Introduction
Hyperthyroidism is a form of thyrotoxicosis
characterized by inappropriately high levels of thyroid hormones
synthesized and secreted by the thyroid (1). Hyperthyroidism is a common endocrine
disorder, which affects 0.5–2% of the population (2). Although hyperthyroidism is not life
threatening, untreated hyperthyroidism can lead to hypertension,
heart failure and bone mass loss, as well as an increase in birth
defects if the patient is pregnant (3). The pathogenic mechanisms underlying
hyperthyroidism are complex and multifactorial, however, several
causes, including autoimmune defects, genetic predisposition and
environmental factors have been well recognized (4). Despite the complexity of the
initiation and development of hyperthyroidism, radioiodine therapy
(RAIT) is currently the most common method for the treatment of
hyperthyroidism in clinical settings due to its safety,
effectiveness and low cost (5,6).
RAIT is based on short-range β radiation from radioactive
iodine-131, which destroys part of the thyroid gland, but retains a
certain quantity of thyroid tissue (7). However, it has long been observed
that hypothyroidism is one of the major side effects of RAIT, which
poses additional risk to the patient. Previous studies have
reported that hypothyroidism arises in 25–40% of patients who are
treated with large doses of radioiodine (8–10),
however, fewer investigations have been performed to examine the
diagnostic markers and the potential pathological mechanisms, which
drive the development of hypothyroidism in certain susceptible
patients following RAIT. Due to the similarity of the ionization
radiation used in cancer treatment, the present study hypothesized
that cellular signaling pathways mediating radiosensitivity and
radioresistance may be associated with the development of
hypothyroidism.
Radiotherapy is one of the major therapeutic
strategies for the treatment of human malignant tumors, however, a
small number of cells are able to survive and gradually
proliferate, reforming tumors with higher resistance to irradiation
(11). Extensive investigations
have demonstrated that multiple signal transduction signaling
pathways, including cell proliferation, apoptosis/anti-apoptosis
and DNA damage response are associated with radioresistance and
radiosensitivity (12). Patients
with ataxia telangiectasia are highly sensitive to irradiation due
to a deficiency of the ataxia-telangiectasia mutated (ATM) protein
(12), a critical factor of the
DNA double strand break repair signaling pathway. Another example
is epidermal growth factor receptor (EGFR), which is frequently
overexpressed in human tumors, and high expression levels of EGFR
are correlated with radioresistance (13,14).
To sensitize tumor cells to radiation, numerous drugs have been
designed and developed to target proteins, which are essential for
cell survival, in order to improve the prognosis of patients with
cancer (15). Since RAIT is an
irradiation-based treatment, it is possible that functional
factors, which are involved in cellular resistance or sensitivity,
may contribute to susceptibility to hypothyroidism following RAIT,
and the identification of these functional factors may improve
current understanding of the molecular mechanism underlying
RAIT-induced hypothyroidism, and improve diagnostic and treatment
methods. To the best of our knowledge, the present study is the
first to screen potential molecular markers of hypothyroidism in
patients by quantitatively analyzing the mRNA expression levels of
selected genes, including EGFR, ATM, TP53, Ku70, B-cell lymphoma 2
(Bcl-2), nuclear factor (NF)-κB, and early growth response protein
1 (Egr-1), which are central in cellular activities and have been
demonstrated to be responsible for radioresistance in human tissues
(16,17). The present study aimed to identify
whether changes in the mRNA expression levels of these genes may
serve as potential prognostic markers of early-stage hypothyroidism
induced by iodine-131 treatment.
