Introduction
Since the beginning of the twentieth century, the
prevalence of mucosal inflammatory diseases, including atopic
dermatitis, has been increasing. atopic dermatitis is more common
in infants and children (10–20%) than in adults (1–3%) in developed
countries (1), and its prevalence
has markedly increased over the last 30–40 years. The onset of
atopic dermatitis is associated with genetic and environmental
factors, including a younger age, and living in urban areas and
climates with low humidity (2,3).
Atopic dermatitis is a common skin disease
characterized by itching, dryness and skin rashes (4). Although the exact cause of atopic
dermatitis remains to be elucidated, it develops following an
abnormal reaction to irritants, including foods and environmental
allergens, which are specific to each individual (2). There is no known cure for atopic
dermatitis, and treatments are limited to improving or suppressing
the symptoms. Since the disease is associated with inflammation and
immune dysfunction, combined treatment with antibiotics and
corticosteroids have reportedly been effective (5). However, this treatment strategy does
not cure the disease, and cannot be used long-term due to their
adverse effects (5). The
application or topical corticosteoid cream produces stretch marks
and thinning of the skin, which compromises epidermal barrier
function, and increases sensitivity to contact allergens and
infection by Staphylococcus aureus (6). Other immunosuppressants, including
tacrolimus and pimecrolimus, are also used as topical preparations
in the treatment of severe atopic dermatitis, and oral
immunosuppressant medications, including ciclosporin, azathioprine
and methotrexate are occasionally prescribed; however, these
treatments have serious side effects, including liver and kidney
damage, and skin cancer (7).
Therefore, the objective of atopic dermatitis treatment is to
reduce the inflammation and hyperactivation of the immune response
to specific allergens, without serious adverse effects.
Natural products have been examined for use as
alternative atopic dermatitis treatments with potent efficacy and
minimal side effects (8–10). Cortex phellodendri (CPE), an
Asian traditional medicine prepared from Phellodendron
amurense, has been used for treating abdominal pain, diarrhea,
gastroenteritis, urinary tract infections and other diseases
(11). Its principal components
are berberine, obacunone and obaculactone. Obaculactone has the
unique immunomodulatory property of inhibiting the
alloantigen-specific expression of T helper cell 1 (Th1) cytokines,
interferon (IFN)-γ, proinflammatory cytokines, tumor necrosis
factor (TNF)-α, interleukin (IL)-2, and IL-6 in mice with skin
allografts (12). The predominant
function of CPE and its components is suppressing inflammation and
scavenging free radicals (9,11,12).
CPE is prepared by drying and salt processing, during which the
bioactive components are altered. A previous study demonstrated
that the contents of obacunone and obaculactone are significantly
different, according to the different processing methods (13). The contents of obaculactone are
increased relative to those of obacunone in wine-fried and
salt-fried products of CPE, compared with raw products (13). Therefore, processing CPE with salt
may improve its efficacy for treating atopic dermatitis, compared
with dried unprocessed CPE. Sanguisorba officinalis (SOE;
great burnet) is known to cool the blood, inhibit bleeding,
decrease temperature and heal wounds, and may be useful in the
treatment of AD (14). A previous
study demonstrated that SOE has similar effects to CPE, and
exhibits anti-inflammatory, anti-oxidant and immunomodulatory
activities (15). The predominant
components of SOE are saponins, including triterpenes and their
glycosides that include ziyuglycoside I, gallic acid and
disaccharide
([5-O-α-D-[3-C-hydroxymethyl]lyxofuranosyl-β-D-[2-C-hydr oxymethyl]
arabino furanose) (15).
Therefore, the present study hypothesized that
salt-processed CPE and SOE may alleviate atopic dermatitis by
improving anti-inflammatory and immunomodulatory activity levels in
experimental animals with AD. The present study examined the
anti-atopic dermatitis activity levels of salt-processed CPE and
SOE in 2,4-dinitrochlorobenzene (DNCB)-treated NC/Nga mice, and
examined the mechanisms underlying the alleviation of atopic
dermatitis symptoms.
Materials and methods
Preparation of extracts
Salt-processed CPE and SOE were purchased from
Kyung-Dong Herb market (Seoul, Korea) in 2010, were confirmed by Dr
Byung Seob Ko (Korean Herbal Medicine Institute, Daejeon, Korea),
and voucher specimens (nos. 2010-04 and 2010-05) were deposited at
the herbarium at the Department of Food and Nutrition, Hoseo
University (Asan, Korea). Salt-processed CPE is commercially
produced by boiling Phellodendron amurense bark and spraying
the bark with 2% salt water prior to drying. Since 1,3-butylene
glycol is an effective solvent for producing skin lotion (16), the salt-processed CPE and SOE (1
kg) were extracted at room temperature for 12 h using 3.3 liters of
1,3-butylene glycol (Sigma-Aldrich, St. Louis, MO, USA), prior to
being filtered with filter paper (Watman; GE Healthcare, Little
Chalfont, UK) and centrifuged at 450 × g at room temperature for 30
min to produce 30% extracts. Over 30% of the salt-processed
extracts of CPE and SOE in 1,3-butylene glycol formed a
precipitate. The supernatants were used for topical application in
the subsequent experiments.
Determination of total phenol, flavonoid
and alkaloid levels
The levels of total phenolic compounds of each 30%
extract of salt-processed CPE and SOE were measured using
Folin-Ciocalteu reagent (97.5% purity; Sigma-Aldrich), and were
expressed as mg gallic acid equivalents·g−1. The
extracts were dissolved in ethanol and the total flavonoid contents
were measured using a previously described method (17) with minor modifications. The extract
was added to 2 N HCl prior to being filtered. The solution was then
mixed with bromocresol green solution (Sigma-Aldrich) and phosphate
buffer (1:5:5) and the mixture was transferred to a separating
funnel. Chloroform (Sigma-Aldrich) was subsequently added and mixed
by vigorous agitation. The chloroform fraction was separated and
its absorbance was measured at 470 nm using a UV/Visible
spectrophotometer (Lambda 850; Perkin Elmer, Waltham, MA, USA).
Berberine chloride (>90% purity; Sigma-Aldrich) was used as a
standard. The total alkaloid content was expressed as mg
berberine/g extract (18).
Animals
A total of 20 male six-week-old NC/Nga mice were
purchased from Charles River Japan (Yokohama, Japan), and
maintained in conventional conditions of a 12 h light/12 h dark
cycle, room temperature of 22–23°C and humidity of 55±15%. The mice
had free access to food and water. All surgical and experimental
procedures were performed according to the guidelines of the Animal
Care and Use Review Committee of Hoseo University (Asan,
Korea).
