE
(for GRP78 and ATP6α; JNK, p-JNK, caspase-12 and β-actin; and CHOP,
respectively; cat. no. KGP113K, KeyGEN Biotech Co., Ltd.), followed
by transfer onto 0.45-µm (pore size) polyvinylidene fluoride
membranes (EMD Millipore, Bedford, MA, USA) through a wet transfer
method in an ice-bath for 2 h. The membranes were blocked with 5%
(v/v) skim milk at room temperature for 1 h, and incubated with
primary antibody diluted with 5% bovine serum albumin (cat. no.
A4737; Sigma-Aldrich) at 4°C overnight. The following primary
antibodies were used: GRP78, CHOP and ATF6α (1:1,000), β-actin
(1:2,000) and, JNK, p-JNK and caspase-12 (1:200). Following washing
the membranes three times, they were incubated with HRP-conjugated
secondary antibodies (1:4,000), diluted with 5% bovine serum
albumin, for 1 h at 37°C and detected using an enhanced
chemiluminescence system (ECL; M&C Gene Technology, Ltd,
Beijing, China). Finally, images were captured using a digital
camera and were analyzed using ImageJ software (version 1.46r;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. One-way analysis of
variance was used to compare differences, followed by Dunnett-t
multiple comparison tests with SPSS 13.0 for Windows (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
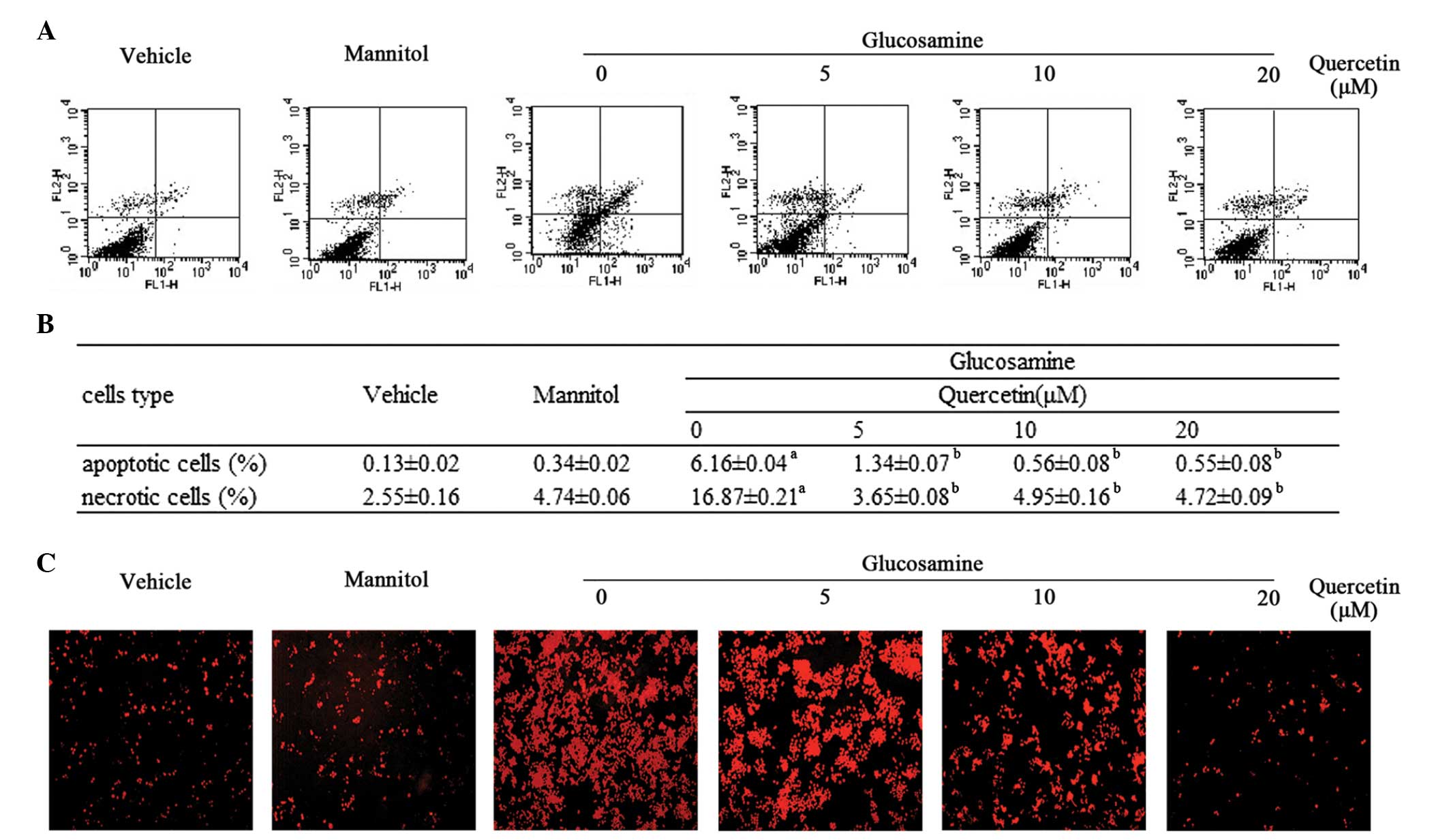
Quercetin protects RAW264.7 cells from
apoptosis and cell death induced by glucosamine
The anti-apoptotic effect of quercetin against
glucosamine in RAW264.7 cells was evaluated using Annexin-V-FITC/PI
double staining and detection using flow cytometry. As shown in
Fig. 1A and B, the treatment of
RAW264.7 cells with 15 mM glucosamine for 24 h induced apoptosis,
compared with the vehicle control cells (6.16±0.04, vs. 0.13±0.02%,
respectively), however, quercetin (5, 10 and 20 µM)
significantly attenuated glucosamine-induced apoptosis in a dose
dependent manner. Consistent with the above, in the RAW264.7 cells
stained with TUNEL, the number of positive cells was markedly
higher in the glucosamine-induced cells, compared with the vehicle
control cells. Representative photomicrograph are presented in
Fig. 1C, with the red
TUNEL-positive cells clearly marked.