Patients and methods
Patients and tissue samples
This present case-cohort study featured a case
cohort and a comparison cohort. A total of 59 patients diagnosed
with hyperthyroidism at the First Affiliated Hospital of Xi'an Jiao
Tong University (Xi'an, China) were randomly selected for the
present study between May and October 2013. Hyperthyroidism was
diagnosed on the basis of the following: i) High metabolic syndrome
including increased heart rate, sudden weight loss or nervousness,
enlarged thyroid gland, hand shaking or swelling or inflammation
around eyes; ii) increased free thyroid hormones, decreased
sensitive thyroid-stimulating hormone (sTSH) and elevated
iodine-131 uptake by the thyroid. Prior to treatment, multiple
indices were measured, including serum thyroid hormone levels,
thyroid antibody levels, and a routine blood test (2 ml samples),
liver and kidney function test, and electrocardiograph were
performed in order to evaluate the physical condition of the
patients. This was operated by the laboratory professionals (using
radioimmunoassay). All patients were asked to avoid consuming
seafood and drugs that may affect iodine-131 uptake 1 week prior to
treatment until 3 months following treatment. Patients were not
included in the present study if they presented with any of the
following: i) Aged ≤12 years; ii) pregnant and lactating; (iii)
presence of thyroid nodules that may be malignant; iv) history of
thyroidectomy. Patients were treated with iodine-131 (Chengdu
Gaotong Isotope Co., Ltd., Sichuan, China) orally in a capsule
form, and the doses were calculated according to the formula
described by Marinelli et al (18). Early-stage hypothyroidism
assessment was performed 3 months following iodine-131 treatment by
evaluating the levels of sTSH and thyroid hormone. The patients
were divided into two groups: An early-stage hypothyroidism group,
including subclinical hypothyroidism; and a non-early-stage
hypothyroidism group, including euthyroid and hyperthyroid
patients.
The comparison cohort included 27 healthy
volunteers. No selected subject had been diagnosed with
hyperthyroidism. All fine needle thyroid tissue specimens,
including those of patients with hyperthyroidism and healthy
volunteers, were collected according to the procedures approved by
the Human Ethics Committee of the Xi'an Medical University, and all
patients and control subjects provided written informed
consent.
mRNA purification and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the biopsy tissue
samples using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the manufacturer's
instructions. The concentration of the purified total RNA was
measured using a Nanodrop 1000 ultraviolet spectrophotometer
(Thermo Fisher Scientific, Wilmington, DE, USA) and the optical
density 260/280 ratios were between 1.8–2.0. Total RNA was reverse
transcribed into cDNA using QTM SYBR® Green Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) in a 10 µl
reaction system containing 500 ng total RNA. The thermocycling
conditions were as follows: 15 min at 37°C and 5 sec at 85°C.
Quantification of the copy number was performed using qPCR with
β-actin gene as the internal reference. qPCR was performed in a 20
µl volume system containing 2 µl cDNA, 12.5 µl
SYBR® Premix Ex Taq Um™ II (Takara Biotechnology, Co.,
Ltd., Dalian, China), 1 µl forward primer and 1 µl
reverse primer, and 3.5 µl dH2O. The primer
sequences of targeted genes and β-actin are presented in Table I. Primers were obtained from Takara
Biotechnology, Co., Ltd. The thermocycling conditions were as
follows: 15 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C for 40
cycles following an initial activating step for 2 sec at 50°C, and
a denaturing step for 10 min at 95°C. A Bio-Rad CFX Manager thermal
Cycler Dice™ real time PCR system was used (Bio-Rad Laboroatories,
Inc.). The relative copy number of target genes was measured using
the 2−ΔΔCt method (19). β-actin was used as an endogenous
reference and each sample was repeated twice, with the mean values
calculated for statistical analysis.
 | Table IPrimer sequences of the target genes
and β-actin for reverse transcription-quantitative polymerase chain
reaction. |
Table I
Primer sequences of the target genes
and β-actin for reverse transcription-quantitative polymerase chain
reaction.