Induction of atopic dermatitis-like skin
lesions
The mice were anesthetized with a mixture of
ketamine and xylazine (100 and 10 mg/kg body weight, respectively;
Bayer AG, Leverkusen, Germany), following which the hair on the
back and right ear were shaved 1 day prior to sensitization. On the
first day, 1% DNCB in acetone/olive oil (3:1; 150 µl per
mouse) was applied to the dorsal skin and right ear, following
which 0.2% DNCB (150 µl per mouse) was applied every other
day for five weeks, as previously described (10). The same volume of acetone/olive oil
vehicle was applied, instead of the DNCB solution, to the controls.
Repeated application of DNCB onto the dorsal region caused apparent
dermatitis in the NC/Nga mice (19).
Topical application of the 1,3-butylene glycol
extracts of the salt-processed CPE and SOE were used to determine
the effect of CPE and SOE on atopic dermatitis. Based on the
preliminary cell-based investigations and the maximum dosage of the
extract, two doses were assigned. The preliminary investigation
demonstrated that 20 and 50 µg/ml of salt-processed CPE and
SOE extracts, respectively, were effective against house mites
(Arthropods of Medical Importance Resource Bank, Yonsei University,
Seoul, Korea) in the HaCaT human keratinocyte cell line (American
Type Culture Collection; Manassas, VA, USA). Therefore, the topical
application of 200 µl 30% 1,3-butylene glycol was considered
to be an effective dosage for use the animal model, when compared
with a previous study (10).
Following the induction of the atopic dermatitis-like skin lesions,
the animals were divided into four groups, each containing 10 mice.
The mice in these groups were then treated topically on the dorsal
skin with a 200 µl dose of one of the following four agents
for five weeks: 1,3-butylene glycol (BG; control); 30% CPE; 30%
SOE; 15% CPE+15% SOE; or 0.1% hydrocortisone butyrate (HC;
Sigma-Aldrich; positive control) twice a day. Mice without
induction of atopic dermatitis-like skin lesions were treated with
1,3-butylene glycol as a normal control. At the end of the study,
rats were anesthetized with ketamine and xylazine (100 and 10 mg/kg
body weight, respectively). Rats were sacrificed and tissues were
collected for further experiments.
Evaluation of skin lesions
The relative dermatitis severity was assessed
macroscopically using a previously described scoring procedure
(20). The total skin severity
scores were assessed weekly and defined as the sum of the
individual scores for each of the following four symptoms: i)
Erythema and hemorrhage, ii) edema, iii) erosion (excoriation) and
iv) scaling (dryness). For each symptom, 0 was defined as
exhibiting no symptoms, 1 as mild symptoms, 2 as moderate symptoms,
and 3 as severe symptoms. To minimize technique variations, a
single investigator performed the measurements throughout each
experiment in a blinded-manner.
Measurement of serum levels of
immunoglobulin (Ig)E and IgG1 and cytokines
Total serum levels of IgE and IgG1 were quantified
using an ELISA Quantification kit (BD Biosciences, San Jose, CA,
USA), according to the manufacturer's instructions. In addition,
the serum concentrations of the TNF-α, IL-4 and IFN-γ cytokines
were quantified using a Mouse Cytokine Enzyme Immunoassay kit
(R&D Systems, Minneapolis, MN, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The dorsal skin tissue samples from five rats of
each group were collected at the end of the experiment, and each
skin tissue sample was individually powdered with a cold steel
mortar and pestle, prior to being mixed with a monophasic solution
of phenol and guanidine isothiocyanate (TRIzol reagent; Invitrogen
Life Technologies, Carlsbad, CA, USA) for total RNA extraction,
according to the manufacturer's instructions. The quantity and
purity of RNA was measured at 260 and 280 nm using a Lambda 850
spectrophotometer (Perkin Elmer, Inc.) and cDNA was reverse
transcribed from 1 µg RNA extracted from the individual rats
using a Superscript III Reverse Transcriptase kit (Invitrogen Life
Technologies). A total of five cDNAs were produced from each group,
and each cDNA was used for RT-qPCR. Equal quantities (1 µg)
of cDNA and primers for specific genes were mixed with
SYBR® Green (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) in duplicate, and amplified using a real-time PCR instrument
(CFX Connect™ Real-Time PCR Detection System; Bio-Rad Laboratories,
Inc.). The following thermocycling conditions were used to perform
the PCR: 55°C for 2 min, 95°C for 10 min followed by 40 cycles of
94°C for 20 sec, 65°C for 30 sec and 72°C for 20 sec. To assess
which genes were associated with inflammation and degradation of
articular cartilage, primers were used to detect the expression
levels of rat-inducible nitric oxide synthase, TNF-α, IL-1β, IL-6,
matrix metalloprotinase (MMP)-3, and MMP-13 genes, as previously
described (21,22). The cycle threshold (CT) for each
sample was subsequently determined. The mRNA expression levels in
the unknown samples were quantified using the comparative CT method
(ΔΔCT method), as previously described by Livak and Schmittgen
(23). ΔCT was calculated using
the following formula: ΔCT = CT (target gene) − CT (endogenous
reference gene, β-actin). The relative fold-change in expression
was calculated using the following equation: ΔΔCt =
ΔCttreatment −ΔCtcontrol. The results were
presented as 2−ΔΔCT.
The following primers were used for the PCR
reactions: Mouse IFN-γ, sense 5′-CGGCACAGTCATTGAAAGCCTA-3′ and
antisense 5′-GTTGCTGATGGCCTGATTGTC-3′; IL-4, sense
5′-TCTCGAATGTACCAGGAGCCATATC-3′ and antisense
5′-AGCACCTTGGAAGCCCTACAGA-3′; IL-13, sense
5′-CAGCTCCCTGGTTCTCTCAC-3′ and antisense
5′-CCACACTCCATACCATGCTG-3′; TNF-α, sense
5′-CCCTCACACTCAGATCATCTTCT-3′ and anti-sense
5′-GCTACGACGTGGGCTACAG-3′; and β-actin, sense
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and anti-sense
5′-ATGGAGCCACCGATCCACA-3′. The primers were designed to surround at
least one intron in order to distinguish between the products
derived from mRNA and genomic DNA.