In addition to its anti-apoptotic property, the
present study also determined the protective effect of quercetin on
glucosamine-induced cell death (Fig.
1A and B). The addition of glucosamine at 15 mM for 24 h
increased cell death, compared with the vehicle group (16.87±0.21,
vs. 2.55±0.16%, respectively). However, incubation of the
glucosamine-induced cells with different doses of quercetin (5, 10
and 20 µM) resulted in a dose-dependent decrease in the
number of necrotic cells.
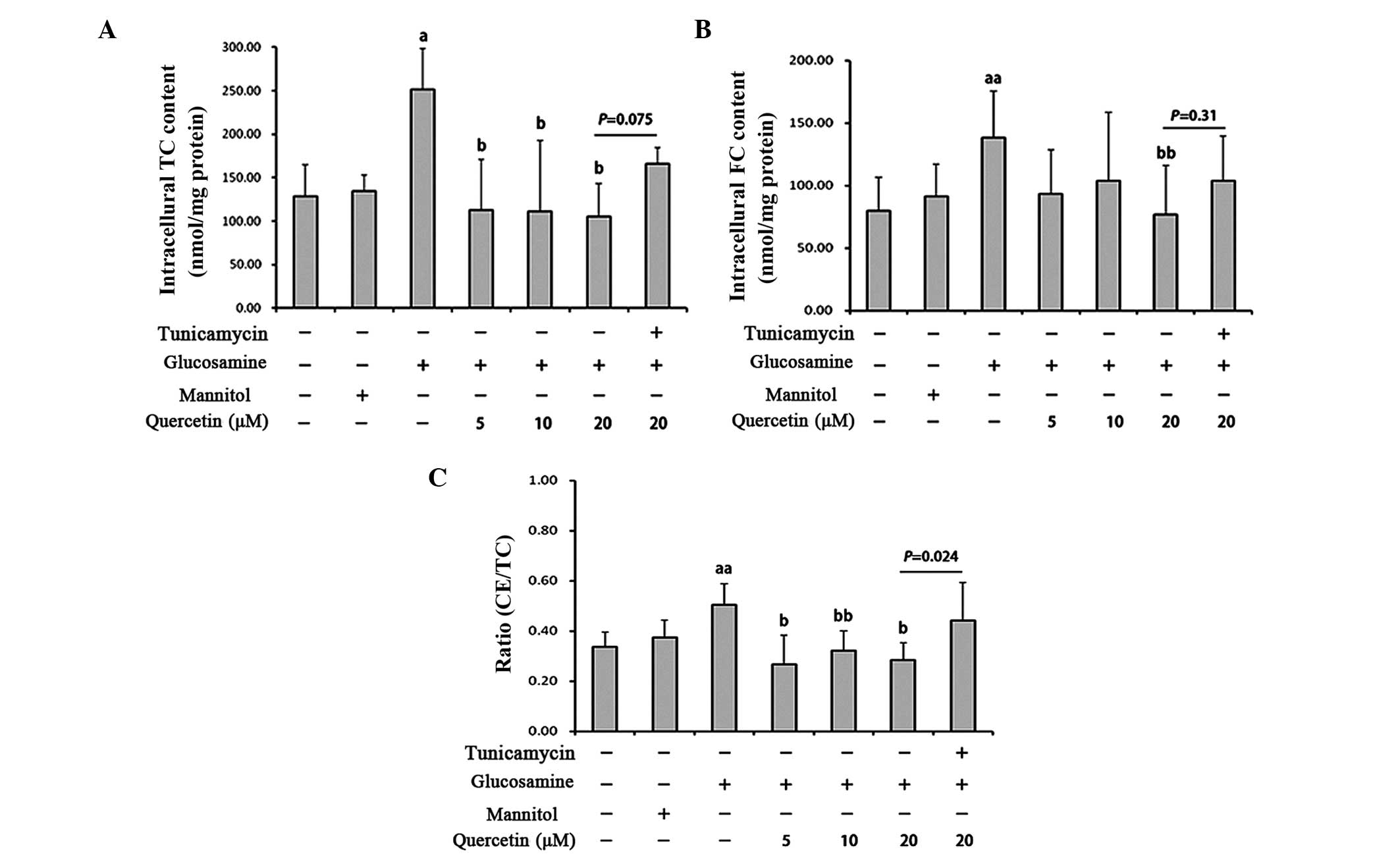
Quercetin attenuates glucosamine-induced
lipid accumulation in RAW264.7 cells
Foam cell formation is a key characteristic of AS
(27) and can be induced by excess
ox-LDL. To determine the effect of quercetin on glucosamine-induced
foam cell formation in the present study, the RAW264.7 macrophages
were incubated with 50 mg/l ox-LDL for 12 h following glucosamine
and quercetin treatment, and subsequent intracellular TC, FC and
cholesteryl ester (CE) quantification were performed. Compared with
the vehicle control, the addition of ox-LDL to the culture medium
including glucosamine amplified foam cell formation as the
intracellular TC content (1.96-fold) and FC content (1.74-fold)
were significantly increased, the ratio of CE/TC was also
significantly increased in the glucosamine-treated group. Treatment
with quercetin significantly decreased the TC content, FC content
and ratio of CE/TC (Fig. 2A–C).