| Gene | Sense | Antisense | Product size
(bp) |
|---|
| p53 |
5′-CTCCTCAGCATCTTACCGAGT-3′ |
5′-GCTGTTCCGTCCCAGTAGATTA-3′ | 239 |
| Bcl-2 |
5′-ATGTGTTGGAGAGCGTCAAC-3′ |
5′-AGAGACAGCCAGGAGAAATCAAAC-3′ | 182 |
| EGFR |
5′-ATCATACGCGGCAGGACCA-3′ |
5′-TCTGACCGGAGGTCCCAAAC-3′ | 187 |
| Egr-1 |
5′-AGAGCATGTGTCAGAGTGTTGTTCC-3′ |
5′-CACATGTCAAGCCATCAGCAAG-3′ | 196 |
| Ku70 |
5′-GCAACCAGAAGTGCCAGCTTA-3′ |
5′-TGAGTGTTTCATAGCATCAAGCAGA-3′ | 86 |
| NF-κB |
5′-TGGCGCAGAAATTAGGTCTGG-3′ |
5′-GATCACTTCAATTGCTTCGGTGTA-3′ | 161 |
| ATM |
5′-TGTGACTTTTCAGGGGATTTG-3′ |
5′-ATAGGAATCAGGGCTTTTGGA-3′ | 121 |
| β-actin |
5′-ACGAGGCCCAGAGCAAGAGA-3′ |
5′-GGTCTTTGCGGATGTCCACG-3′ | 96 |
Statistical analysis
Statistical significance was examined using
Student's t-test for comparison between two different groups.
P<0.05 was considered to indicate a statistically significant
difference when comparing two groups. Correlation between the
changes in mRNA expression and the susceptibility of early-stage
hypothyroidism was examined using multivariate logistic regression
analysis. The level of gene expression was presented as mean ±
standard deviation. All statistical analyses were performed using
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA), and the type I error was
set at 5%.
Results
mRNA expression levels of target genes in
patients with hyperthyroidism and normal healthy subjects