Histological analysis
Dorsal skin tissue samples were harvested 24 h
following final DNCB administration on day 35, and fixed in 10%
buffered-neutral formaldehyde (Sigma-Aldrich) and embedded in
paraffin wax (Leica Microsystems, Wetzlar, Germany). Histological
skin tissue sections (6 µm) were stained with hematoxylin
and eosin (Sigma-Aldrich), in order to count the number of
eosinophils. The sections were also stained with 0.5% toluidine
blue (Sigma-Aldrich) in order to determine the number of mast
cells. The cell counts were performed using microscope (Axio Imager
2; Carl Zeiss AG, Oberkochen, Germany) in six consecutive
microscopic fields at ×400 magnification.
Statistical analysis
Statistical analysis was performed using SAS
software (version 9.3; SAS Institute Inc., Cary, NC, USA) and all
results are expressed as the mean ± standard deviation. The
biological and metabolic effects of CPE, SOE, CPE + SOE, HC
(positive control), and vehicle (negative control) were compared
using one-way analysis of variance. Significant differences in the
treatment effects among the groups were identified using Tukey's
test. The significance of differences between the mice with and
without atopic dermatitis-like skin lesion were determined using a
two-sample Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Total phenol and flavonoid levels
CPE was predominantly composed of alkaloids,
particularly berberine, and low levels of phenols and flavonoids.
However, SOE was predominantly composed of phenols and flavonoids
(Table I).
 | Table IContents of phenolic compounds and
flavonoids. |
Table I
Contents of phenolic compounds and
flavonoids.
| Content | Water extract of
Korean mistletoe (mg/g dry weight) | Sanguisorba
officinalis (mg/g dry weight) |
|---|
| Total phenols | 1.43±0.09 | 71.4±2.37 |
| Total
flavonoids | 0.89±0.10 | 75.5±3.57 |
| Total
alkaloids | 154±19 | 0.08±0.01 |
Clinical severity of skin lesions
Following repeated topical applications of DNCB on
the backs of the NC/Nga mice atopic dermatitis lesions, the dorsal
skin of the mice in the control group that developed DNCB-induced
atopic dermatitis exhibited hypertrophy, hyperkeratosis,
intercellular edema and liquefaction degeneration of the basal
layer (data not shown). Clinical severity was calculated from the
sums of the scores for erythema and hemorrhage, edema, erosion
(excoriation) and scaling (dryness). The clinical severity of the
skin lesions in the control group increased markedly until three
weeks post-topical application of DNCB, however, no further
exacerbated was observed at four weeks (Fig. 1). Hypertrophy, hyperkeratosis,
intercellular edema and liquefaction degeneration of the basal
layer in the dorsal skin tissue samples were alleviated by
treatments with CP and SOE to a similar extent, and were alleviated
more by SOE+CPE, compared with the control group (data not shown).
Treatment with CPE and SOE decreased the severity of atopic
dermatitis symptoms after four weeks. In addition, treatment with
CPE and SOE synergistically slowed symptom progression and
exhibited similar activity levels to treatment with HC over time
(Fig. 1).
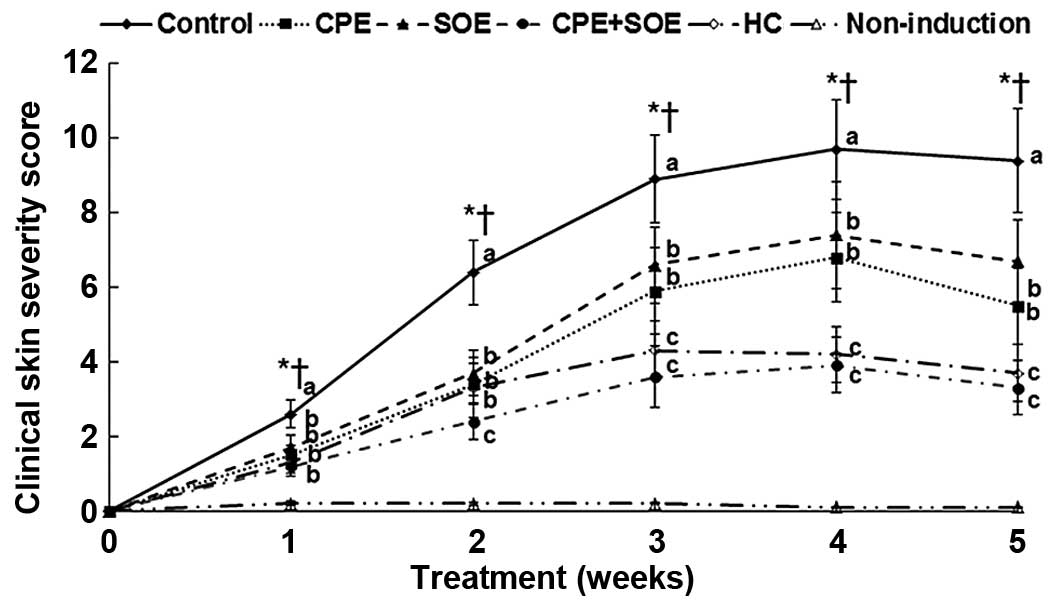 | Figure 1Changes in total skin severity scores
of AD in NC/Nga mice. AD was induced in NC/Nga mice by topical
application of DNCB to the dorsal skin and the ear, which was also
topically treated with BG (control), 30% salt-treated CPE, 30% SOE,
15% CPE+15% SOE, or 0.1% HC (positive control) on the lesions twice
a day for five weeks. Mice without DNCB were treated with BG as a
normal control. The total skin severity score was defined as the
sum of the individual scores for each of the following four signs:
i) Erythema and hemorrhage, ii) edema, iii) erosion (excoriation)
and iv) scaling (dryness) every week. Score 0, no symptoms; 1, mild
symptoms; 2, moderate symptoms; 3, severe symptoms. Each value
represents the mean ± standard deviation of 10 mice in each group.
*P<0.05, among the various treatments in NC/Nga mice.
a–cValues with different superscripts were significantly
different among the groups of NC/Nga mice. †P<0.05,
BG control, vs. non-induction control. AD, atopic dermatitis; DBCN,
2,4-dini-trochlorobenzene; BG, 1,3-butylene glycol; CPE, Cortex
phellodendri; SOE, Sanguisorba officinalis; HC,
hydrocortisone. |
Expression levels of circulating IgE,
IgG1, TNF-α, IL-4 and INF-γ
Following topical application of DNCB onto the
dorsal skin of the mice, the serum expression levels of IgE, an
activator of mast cells, increased by ~7 and 15-fold at 2 and 5
weeks, compared with the control. Compared with the control, the
increase in serum levels of IgE were suppressed by treatment with
SOE CPE, and suppressed further by treatment with SOE and CPE
combined, compared with SOE and CPE alone, and this suppression
level was similar to that induced by treatment with HC (Fig. 2A). Serum levels of IgG1 increased
in the control group by 3.3-fold after two weeks, and these
expression levels decreased to 2.3-fold after five weeks (Fig. 2B). Treatment with SOE, CPE, and CPE
and SOE combined decreased the serum levels of IgG1, and the
decrease in expression levels induced by treatment with CPE and SOE
combined were similar to those observed following treatment with HC
after 2 and 5 weeks (Fig. 2B). In
addition, treatment with CPE and SOE combined, and treatment with
HC decreased the serum expression levels of IgG1 to similar levels
as the non-induction group.