However, following treatment with tunicamycin, an ER
stress-inducing agent, these effects of quercetin disappeared.
Tunicamycin treatment of the cells treated with quercetin and
glucosamine led to a significant increase in intracellular TC
content and CE/TC ratio, compared with those without tunicamycin
(Fig. 2A–C). These results
indicated that quercetin prevented the amplification of
ox-LDL-induced foam cell formation by glucosamine via ER stress in
the RAW264.7 cells.
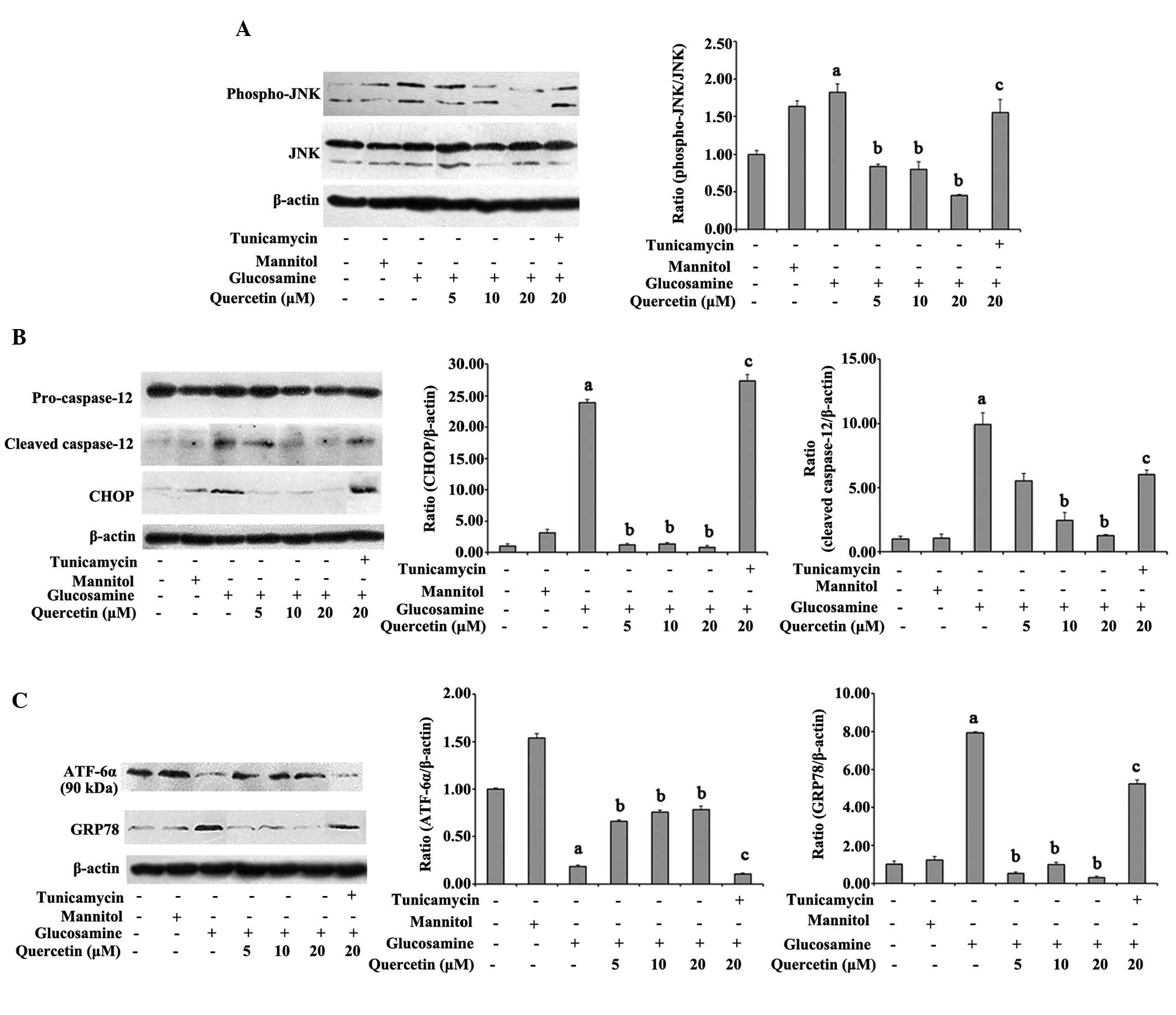
Effect of quercetin on the expression of
p-JNK and JNK
JNK activation is known to affect the cell-death
mechanism which is involved in ER stress (28–30).
To investigate whether JNK was involved in the effect of quercetin
on ER stress, the present study detected the expression of pJNK and
JNK. The results demonstrated that p-JNK in the glucosamine-treated
cells, the active form of JNK, was significantly increased,
compared with that of the vehicle control cells (P<0.05).
However, quercetin significantly inhibited the phosphorylation of
JNK in a dose-dependent manner. In addition, JNK phosphorylation
was not reversed by quercetin in the presence of tunicamycin
(Fig. 3A).
Effect of quercetin on the expression
levels of CHOP and caspase-12
In addition to JNK activation, C/EBP homologous
proteins and caspase-12 are the other two apoptotic pathways
induced by ER stress (31). To
determine the effects of quercetin on CHOP and caspase-12, western
blot analysis was performed. Glucosamine treatment resulted in a
significant increase in the expression level of CHOP, compared with
the vehicle control (23.9-fold). The levels of cleaved caspase-12
were also markedly elevated in the glucosamine-treated cells,
compared with the vehicle control (P<0.05). Notably, 10 and 20
µM quercetin significantly decreased the expression of CHOP
and the activation of caspase-12, compared with the
glucosamine-treated cells (P<0.05). No significant changes in
the expression levels of CHOP or cleaved caspase-12 were observed
between the glucosamine-treated cells and quercetin-treated cells
in the presence of tunicamycin (P>0.05; Fig. 3B).