A total of 59 patients diagnosed with
hyperthyroidism and 27 healthy subjects were included in the
present study. To measure the mRNA expression levels of the
selected target genes, seven sets of primers were designed, based
on the National Center for Biotechnology database (http://www.ncbi.nlm.nih.gov/), and a pair of primers
was designed for β-actin as an endogenous reference to normalize
the expression levels of genes. Using fine needle biopsy, tissue
samples from all patients (pre-treatment group) and volunteers
(control group) were collected, and total the mRNA from each sample
were extracted and reverse transcribed into cDNA. The cDNA products
were then amplified using qPCR, followed by quantification of the
mRNA expression levels using Bio-Rad CFX Manager. Compared with the
control group, the mRNA expression levels of Ku70 and EGFR were
significantly higher, and those of TP53 were marginally higher in
patients with hyperthyroidism; however, the mRNA expression levels
of Bcl-2, NF-κB and Egr-1 were markedly lower, and those of ATM
were marginally lower in the patients with hyperthyroidism,
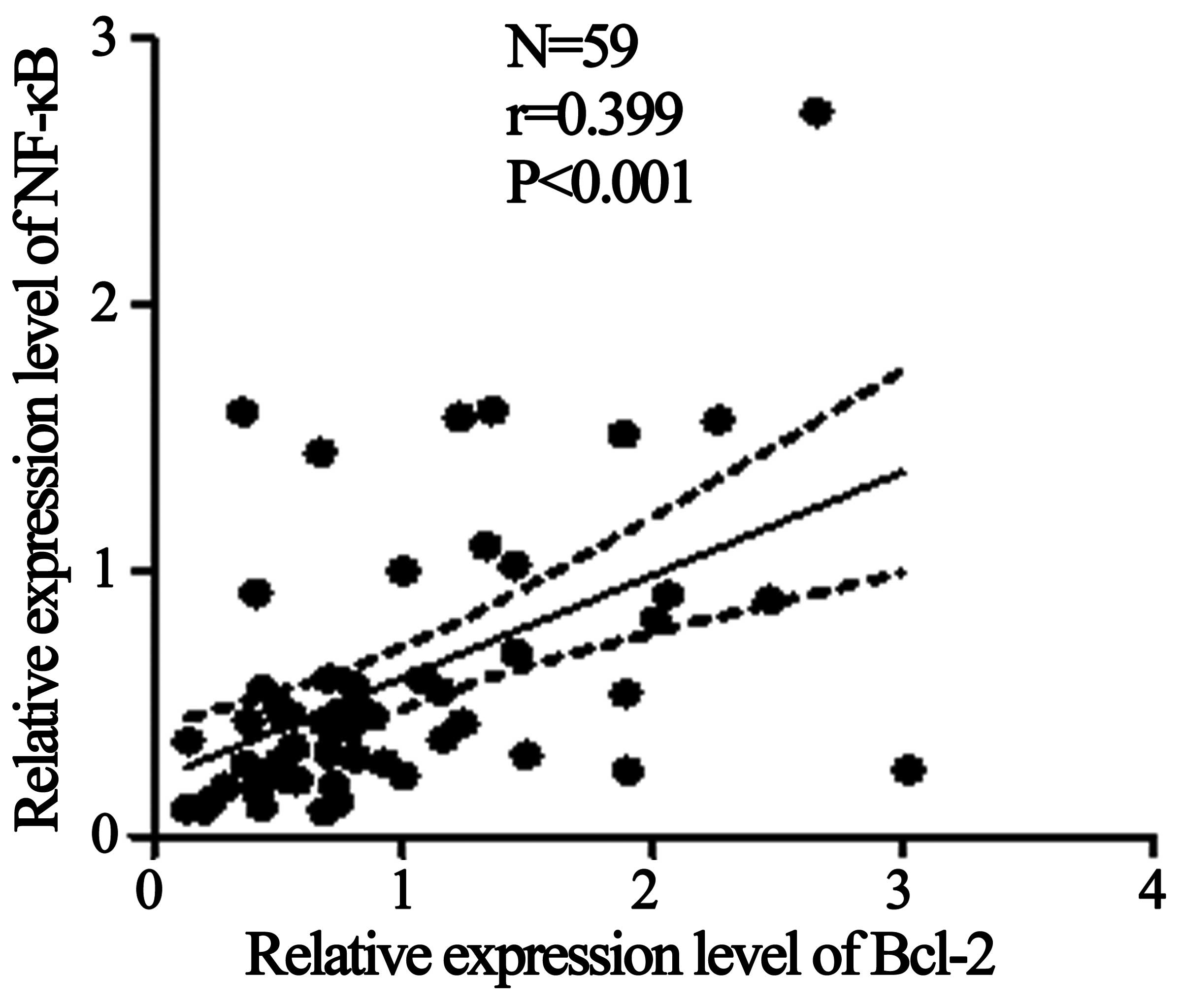
compared with the healthy control group (Fig. 1). Furthermore, regression analysis
demonstrated that the mRNA expression levels of Bcl-2 and NF-κB
were associated (R=0.399; P<0.001; Fig. 2) in the samples of patients with
hyperthyroidism (Fig. 2).