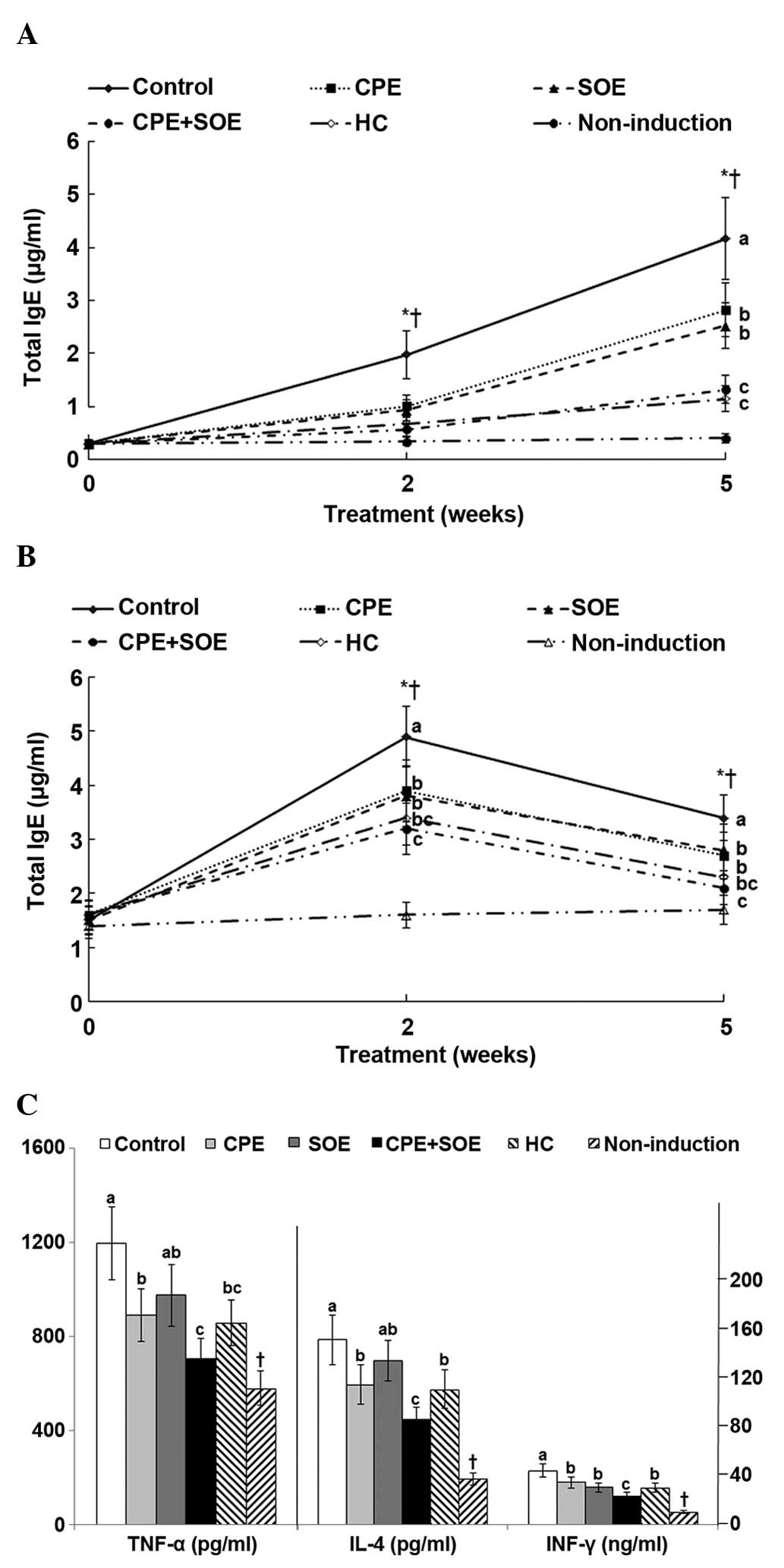 | Figure 2Serum levels of IgG1, IgE, IL-4 and
IFN-γ at the end of the experimental periods. AD was induced in
NC/Nga mice by topical application of DNCB to the dorsal skin of
the ear, which was also topically treated with BG (control), 30%
salt-treated CPE, 30% SOE, 15% CPE+15% SOE, or 0.1% HC (positive
control) on the lesions twice a day for five weeks. Mice without
DNCB application were treated with BG as a normal control.
Following five weeks of treatment, serum was separated to measure
immunoglobulins and cytokines. (A) Serum levels of IgE. (B) Serum
levels of IgG1. (C) Serum levels of TNF-α, IL-4 and INF-γ. Each
value represents the mean ± standard deviation of 10 mice in each
group. *P<0.05, among the various treatments in the
NC/Nga mice. a–cValues with different superscripts were
significantly different among the groups of NC/Nga mice according
to Tukey's test. †P<0.05, BG control, vs. non-induced
control. AD, atopic dermatitis; Ig, immunoglobulin; IL,
interleukin; IFN, interferon; DBCN, 2,4-dinitrochlorobenzene; BG,
1,3-butylene glycol; CPE, Cortex phellodendri; SOE,
Sanguisorba officinalis; HC, hydrocortisone. |
Mast cells secrete TNF-α and activate Th2-derived
cytokines, including IL-4, IL-6 and IL-13. The serum levels of
TNF-α in the control group increased significantly by 2.1-fold,
compared with those of the non-induction group (Fig. 2C). The levels of TNF-α expression
levels were suppressed by treatment with CPE, however treatment
with SOE and CPE combined inhibited them to similar levels as those
induced following treatment with HC. In addition, the serum levels
of IL-4 and IFN-γ were higher in the control group, compared with
the non-induction group; IL-4 and IFN-γ expression was suppressed
by CPE, and this suppression was markedly increased by treatment
with SOE and CPE combined, which reached similar expression
suppression as that induced by treatment with HC (Fig. 2C). SOE also inhibited the serum
expression levels of INF-γ, as compared with the control group, but
did not inhibit the expression of IL-4. These results suggest that
both Th2 and Th1 were activated during the experiment.