Quercetin protects against the
glucosamine-induced activation of ATF6α and GRP78
In order to identify the potential effect of
quercetin on glucosamine-induced ER stress in RAW264.7 macrophages,
the present study measured the expression levels of the ER stress
marker, GRP78, and the ER membrane-anchored transcription factor,
p90-ATF6α. As shown in Fig. 3C,
the glucosamine-treated cells exhibited activation of ER stress
markers, with the cells in glucosamine group exhibiting a
significant 7.94-fold increase in GRP78, compared with the vehicle
control cells. In addition, the expression levels of p90-ATF6α, the
inactive form of ATF6α, were markedly decreased in the
glucosamine-treated cells, by 0.183-fold, compared with the vehicle
control. Notably, quercetin restored these changes in the levels of
GRP78 and p90-ATF6α, whereas tunicamycin suppressed the effects of
quercetin on the expression levels of GRP78 and p90-ATF6α (Fig. 3C).
Discussion
Animal studies have suggested that hyperglycemia is
an independent risk factor of AS. The conditions of hyperglycemia
give rise to the accumulation of glucosamine in the vessel wall and
accelerate AS (8,32). In human aortic smooth muscle cells
(HASMC), monocytes and hepatocytes, glucosamine disturbs lipid
metabolism, leading to the accumulation of cholesterol in cultured
cells (8), and lipid deposition
and macrophage apoptosis are key in the development of AS (33–35).
Thus, the inhibition of lipid dysregulation and macrophage
apoptosis may be effective strategy in preventing the development
and progression of diabetic atherosclerosis. Quercetin is reported
to possess properties against diabetes and CVD (10–14,19).
It can also suppress macrophage apoptosis and foam cell formation
induced by tunicamycin and ox-LDL (24). However, the effect of quercetin on
glucosamine-induced lipid dysregulation and macrophage apoptosis,
and the possible mechanism remained to be elucidated. The present
study demonstrate that quercetin attenuated glucosamine-induced
apoptosis in RAW264.7 macrophages, and suppressing the ER stress
pathway by inhibiting ATF6 activation may be one of the mechanisms.
In addition, quercetin prevented the formation of foam cells
through the ER stress pathway, which may be another cytoprotective
mechanism.
Lipid accumulation is one of the predominant
characteristics of AS. Free cholesterol loading has been
demonstrated to be associated with the activation and apoptosis of
macrophages (33). Although a
previous study demonstrated that intraperitoneal glucosamine
decreased AS in ApoE−/− mice (36), glucosamine supplementation has been
observed to increase the initiation of AS after 5 weeks, and
cholesterol and triglyceride concentrations increase significantly
following glucosamine treatment for 20 weeks (4). HASMC and HepG2 cells treated with
glucosamine exhibit increased intracellular levels of free
cholesterol (8). Quercetin has
been reported to interfere with key atherogenic progresses of
macrophages, including foam cell formation (21), anti-oxidation (37), anti-inflammation (17) and the regulation of several genes
involved in lipid metabolism (20,38).
The present study demonstrated that the effects of 50 mg/l ox-LDL
added to macrophages were aggravated by glucosamine treatment, as
intracellular TC and FC contents increased markedly following
glucosamine-treatment, with a concomitant increase in the CE rate
of TC (50.5%). These results were characteristic of foam cells
(39). By contrast, the
supplementation of quercetin to the glucosamine and ox-LDL-induced
macrophages alleviated ox-LDL uptake and suppressed the formation
of foam cells in the RAW264.7 cells. Of note, classical ER-stress
inducer treatment reversed the beneficial effects of quercetin on
ox-LDL uptake and the formation of foam cells. These results
suggested that ER stress may be involved in the glucosamine-induced
dysregulation of lipid metabolism.
Increasing evidence supports the suggestion that
glucosamine induces the apoprosis of various cels, including rat
mesangial cells (40) and
pancreatic β cells (41). Although
limited information is available describing the harmful effects of
glucosamine on macrophages, elevated glucose increases flux through
the hexosamine biosynthetic pathway, which ultimately leads to
increased glucosamine concentrations (42–44).
In a study investigating subjects with type 2 diabetes, increased
levels of macrophage apoptosis were observed in their
atherosclerotic lesions, and this may be a risk factor of plaque
destabilization and acute clinical events (45). In addition, Khan (32) detected increased O-GlcNAc staining,
an indicator of intracellular glucosamine concentration (46), in macrophage/foam cells of the
atherosclerotic lesions in hyperglycemic ApoE−/− mice, compared
with normoglycemic rice. As mentioned previously, quercetin has
been observed to prevent macrophages from apoptosis induction by
high glucose (26), ox-LDL
(24) and
H2O2 (37).
However, whether quercetin inhibits glucosamine-induced macrophage
apoptosis had not previously reported. The results of the present
study suggested that macrophages treated with 15 mM glucosamine for
24 h exhibited increased apoptotic and necrotic rates, compared
with cells in the vehicle control group. However, the addition of
quercetin to the glucosamine-treated macrophages significantly
decreased the apoptotic and necrotic rates of the cells.
It has been reported that quercetin protects
multiple types of cells from apoptosis through various mechanisms.
Roshanzamir et al (47)
reported that quercetin inhibits H2O2-induced
SK-N-MC cell death through the downregulation of HIF-1α, Foxo-3a
and NICD, as well as pro-apoptotic factors, including p53 and Bax.