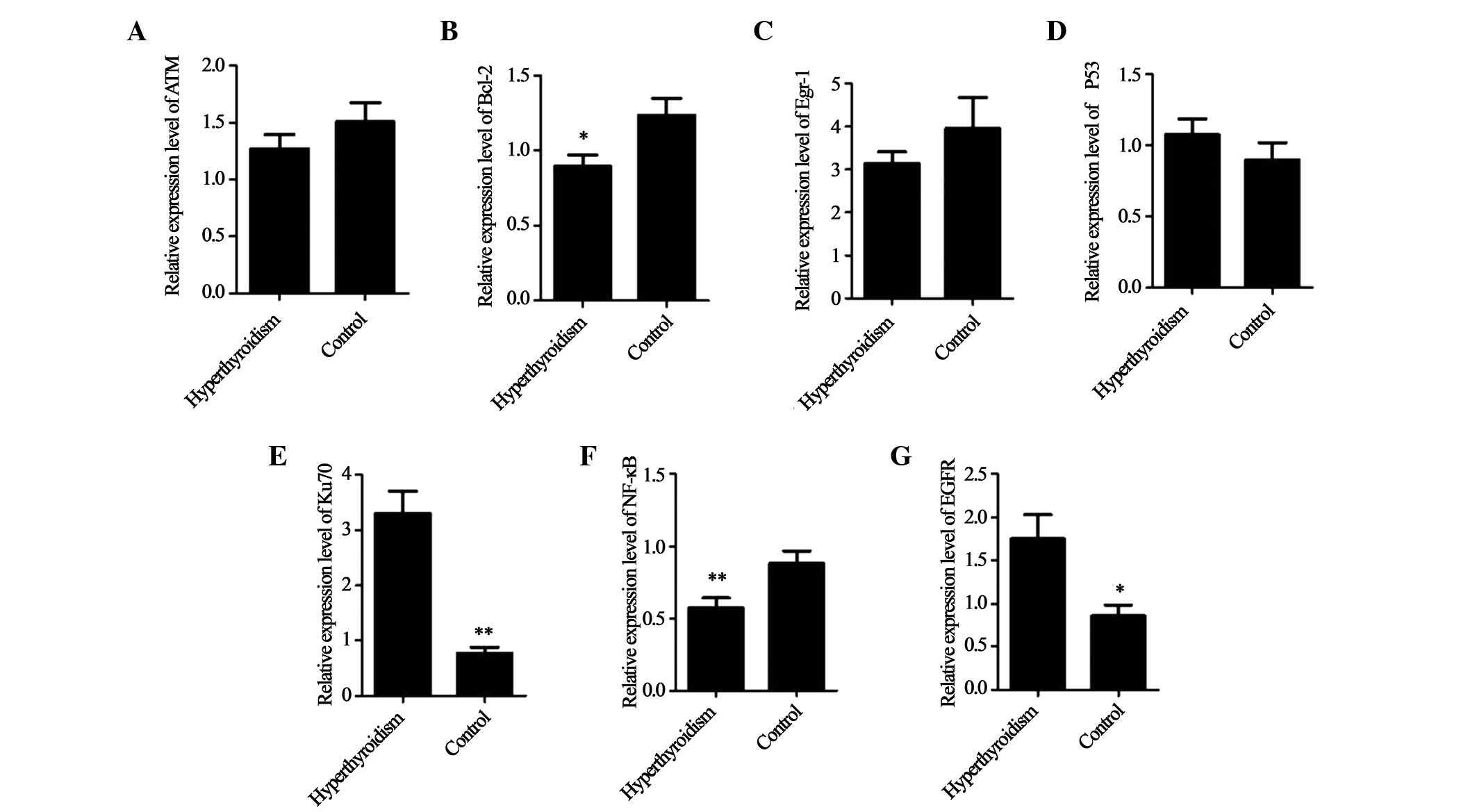 | Figure 1Mean mRNA expression levels of target
genes in the hyperthyroidism and control groups. The relative mRNA
expression levels of (A) ATM, (B) Bcl-2, (C) Egr-1, (D) TP53, (E)
Ku70, (F) NF-κB and (G) EGFR. *P<0.05, and
**P<0.01, vs. control group. ATM, ataxia
telangiectasia mutated; Bcl-2, B-cell lymphoma 2; Egr-1, early
growth response 1; NF-κB, nuclear factor κB; EGFR, epidermal growth
factor receptor. |
Comparison of target mRNA expression
levels in early and non-early-stage hypothyroidism
Iodine-131 was administered orally to all patients,
the dose of which was to the previously a formula previously
described by Marinelli et al (18). At 3 months post-treatment, the