Histological findings and mast cell
number in the inflamed skin tissue samples
As determined using histological staining, skin
thickness was markedly higher in the control group, compared with
the non-induction group. Treatment with CPE or SOE alone decreased
skin thickness, however, treatment with SOE and CPE combined
markedly decreased skin thickness to similar levels as treatment
with HC, as compared with treatment with SOE or CPE alone (Fig. 3A).
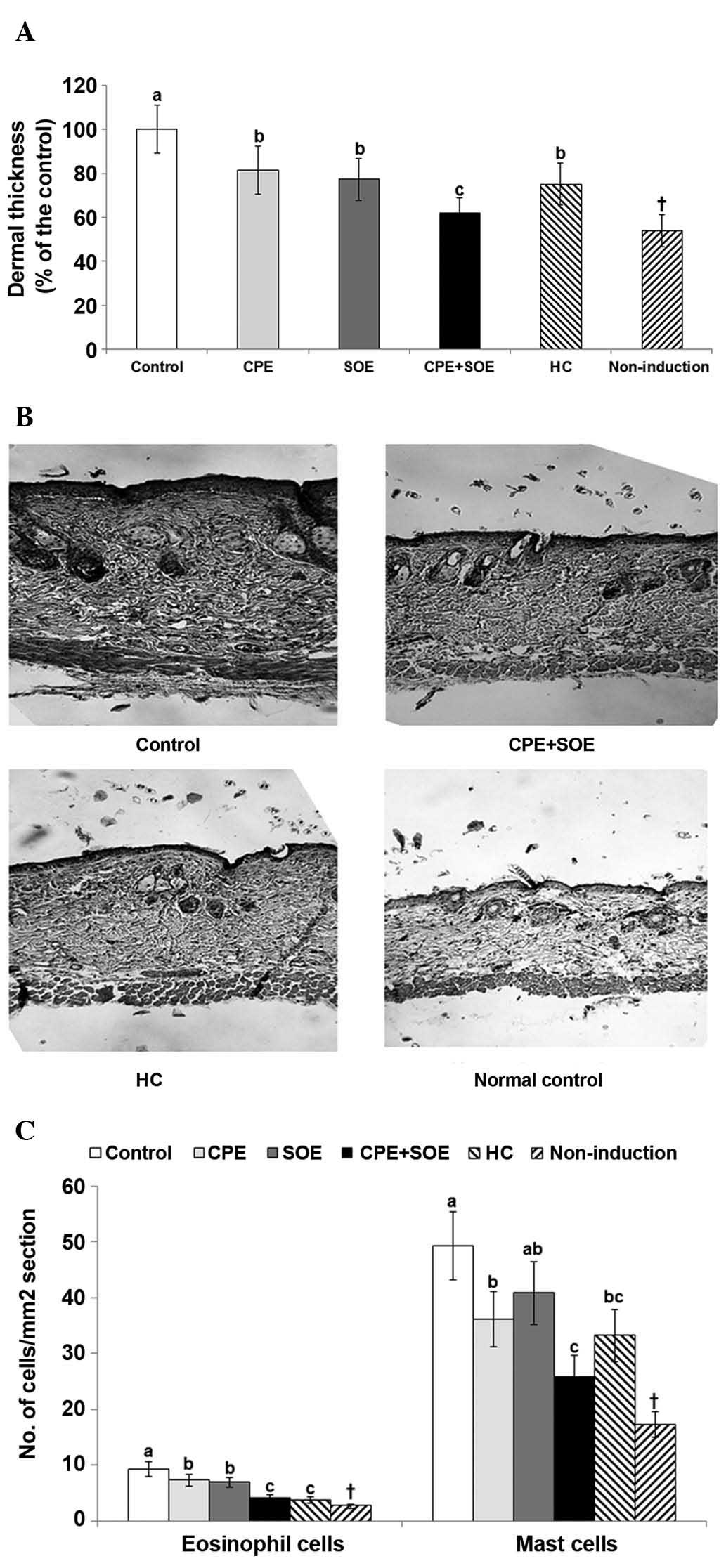 | Figure 3Numbers of mast cells and eosinophils
in the dorsal skin, determined using histopathological analysis of
AD, which was induced in NC/Nga mice by the topical application of
DNCB to the dorsal skin. The skin was also topically treated with
either BG (control), 30% salt-treated CPE, 30% SOE, 15% CPE+15%
SOE, or 0.1% HC (positive control) on the lesions twice a day for
five weeks. Mice without DNCB application were treated with BG and
used as a normal control. Following five weeks of treatments the
dorsal skin was fixed with 10% formaldehyde and embedded in
paraffin. The skin sections were stained with hematoxylin and eosin
and toluidine blue, and the number of eosinophils and mast cells
were counted under a microscope in the respective stained sections.
Each value is expressed as the mean ± standard deviation of five
mice in each group. (A) Dermal thickness of the dorsal skin. (B)
Histology of the dorsal skin sections following staining with
hematoxylin and eosin (magnification, ×400). (C) Number of
eosinophils and mast cells. *P<0.05 among the various
treatments in the NC/Nga mice. a–cValues with different
superscripts were significantly different among the groups of
NC/Nga mice according to Tukey's test. †P<0.05 BG
control, vs. non-induced control. AD, atopic dermatitis; DBCN,
2,4-dinitrochlorobenzene; BG, 1,3-butylen glycol; CPE, Cortex
phellodendri; SOE, Sanguisorba officinalis; HC,
hydrocortisone. |
As seen in Fig. 3B,
the infiltration of inflammatory cells, including mast cells and
eosinophils was greater in the control group than in the normal
control group that did not exhibit atopic dermatitis. The number of
mast cells and eosinophils markedly increased in the skin lesions
of the control group, compared with the non-induction group
(Fig. 3C). SOE and CPE alone
decreased the number of eosinophils in the DNCB-treated mice, and
treatment with SOE+CPE further decreased the number of eosinophils,
which was similar to that observed following treatment with HC.
However, the number of mast cells was decreased by CPE, but not
SOE, compared with the control group, whereas treatment with CPE
and SOE synergistically inhibited the number of mast cells, and
this decrease was marginally greater compared with treatment with
HC (Fig. 3C).
mRNA expression levels of cytokines
To investigate cytokine production in the dorsal
skin lesions, the mRNA expression levels of the TNF-α, IL-4, IL-6,
IL-13 and INF-γ cytokines were quantified. The dorsal skin of the
DNCB-treated control mice exhibited significantly higher expression
levels of TNF-α, IL-4, IL-6, IL-13, and IFN-γ, compared with those
of the non-induction group (Fig.