Suganya et al (48)
observed that quercetin has an anti-apoptotic effect against
tunicamycin-induced endothelial cell toxicity through the
ER-stress/CHOP pathway, which involved an increase in the
expression of B cell lymphoma-2 (Bcl-2) and reduction in the
expression of Bcl-2-associated X protein (Bax). Similar results
have been observed in RAW264.7 macrophages (24). Our previous study suggested that
quercetin prevents β-cell death through the mitochondrial pathway
and nuclear factor-κB signaling in RINm5F rat insulinoma cells
(49). Therefore, the biological
effects of quercetin on cells remain to be fully elucidated. In the
present study, it was observed that quercetin significantly reduced
the expression of CHOP and suppressed the phosphorylation of JNK
and activation of caspase-12 by inhibiting the expression of GRP78
and activation of ATF6α in the glucosamine-treated RAW264.7
macrophages. Tunicamycin is a typical ER stress inducer via
interfering with N-linked protein glycosylation in ER (50). The present study also found that
quercetin did not attenuate glucosamine-induced lipid accumulation
in tunicamycin-pretreated cells. Compared with the
glucosamine-induced cells without tunicamycin, quercetin had no
significant effect on the expression of CHOP, the activities of JNK
and caspase-12, GRP78 or full length ATF6α following tunicamycin
treatment. However, no detailed indicator of ER stress-lipid
metabolism or other ER stress markers, including as protein
kinase-like ER kinase or intercellular adhesion molecule-1 (IRE1)
were detected. These data indicated that the ER stress pathway is
involved in the beneficial effects of quercetin on
glucosamine-induced cell damage.
The ER is located in the cytoplasm close to the cell
nucleus, and is a vital organelle responsible for protein
biosynthesis, post-translational modifications, folding and
assembly of polypeptide chains, intracellular calcium storage and
controlling lipid biosynthesis (51). Multiple stimuli, including
hyperglycemia (52), hypoxia
(53) and chemical toxicity
(54) can lead to ER dysfunction
and ER stress, which is an adaptive signaling mechanism, termed the
unfolded protein response, to restore ER homeostasis. However,
continuous and serious ER stress triggers a series of processes
necessary for apoptosis, including CHOP, JNK and caspase-12
signaling (55,56). Accumulated evidence indicates the
role of CHOP on various aspects of cell damage associated with ER
stress (57,58). CHOP−/− animals exhibit
four-fold lower levels of TUNEL-positive cells in the proximal tube
epithelium (57).
CHOP−/−/ApoE−/− mice exhibit minimal
apoptosis of macrophages in plaques and, compared with high
cholesterol-fed CHOP−/−/ApoE−/− mice, high
cholesterol-fed CHOP+/+/ApoE−/− mice exhibit
increased rupture of atherosclerotic plaques. Tsukano et al
(58) suggested that ER
stress-CHOP-Bax-mediated apoptosis contributes to these changes.
JNK activation is key in the process of cell apoptosis induced by
ER stress (28). In response to ER
stress, IRE-1α activates JNK directly (59). Caspase-12, a caspase localized to
the ER, is activated to generate cleaved caspase-12 induced by ER
stress (60). Nakagawa et
al (61) suggested that
treatment of PC12 cells or embryonic fibroblast cells with
tunicamycin and thapsigargin induces effective cleavage of
caspase-12. Furthermore, in vivo, caspase-12−/−
mice were found to be resistant to ER stress-induced apoptosis. In
the present study, it was demonstrated that glucosamine-treatment
increased the expression of CHOP, induced the cleavage of
caspase-12 and activated JNK in the RAW264.7 cells. However,
quer-cetin significantly inhibited the cleavage of caspase-12 and
the activation of JNK, and suppressed the expression levels of
CHOP, whereas these changes were reversed by tunica-mycin, which
has been used in the analyses of CHOP- or caspase-12-deficient mice
(57,61). Therefore, the effects of quercetin
contributed to the recovery of RAW264.7 cell damage by
glucosamine.
ATF6 is one of three ER stress sensors, and is an
ER-resident transmembrane protein, which is vital for proper
protein folding and assembly (55,62).
ER stress results in the dissociation of GRP78 from the ER membrane
proteins, including ATF6, and activated ATF6 translocates to the
golgi for cleavage and causes activation of downstream signaling
pathways (51). ATF6 consists of
ATF6α and ATF6β in mammalian cells, however, previous studies have
suggested that ATF6α is more sensitive to ER stress (63,64),
Rutkowski et al (65)
observed that ATF6α-deficient mice were induced with unresolved ER
stress following tunicamycin injection, and these alterations
increased the expression of CHOP. Yamamoto et al (64) demosntrated that the levels of
triacylglycerol and TC in the liver of ATF6α−/− mice
were significantly increased, compared with those of
ATF6α+/+ mice following tunicamycin injection, and that
tunicamycin injection caused the accumulation of neutral lipids in
the liver of the ATF6α−/− mice. Yao et al
(24) suggested that quercetin
inhibits the translocation of ATF6 from the cytoplasm into the
nucleus in RAW264.7 cells. In the present study, it was revealed
that tunicamycin treatment also caused significant lipid
accumulation in glucosamine-treated cells treated with quercetin.
Notably, glucosamine treatment significantly increased the
expression of GRP78 and induced the activation of ATF6α, and
decreased the expression levels of 90 kDa ATF6α (full-length),
which is an inactive form of ATF6α. However, the expression of
ATF6α in the quercetin-treated RAW264.7 cells were increased
significantly, compared with the glucosamine-treated cells.
Quercetin also alleviated the expression of GRP78, whereas,
tunicamycin treatment inhibited these effects of quercetin.