serum indices were measured, and 30 patients were identified with
early-stage hypothyroidism symptoms, including decreased levels of
FTH and increased levels of sTSH (including subclinical
hypothyroidism). Subsequently, the 59 patients were divided into an
early-hypothyroidism group, which included the 30 patients with
symptoms of hypothyroidism; and a non-early-stage hypothyroidism
group, which included the remaining 29 patients who continued to
exhibit hyperthyroidism. The mRNA expression levels of the target
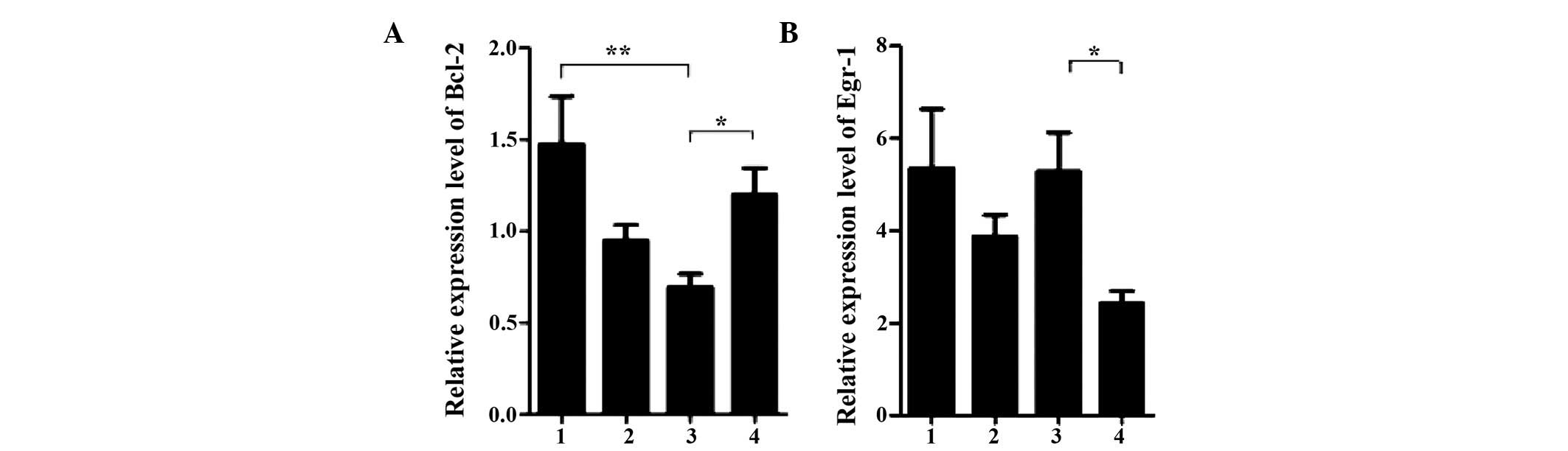
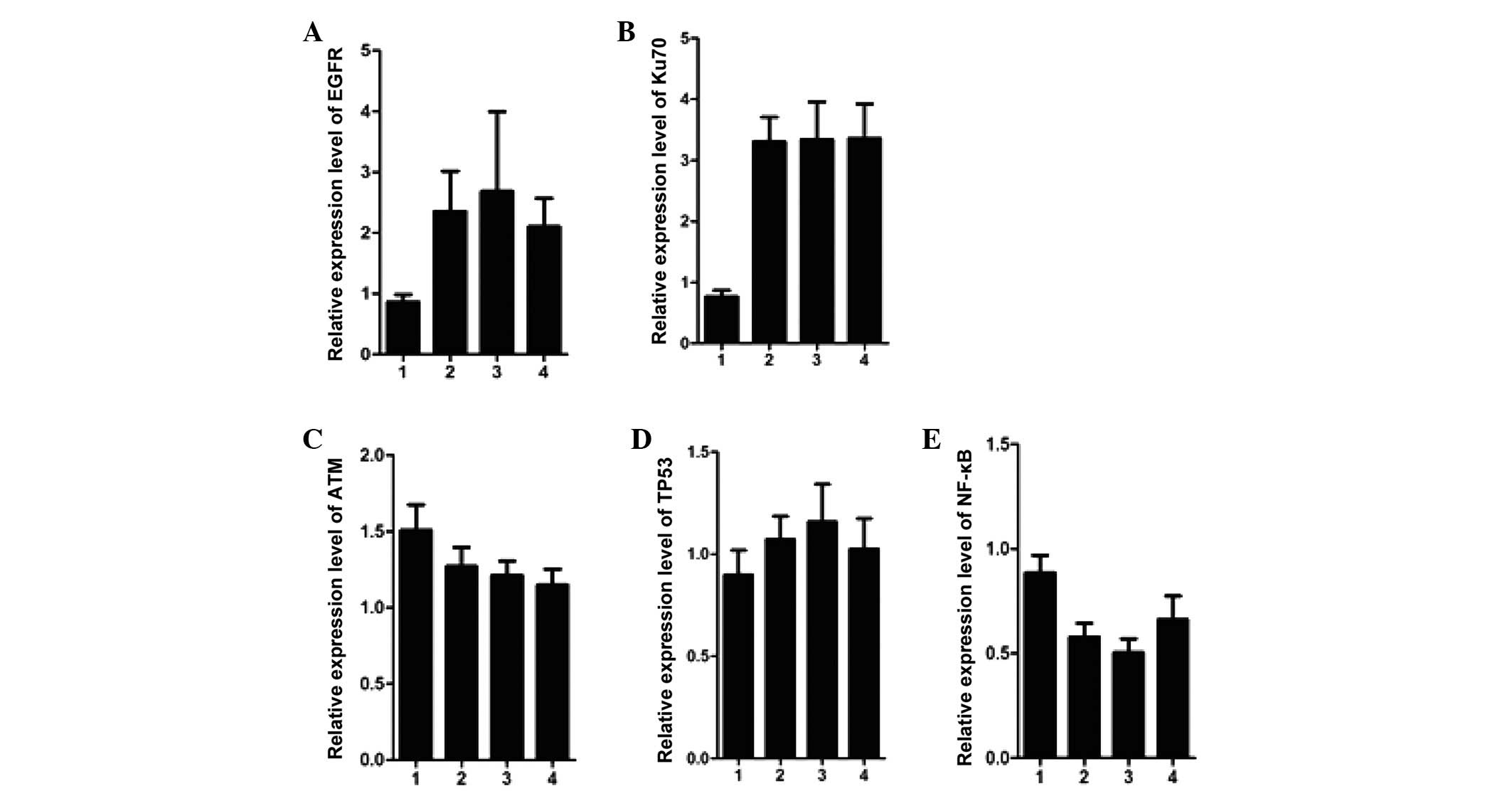
genes in the groups were then investigated. Notably, among the
target genes, the mRNA expression levels of Bcl-2 were
significantly lower in the patients of the early-hypothyroidism
group, compared with the patients of the non-early-stage
hypothyroidism group, and these expression levels were even lower
than those of the patients of the pre-treatment and control groups
(Fig. 3A). This suggested that the
decrease in the expression of Bcl-2 mRNA may be associated with the
onset of early-stage hypothyroidism. In addition to Bcl-2, distinct
changes in the mRNA expression levels of Egr-1 were observed. The
mRNA expression levels of Egr-1 were markedly increased in the
early-hypothyroidism group, compared with the non-early-stage
hypothyroidism group, and these expression levels were comparable
to those in the control group (Fig.