4A and 4B). CPE, but not SOE,
suppressed the mRNA expression of TNF-α, IL-4, IL-6, IL-13 and
IFN-γ, and treatment with CPE+SOE further decreased their
expression levels (Fig. 4A and
4B). The suppression of TNF-α,
IL-4, IL-6, IL-13 and IFN-γ following treatment with CPE and SOE
combined was marginally more effective than treatment with the HC a
positive control (Fig. 4A and
4B).
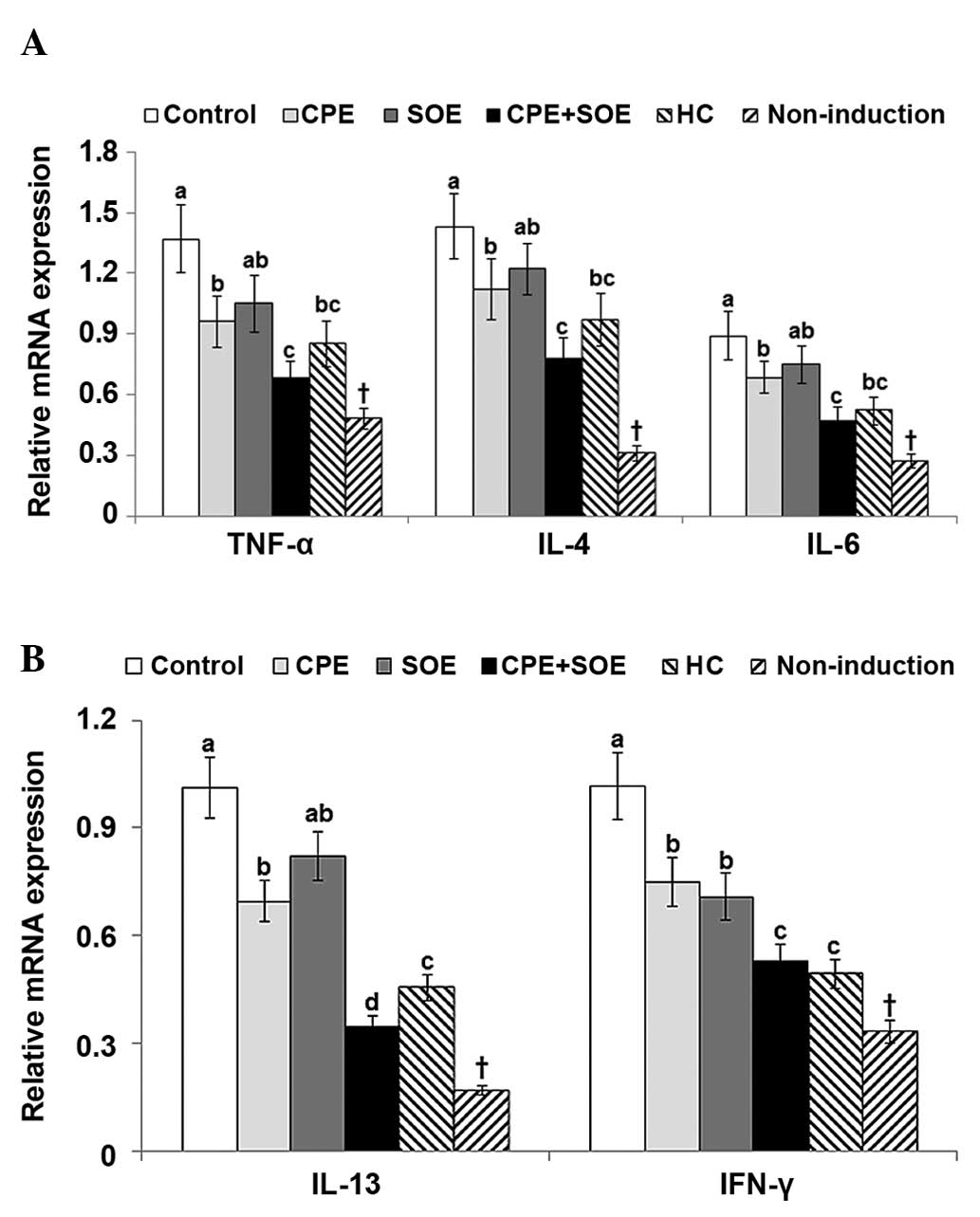 | Figure 4Expression levels of TNF-α, IL-4,
IL-6, IL-13 and IFN-γ in the dorsal skin. AD was induced in NC/Nga
mice by topical application of DNCB to the dorsal skin of the ear,
which was also topically treated with BG (control), 30%
salt-treated CPE, 30% SOE, 15% CPE+15% SOE, or 0.1% HC (positive
control) on the lesions twice a day for five weeks. Mice without
DNCB application were treated with BG as a normal control.
Following five weeks of treatment, total RNA was extracted from the
dorsal skin, from which cDNA was reverse transcribed. mRNA
expression levels were measured using reverse
transcription-quantitative polymerase chain reaction, and their
relative expression levels were standardized according to
respective mRNA levels of β-actin. Each value represents the mean ±
standard deviation of five mice in each group. (A) mRNA expression
levels of TNF-α, IL-4 and IL-6. (B) mRNA expression levels of IL-13
and INF-γ. *P<0.05 among the various treatments in
the NC/Nga mice. a,b,cP<0.05 among NC/Nga mice,
determined using Tukey's test. †P<0.05 BG control,
vs. non-induced control. AD, atopic dermatitis; Ig, immunoglobulin;
IL, interleukin; IFN, interferon; DBCN, 2,4-dinitrochlorobenzene;
BG, 1,3-butylene glycol; CPE, Cortex phellodendri; SOE,
Sanguisorba officinalis; HC, hydrocortisone. |
Discussion
The present study demonstrated that CPE alleviated
the clinical severity of atopic dermatitis by decreasing the number
of mast cells, as well as the serum levels of serum TNF-α, IL-4 and
IFN-γ and various cytokines in the dorsal lesions of NC/Nga mice,
compared with the control group. However, SOE alone did not improve
the clinical severity of atopic dermatitis, although serum levels
of IgE and IgG1 were suppressed to similar levels as those observed
following treatment with CPE. Treatment with CPE+SOE
synergistically improved the clinical severity of the atopic
dermatitis skin lesions by decreasing the serum levels of IgE,
IgG1, TNF-α, IL-4 and IFN-γ, and the mRNA expression levels of
TNF-α, IL-4, IL-13 and IFN-γ. The suppression of cytokine
production by CPE+SOE combined was marginally higher compared with
that observed following treatment with HC. Therefore, these results
suggested that CPE and SOE may be an effective alternative
treatment strategy for the management of atopic dermatitis.