In conclusion, the present study suggested that
apoptosis and lipid accumulation in RAW264.7 cells induced by
glucosamine were partially due to ER stress. Quercetin
supplementation inhibited ER stress by decreasing the expression of
CHOP, and inhibiting the activation of JNK and caspase-12. Although
there may be other mechanisms, the results suggested that the
inhibitory effects of quercetin on GRP78 and ATF6α activation
contributed, at least in part, to the above alterations. These
findings support the anti-atherosclerotic effects and mechanisms of
quercetin upon diabetes in vitro. However, a series of
questions remain due to the complex and unclear mechanism of ER
stress. Further in vitro and vivo investigations on
the effect of quercetin on ER stress are necessary to further
elucidate the mechanisms of atherosclerosis in diabetes and the
identification of novel targets for therapy.
Abbreviations:
|
AS
|
atherosclerosis
|
|
ATF6
|
activating transcriptional factor
6
|
|
CHOP
|
C/EBP homologous protein
|
|
CVD
|
cardiovascular disease
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose regulated protein 78
|
|
IRE1
|
inositol requiring enzyme-1
|
|
JNK
|
c-Jun N-terminal kinase
|
|
Phospho-JNK
|
phosphorylated-JNK
|
|
TUNEL
|
terminal-deoxynucleoitidyl transferase
mediated dUTP nick end labeling
|
Acknowledgments
This study was supported by a research grant from
the National Natural Science Foundation of China (grant. no.
81172652).
References
|
1
|
Stamler J, Vaccaro O, Neaton JD and
Wentworth D: Diabetes, other risk factors and 12-yr cardiovascular
mortality for men screened in the multiple risk factor intervention
trial. Diabetes Care. 16:434–444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tannock LR, Kirk EA, King VL, LeBoeuf R,
Wight TN and Chait A: Glucosamine supplementation accelerates early
but not late atherosclerosis in LDL receptor-deficient mice. J
Nutr. 136:2856–2861. 2006.PubMed/NCBI
|
|
5
|
Largo R, Martínez-Calatrava MJ,
Sánchez-Pernaute O, Marcos ME, Moreno-Rubio J, Aparicio C, Egido J
and Herrero-Beaumont G: Effect of a high dose of glucosamine on
systemic and tissue inflammation in an experimental model of
atherosclerosis aggravated by chronic arthritis. Am J Physiol Heart
Circ Physiol. 297:H268–H276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nowak A, Szcześniak L, Rychlewski T,
Dylewicz P and Przywarska I: Glucosamine levels in people with
ischaemic heart disease with and without type II diabetes. Pol Arch
Med Wewn. 100:419–425. 1998.
|
|
7
|
Beriault DR, Sharma S, Shi Y, Khan MI and
Werstuck GH: Glucosamine-supplementation promotes endoplasmic
reticulum stress, hepatic steatosis and accelerated atherogenesis
in apoE−/− mice. Atherosclerosis. 219:134–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werstuck GH, Khan MI, Femia G, Kim AJ,
Tedesco V, Trigatti B and Shi Y: Glucosamine-induced endoplasmic
reticulum dysfunction is associated with accelerated
atherosclerosis in a hyperglycemic mouse model. Diabetes.
55:93–101. 2006. View Article : Google Scholar
|
|
9
|
Qiu W, Avramoglu RK, Rutledge AC, Tsai J
and Adeli K: Mechanisms of glucosamine-induced suppression of the
hepatic assembly and secretion of apolipoprotein B-100-containing
lipo-proteins. J Lipid Res. 47:1749–1761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egert S, Bosy-Westphal A, Seiberl J,
Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J,
Schrezenmeir J, Rimbach G, et al: Quercetin reduces systolic blood
pressure and plasma oxidised low-density lipoprotein concentrations
in overweight subjects with a high-cardiovascular disease risk
phenotype: A double-blinded, placebo-controlled cross-over study.
Br J Nutr. 102:1065–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Larson A, Witman MA, Guo Y, Ives S,
Richardson RS, Bruno RS, Jalili T and Symons JD: Acute,
quercetin-induced reductions in blood pressure in hypertensive
individuals are not secondary to lower plasma
angiotensin-converting enzyme activity or endo-thelin-1: Nitric
oxide. Nutr Res. 32:557–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edwards RL, Lyon T, Litwin SE, Rabovsky A,
Symons JD and Jalili T: Quercetin reduces blood pressure in
hypertensive subjects. J Nutr. 137:2405–2411. 2007.PubMed/NCBI
|
|
13
|
Pfeuffer M, Auinger A, Bley U,
Kraus-Stojanowic I, Laue C, Winkler P, Rüfer CE, Frank J,
Bösch-Saadatmandi C, Rimbach G and Schrezenmeir J: Effect of
quercetin on traits of the metabolic syndrome, endothelial function
and inflammation in men with different APOE isoforms. Nutr Metab
Cardiovasc Dis. 23:403–409. 2013. View Article : Google Scholar
|
|
14
|
Knab AM, Shanely RA, Henson DA, Jin F,
Heinz SA, Austin MD and Nieman DC: Influence of quercetin
supplementation on disease risk factors in community-dwelling
adults. J Am Diet Assoc. 111:542–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleemann R, Verschuren L, Morrison M,
Zadelaar S, van Erk MJ, Wielinga PY and Kooistra T:
Anti-inflammatory, anti-proliferative and anti-atherosclerotic
effects of quercetin in human in vitro and in vivo models.
Atherosclerosis. 218:44–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garelnabi M, Mahini H and Wilson T:
Quercetin intake with exercise modulates lipoprotein metabolism and
reduces atherosclerosis plaque formation. J Int Soc Sports Nutr.