Atopic dermatitis is a chronic inflammatory skin
disease that develops in response to specific antigens. The skin is
a host defense system against microbial invasion and allergen
penetration (1). Although the
cause of atopic dermatitis remains controversial, defects in the
epidermal barrier function contribute to the activation of the
immune response and pro-inflammatory cytokine release, which
results in atopic dermatitis (1,2,4).
Antigens disrupt the epidermal barrier and sensitize inflammatory
dendritic epidermal cells to Th lymphocytes in the skin (1,4). The
Th cells release pro and anti-inflammatory cytokines that usually
exacerbate the symptoms. Therefore, atopic dermatitis is treated
predominantly with various emollients that improve skin appearance
and dryness, or skin barrier repair and anti-inflammatory
agents.
NC/Nga mice is a well-known animal model for atopic
dermatitis, and atopic dermatitis-like skin lesions induced by
allergens in NC/Nga mice are similar to those in human atopic
dermatitis (24). The symptoms of
atopic dermatitis are erythema and hemorrhage, followed by edema,
superficial erosion, deep excoriation, alopecia and dryness in the
skin (10,24). In the present study, DNCB-treated
NC/Nga mice were used as an atopic dermatitis animal model.
Although NC/Nga mice are susceptible to developing atopic
dermatitis, an allergen, such as DNCB, is required in order to
induce apparent symptoms. The mice in the control group developed
moderate to severe symptoms of erythema and hemorrhage, edema,
erosion (excoriation) and scaling (dryness). The clinical severity
of the skin lesions were attenuated by CPE, and to a lesser extent
by SOE, whereas treatment with CPE and SOE synergistically improved
symptom severity. The clinical severity of atopic dermatitis was
associated with the hyperproduction of circulating IgE by B
lymphocytes and inflammatory cytokines from Th lymphocytes.
Among the clinical symptoms, itching is a common
symptom of atopic dermatitis, and due to itching, scratching of the
skin with toenails appears to be the crucial factor in causing
dermatitis, and results in the elevation of serum concentrations of
IgE and the number of mast cells (25). Serum levels of IgE are considered
an important marker of atopic dermatitis, as the majority of
patients with atopic dermatitis exhibit significantly elevated
serum levels of IgE, compared with patients with non-atopic
dermatitis (26,27). IgE binds to the surface of mast
cells in order to induce the release of histamine and cytokines,
which aggravate atopic dermatitis symptoms. In addition, atopic
dermatitis lesions are dominated by infiltrating Th2 cells in the
acute phase, which release cytokines, including IL-4, IL-5 and
IL-13, whereas in chronic atopic dermatitis lesions, there is a
switch towards a Th1 phenotype, which leads to IFN-γ secretion
(28). Several studies have
demonstrated that patients with atopic dermatitis lesions exhibit
elevated serum levels of IgE, TNF-α, IL-4, IL-6 and INF-γ, compared
with healthy individuals, indicating that Th2 and Th1 lymphocytes
are activated in patients with atopic dermatitis (21,29).
Similar to these investigations in humans, the present study
demonstrated that serum levels of IgE, TNF-α, IL-4 and INF-γ were
significantly increased in the control mice that developed atopic
dermatitis, and treatment with CPE and SOE synergistically lowered
serum levels of IgE, as well as those of serum TNF-α, IL-4 and
INF-γ. The results suggested that NC/Nga mcie challenged with DNCB
activated Th2 and Th1 lymphocytes at week 5 of the experimental
period. The decrease in cytokine levels induced by CPE+SOE was
greater, compared with HC treatment. Topical corticosteroid
treatment is known to suppress the initial step, which activates Th
lymphocytes and consequently suppresses the release of
pro-inflammatory cytokines (5,22).
These results suggested that corticosteroids attenuated atopic
dermatitis symptoms by inhibiting the initial step of Th lymphocyte
activation and not by directly inhibiting the secretion of
inflammatory cytokines. Therefore, treatment with CPE+SOE may
provide improved treatment for atopic dermatitis than
corticosteroids.
Similar to serum levels of cytokines, allergens
induce atopic dermatitis as a result of complex immune and
inflammatory responses driven by the release of proinflammatory
cytokines and chemokines in the skin. Inflammation damages the skin
barrier and continues to elevate the levels of cytokines released
from mast cells and Th2 and Th1 lymphocytes, thereby exacerbating
atopic dermatitis (29,30). The chronic phase of atopic
dermatitis is characterized by lichenification of skin,
infiltration of Th1 cells and tissue remodeling, with increased
collagen deposition and dermal thickening (31). The histological observations of the
present study demonstrated that the NC/Nga mice challenged with
DNCB exhibited increased dermal thickness, and higher numbers of
mast cells and eosinophils, compared with non-induced mice. These
changes were associated with increased mRNA expression levels of
cytokines, including TNF-α, IL-4, IL-6, IL-13 and INF-γ in the
dorsal skin lesion samples. Previous studies have demonstrated
similar results following herbal treatments of atopic dermatitis
lesions (8,10). The results of the present study
also demonstrated that treatment with CPE+SOE synergistically
reduced dermal thickness and the number of mast cells and
eosinophils, whereas the mRNA expression levels of cytokines in the
dorsal lesion were markedly suppressed by treatment with CPE+SOE.
HG also improved dermal thickness and suppressed cytokine
expression in the dorsal skin tissue lesions. However, treatment
with CPE+SOE had more marked effects than treatment with HG.
In conclusion, compared with the control group,
treatment with CPE alone alleviated the clinical severity of atopic
dermatitis symptoms, with decreased numbers of mast cells, serum
levels of TNF-α, IL-4 and INF-γ, and expression levels of cytokines
in the dorsal lesions of the mice. However, treatment with SOE did
not result in decreased expression levels, although serum levels of
IgE and IgG1 were suppressed to similar levels as those observed
following treatment with CPE. Furthermore, treatment with CPE+SOE
synergistically relieved the clinical severity of the symptoms,
suppressed serum levels of IgE, IgG1, TNF-α, IL-4 and IFN-γ, and
decreased the mRNA expression levels of TNF-α, IL-4, IL-13 and
IFN-γ in the dorsal skin lesions. The improvements in symptoms
following treatment with CPE+SOE combined were more marked than
those following treatment with HC in reducing dermal thickness and
suppressing cytokine production. In conclusion, synergistic
treatment with CPE+SOE may be an effective alternative therapeutic
strategy for the management of atopic dermatitis.
Acknowledgments
The present study was supported by the Academic
Research fund of Hoseo University (Asan, China) in 2013 (no.
2013-0387).
References
|
1
|
Williams HC: Clinical practice. Atopic
dermatitis. N Engl J Med. 352:2314–2324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung DYM: New insights into the complex
gene-environment interactions evolving into atopic dermatitis. J
Allergy Clin Immunol. 118:37–39. 2006. View Article : Google Scholar
|
|
3
|
Esparza-Gordillo J, Weidinger S,
Fölster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, Rohde K,
Marenholz I, Schulz F, Kerscher T, et al: A common variant on
chromosome 11q13 is associated with atopic dermatitis. Nat Genet.
41:596–601. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leung AK and Barber KA: Managing childhood
atopic dermatitis. Adv Ther. 20:129–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cork MJ, Robinson DA, Vasilopoulos Y,
Ferguson A, Moustafa M, MacGowan A, Duff GW, Ward SJ and
Tazi-Ahnini R: New perspectives on epidermal barrier dysfunction in
atopic dermatitis: Gene-environment interactions. J Allergy Clin
Immunol. 118:3–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Danby SG and Cork MJ: The effects of
pimecrolimus on the innate immune response in atopic dermatitis. Br
J Dermatol. 168:235–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EC, Lee HS, Kim SK, Choi MS, Lee S,
Han JB, An HJ, Um JY, Kim HM, Lee NY, et al: The bark of Betula
platyphylla var.japonica inhibits the development of atopic
dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol.
116:270–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi YY, Kim MH, Han JM, Hong J, Lee TH,
Kim SH and Yang WM: The anti-inflammatory potential of Cortex
Phellodendron in vivo and in vitro: Down-regulation of No and iNOS
through suppression of NF-κB and MAPK activation. Int
Immunopharmacol. 19:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park S, Lee JB and Kang S: Topical
application of Chrysanthemum indicum L. Attenuates the development
of atopic dermatitis-like skin lesions by suppressing serum IgE
levels, IFN-γ and IL-4 in Nc/Nga mice. Evid Based Complement
Alternat Med. 2012:8219672012. View Article : Google Scholar
|
|
11
|
Xian YF, Mao QQ, Ip SP, Lin ZX and Che CT:
Comparison on the anti-inflammatory effect of cortex Phellodendri
chinensis and cortex Phellodendri amurensis in
12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J
Ethnopharmacol. 137:1425–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong F, Shen Y, Zhang Q, Sun Y, Tang J,
Tao F and Xu Q: Obaculactone suppresses Th1 effector cell function
through down-regulation of T-bet and prolongs skin graft survival
in mice. Biochem Pharmacol. 80:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C: Determination of obacunone and
obaculactone in different processing products of Phellodendria
murensis cortex. Zhong Yao Cai. 36:205–208. 2013.In Chinese.
PubMed/NCBI
|
|
14
|
Cai Z, Li W, Wang H, Yan W, Zhou Y, Wang
G, Cui J and Wang F: Anti-tumor and immunomodulating activities of
a polysaccharide from the root of Sanguisorba officinalis L. Int J
Biol Macromol. 51:484–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu T, Lee YJ, Yang HM, Han S, Kim JH, Lee
Y, Kim C, Han MH, Kim MY, Lee J and Cho JY: Inhibitory effect of
Sanguisorba officinalis ethanol extract on NO and PGE2
production is mediated by suppression of NF-κB and AP-1 activation
signaling cascade. J Ethnopharmacol. 134:11–17. 2011. View Article : Google Scholar
|
|
16
|
Hayakawa R: Human closed patch test. Skin
Res. 26:1119–1127. 1984.In Japanese.
|
|
17
|
Zhishen J, Mengcheng T and Jianming W: The
determination of flavonoid contents in mulberry and their
scavenging effects on superoxide radicals. Food Chem. 64:555–559.
1999. View Article : Google Scholar
|
|
18
|
Rao TM, Rao BG and Rao YV: Antioxidant
activity of Spilanthes acmella extracts. Int J Phytopharmacol.
3:216–220. 2012.
|
|
19
|
Mills LB, Mordan LJ, Roth HL, Winger EE
and Epstein WL: Treatment of severe atopic dermatitis by topical
immune modulation using dinitrochlorobenzene. J Am Acad Dermatol.
42:687–689. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kunz B, Oranje AP, Labrèze L, Stalder JF,
Ring J and Taïeb A: Clinical validation and guidelines for the
SCORAD index: Consensus report of the european task force on atopic
dermatitis. Dermatology. 195:10–19. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ong PY and Leung DY: Immune dysregulation
in atopic dermatitis. Curr Allergy Asthma Rep. 6:384–389. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abramovits W: A clinician's paradigm in
the treatment of atopic dermatitis. J Am Acad Dermatol. 53(1 Suppl
1): S70–S77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Matsuda H, Watanabe N, Geba GP, Sperl J,
Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW and
Ra C: Development of atopic dermatitis-like skin lesion with IgE
hyperproduction in NC/Nga mice. Int Immunol. 9:461–466. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi N, Arai I, Honma Y, Hashimoto Y,
Harada M, Futaki N, Sugimoto M and Nakaike S: Scratching behavior
in spontaneous- or allergic contact-induced dermatitis in NC/Nga
mice. Exp Dermatol. 14:830–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashimoto Y, Takaoka A, Sugimoto M, Honma
Y, Sakurai T, Futaki N and Arai I: Itch-associated scratching
contributes to the development of dermatitis and
hyperimmunoglobulinaemia E in NC/Nga mice. Exp Dermatol.
20:820–825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vailes LD, Perzanowski MS, Wheatley LM,
Platts-Mills TA and Chapman MD: IgE and IgG antibody responses to
recombinant Alt a 1 as a marker of sensitization to alternaria in
asthma and atopic dermatitis. Clin Exp Allergy. 31:1891–1895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grewe M, Bruijnzeel-Koomen CA, Schöpf E,
Thepen T, Langeveld-Wildschut AG, Ruzicka T and Krutmann J: A role
for Th1 and Th2 cells in the immunopathogenesis of atopic
dermatitis. Immunol Today. 19:359–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Martinez O, Overbergh L, Mathieu
C, Prabhakar BS and Chan LS: Early up-regulation of Th2 cytokines
and late surge of Th1 cytokines in an atopic dermatitis model. Clin
Exp Immunol. 138:375–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamanaka K and Mizutani H: The role of
cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr
Probl Dermatol. 41:80–92. 2011. View Article : Google Scholar : PubMed/NCBI
|


