11:222014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhaskar S, Kumar KS, Krishnan K and Antony
H: Quercetin alleviates hypercholesterolemic diet induced
inflammation during progression and regression of atherosclerosis
in rabbits. Nutrition. 29:219–229. 2013. View Article : Google Scholar
|
|
18
|
Juźwiak S, Wójcicki J, Mokrzycki K,
Marchlewicz M, Białecka M, Wenda-Rózewicka L, Gawrońska-Szklarz B
and Droździk M: Effect of quercetin on experimental hyperlipidemia
and atherosclerosis in rabbits. Pharmacol Rep. 57:604–609.
2005.
|
|
19
|
Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM
and Kim JI: Quercetin attenuates fasting and postprandial
hyperglycemia in animal models of diabetes mellitus. Nutr Res
Pract. 5:107–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawai Y, Nishikawa T, Shiba Y, Saito S,
Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K and Terao J:
Macrophage as a target of quercetin glucuronides in human
atherosclerotic arteries: Implication in the anti-atherosclerotic
mechanism of dietary flavonoids. J Biol Chem. 283:9424–9434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lara-Guzman OJ, Tabares-Guevara JH,
Leon-Varela YM, Álvarez RM, Roldan M, Sierra JA, Londoño-Londoño JA
and Ramirez-Pineda JR: Proatherogenic macrophage activities are
targeted by the flavonoid quercetin. J Pharmacol Exp Ther.
343:296–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao W, Fukuoka S, Iwai C, Liu J, Sharma
VK, Sheu SS, Fu M and Liang CS: Cardiomyocyte apoptosis in
autoimmune cardiomyopathy: Mediated via endoplasmic reticulum
stress and exaggerated by norepinephrine. Am J Physiol Heart Circ
Physiol. 293:H1636–H645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada K, Minamino T, Tsukamoto Y, Liao Y,
Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani
T, et al: Prolonged endoplasmic reticulum stress in hypertrophic
and failing heart after aortic constriction: Possible contribution
of endoplasmic reticulum stress to cardiac myocyte apoptosis.
Circulation. 110:705–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao S, Sang H, Song G, Yang N, Liu Q,
Zhang Y, Jiao P, Zong C and Qin S: Quercetin protects macrophages
from oxidized low-density lipoprotein-induced apoptosis by
inhibiting the endoplasmic reticulum stress-C/EBP homologous
protein pathway. Exp Biol Med (Maywood). 237:822–831. 2012.
View Article : Google Scholar
|
|
25
|
Liu CM, Zheng GH, Ming QL, Sun JM and
Cheng C: Protective effect of quercetin on lead-induced oxidative
stress and endoplasmic reticulum stress in rat liver via the
IRE1/JNK and PI3K/Akt pathway. Free Radic Res. 47:192–201. 2013.
View Article : Google Scholar
|
|
26
|
Chao CL, Hou YC, Chao PD, Weng CS and Ho
FM: The antioxidant effects of quercetin metabolites on the
prevention of high glucose-induced apoptosis of human umbilical
vein endothelial cells. Br J Nutr. 101:1165–1170. 2009. View Article : Google Scholar
|
|
27
|
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y,
Meng G, Xie L, Wang J, Xiao Y, et al: Celastrol prevents
atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS
One. 8:e654772013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fribley A, Zhang K and Kaufman RJ:
Regulation of apoptosis by the unfolded protein response. Methods
Mol Biol. 559:191–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan MI, Pichna BA, Shi Y, Bowes AJ and
Werstuck GH: Evidence supporting a role for endoplasmic reticulum
stress in the development of atherosclerosis in a hyperglycaemic
mouse model. Antioxid Redox Signal. 11:2289–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tabas I: Apoptosis and plaque
destabilization in atherosclerosis: The role of macrophage
apoptosis induced by cholesterol. Cell Death Differ. 11(Suppl 1):
S12–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hansson GK: Inflammation, atherosclerosis
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han S, Liang CP, DeVries-Seimon T,
Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I and
Tall AR: Macrophage insulin receptor deficiency increases ER
stress-induced apoptosis and necrotic core formation in advanced
atherosclerotic lesions. Cell Metab. 3:257–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan W, Paka L and Pillarisetti S:
Distinct effects of glucose and glucosamine on vascular endothelial
and smooth muscle cells: Evidence for a protective role for
glucosamine in atherosclerosis. Cardiovasc Diabetol. 4:162005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chow JM, Shen SC, Huan SK, Lin HY and Chen
YC: Quercetin, but not rutin and quercitrin, prevention of
H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase
1 gene expression in macrophages. Biochem Pharmacol. 69:1839–1851.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garige M, Gong M, Varatharajalu R and
Lakshman MR: Quercetin up-regulates paraoxonase 1 gene expression
via sterol regulatory element binding protein 2 that translocates
from the endoplasmic reticulum to the nucleus where it specifically
interacts with sterol responsive element-like sequence in
paraoxonase 1 promoter in HuH7 liver cells. Metabolism.
59:1372–1378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fogelman AM, Shechter I, Seager J, Hokom
M, Child JS and Edwards PA: Malondialdehyde alteration of low
density lipoproteins leads to cholesteryl ester accumulation in
human monocyte-macrophages. Proc Natl Acad Sci USA. 77:2214–2218.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bao L, Cai X, Zhang Z and Li Y: Grape seed
procyanidin B2 ameliorates mitochondrial dysfunction and inhibits
apoptosis via the AMP-activated protein kinase-silent mating type
information regulation 2 homologue 1-PPARγ co-activator-1α axis in
rat mesangial cells under high-dose glucosamine. Br J Nutr. 1–10.
2014.Epub Ahead of Print.
|
|
41
|
Kang ES, Han D, Park J, Kwak TK, Oh MA,
Lee SA, Choi S, Park ZY, Kim Y and Lee JW: O-GlcNAc modulation at
Akt1 Ser473 correlates with apoptosis of murine pancreatic beta
cells. Exp Cell Res. 314:2238–2248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma J and Hart GW: Protein O-GlcNAcylation
in diabetes and diabetic complications. Expert Rev Proteomics.
10:365–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hawkins M, Angelov I, Liu R, Barzilai N
and Rossetti L: The tissue concentration of UDP-N-acetylglucosamine
modulates the stimulatory effect of insulin on skeletal muscle
glucose uptake. J Biol Chem. 272:4889–4895. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marshall S, Bacote V and Traxinger RR:
Discovery of a metabolic pathway mediating glucose-induced
desensitization of the glucose transport system. Role of hexosamine
biosyn-thetic in the induction of insulin resistance. J Biol Chem.
266:4706–4712. 1991.PubMed/NCBI
|
|
45
|
Burke AP, Kolodgie FD, Zieske A, Fowler
DR, Weber DK, Varghese PJ, Farb A and Virmani R: Morphologic
findings of coronary atherosclerotic plaques in diabetics: A
postmortem study. Arterioscler Thromb Vasc Biol. 24:1266–1271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han I, Oh ES and Kudlow JE: Responsiveness
of the state of O-linked N-acetylglucosamine modification of
nuclear pore protein p62 to the extracellular glucose
concentration. Biochem J. 15:109–114. 2000. View Article : Google Scholar
|
|
47
|
Roshanzamir F and Yazdanparast R:
Quercetin attenuates cell apoptosis of oxidant-stressed SK-N-MC
cells while suppressing up-regulation of the defensive element,
HIF-1α. Neuroscience. 277:780–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Suganya N, Bhakkiyalakshmi E,
Suriyanarayanan S, Paulm urugan R and Ramkumar KM: Quercetin
ameliorates tunic-amycin-induced endoplasmic reticulum stress in
endothelial cells. Cell Prolif. 47:231–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dai X, Ding Y, Zhang Z, Cai X and Li Y:
Quercetin and quercitrin protect against cytokine-induced injuries
in RINm5F β-cells via the mitochondrial pathway and NF-κB
signaling. Int J Mol Med. 31:265–271. 2013.
|
|
50
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chistiakov DA, Sobenin IA, Orekhov AN and
Bobryshev YV: Role of endoplasmic reticulum stress in
atherosclerosis and diabetic macrovascular complications. Biomed
Res Int. 6101402014.PubMed/NCBI
|
|
52
|
Beriault DR, Sharma S, Shi Y, Khan MI and
Werstuck GH: Glucosamine-supplementation promotes endoplasmic
reticulum stress, hepatic steatosis and accelerated atherogenesis
in apoE−/− mice. Atherosclerosis. 219:134–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu CM, Zheng GH, Ming QL, Sun JM and
Cheng C: Protective effect of quercetin on lead-induced oxidative
stress and endoplasmic reticulum stress in rat liver via the
IRE1/JNK and PI3K/Akt pathway. Free Radic Res. 47:192–201. 2013.
View Article : Google Scholar
|
|
54
|
Xu LH, Xie H, Shi ZH, Du LD, Wing YK, Li
AM, Ke Y and Yung WH: Critical role of endoplasmic reticulum stress
in chronic intermittent hypoxia-induced deficits in synaptic
plasticity and long-term memory. Antioxid Redox Signal. May
8–2015.Epub ahead of print. View Article : Google Scholar :
|
|
55
|
van der Kallen CJ, van Greevenbroek MM,
Stehouwer CD and Schalkwijk CG: Endoplasmic reticulum
stress-induced apoptosis in the development of diabetes: Is there a
role for adipose tissue and liver? Apoptosis. 14:1424–1434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Minamino T and Kitakaze M: ER stress in
cardiovascular disease. J Mol Cell Cardiol. 48:1105–1110. 2010.
View Article : Google Scholar
|
|
57
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tsukano H, Gotoh T, Endo M, Miyata K,
Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, et al:
The endoplasmic reticulum stress-C/EBP homologous protein
pathway-mediated apoptosis in macrophages contributes to the
instability of atherosclerotic plaques. Arterioscler Thromb Vasc
Biol. 30:1925–1932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu P, Han Z, Couvillon AD, Kaufman RJ and
Exton JH: Autocrine tumor necrosis factor alpha links endoplasmic
reticulum stress to the membrane death receptor pathway through
IRE1alpha-mediated NF-kappaB activation and down-regulation of
TRAF2 expression. Mol Cell Biol. 26:3071–3084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen S, Zhao Y, Zhang Y and Zhang D:
Fucoidan induces cancer cell apoptosis by modulating the
endoplasmic reticulum stress cascades. PLoS One. 9:e1081572014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tabas I: The role of endoplasmic reticulum
stress in the progression of atherosclerosis. Circ Res.
107:839–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamamoto K, Sato T, Matsui T, Sato M,
Okada T, Yoshida H, Harada A and Mori K: Transcriptional induction
of mammalian ER quality control proteins is mediated by single or
combined action of ATF6alpha and XBP1. Dev Cell. 13:365–376. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamamoto K, Takahara K, Oyadomari S, Okada
T, Sato T, Harada A and Mori K: Induction of liver steatosis and
lipid droplet formation in ATF6alpha-knockout mice burdened with
pharmacological endoplasmic reticulum stress. Mol Biol Cell.
21:2975–2986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rutkowski DT, Wu J, Back SH, Callaghan MU,
Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al:
UPR pathways combine to prevent hepatic steatosis caused by ER
stress-mediated suppression of transcriptional master regulators.
Dev Cell. 15:829–840. 2008. View Article : Google Scholar : PubMed/NCBI
|