3B). Although the present study did not provide further
experimental data to address the potential significance of the
increase in Egr-1 expression levels in patients with
hypothyroidism, the association between Egr-1 and hypothyroidism
merits further research. Notably, the mRNA expression levels of
Ku70 and EGFR which were high in the pre-treatment group maintained
these high levels in the early-hypothyroidism and non-early-stage
hypothyroidism groups, which suggested that the increase was likely
to be associated with the initiation of hyperthyroidism (Fig. 4A and B). However, the mRNA
expression levels of the other genes, including ATM, TP53 and NF-κB
exhibited only marginal differences between the groups (Fig. 4C–E).
mRNA expression levels of Bcl-2 and Egr-1
are associated with susceptibility to hypothyroidism
The present study further investigated whether the
changes in mRNA expression levels were associated with
susceptibility to hypothyroidism through a multiple logistic
regression model. The statistical differences in the mRNA
expression levels of ATM, TP53, EGFR, NF-κB and Ku70 were not
significant between the early-stage hypothyroidism group and the
non-early-stage hypothyroidism group. However, the model revealed
that the regression coefficient of Egr-1 [hazard ration (HR),
6.432; 95% CI, 1.106–2.197] and Bcl-2 (HR, 6.193; 95% CI,
0.086–0.747) were positively and negatively correlated with the
occurrence of early-stage hypothyroidism, respectively (Table II). These results suggested that
increasing the expression of Egr-1 is likely to increase the
likelihood of developing early-hypothy roidism, whereas the
susceptibility to early-hypothyroidism is likely to be reduced if
the expression of Bcl-2 is suppressed.
 | Table IIVariables and constants in the
logistic resection equation. |
Table II
Variables and constants in the
logistic resection equation.
| Factor | B | SE | HR | P-value | CI (RC) 95%
lower | CI (RC) 95%
upper |
|---|
| Variable | | | | | | |
|
NF-κB | −0.819 | 0.748 | 0.200 | 0.273 | 0.102 | 0.909 |
|
Bcl-2 | −1.373 | 0.552 | 6.193 | 0.013 | 0.086 | 0.747 |
|
Egr-1 | 0.444 | 0.175 | 6.432 | 0.011 | 1.106 | 2.197 |
| Constant | −0.156 | 0.628 | 0.062 | 0.804 | | |
Discussion
It has long been recognized that each human disease
has an underlying molecular mechanism, and the elucidation of these
mechanisms is directly associated with the understanding of the
cause, process and treatment of these diseases. Of numerous
strategies, the identification of susceptible genes with expression
levels, which are significantly altered in patients is one of the
most widely used methods to investigate the basis of diseases in
humans. Hyperthyroidism is one of most common autoimmune thyroid
diseases, and several genes, including thyroid stimulating hormone
receptor and thyroglobulin, which belong to thyroid-specific genes;
and human leukocyte antigen (HLA) class II, cytotoxic
T-lymphocyte-associated protein 4 and PTPN22, which belong to
immunoregulatory genes, have been identified and recognized as risk
factors of hypothyroidism (4).
Other genes, including HLA class I, HLA-C, HLA-B, CD40 and Fc
receptor-like 3 have also been subsequently identified, which has
provided insight into the molecular mechanisms underlying the
immunopathogenesis of hyperthyroidism (4). Iodine-131 is currently the
predominant drug used to treat hyperthyroidism, and one of
side-effects, hypothyroidism, is almost always associated with this
treatment strategy (6,20–23).
However, to the best of our knowledge, the unstable gene
expression, which may be associated with hypothyroidism has not
been investigated. As iodine-131 treatment induces genomic damage,
demonstrated by the previous observation of increased micronuclei,
and differences in individual radiosensitivity regulated by
specific genes are the predominant factors that affect the efficacy
of iodine-131 treatment (24), the
present study hypothesized that radioresistant/radiosensitive
and/or cell proliferation-associated genes may be involved in the
pathologic process of iodine-131-dependent hypothyroidism. Several
genes, including EGFR, ATM, TP53, Ku70, Bcl-2, NF-κB and Egr-1,
were selected in the present study, and their mRNA expression
levels were quantified. Irradiation-induced DNA double strand
breaks (DSBs) are the most life-threatening form of DNA damage, and
ATM, Ku70 and TP53 are important in the DSB responses (25). ATM is recruited to DSB sites in the
initial stage of DNA damage response and is activated through the
phosphorylation of serine 1981 (26). This activated ATM then amplifies
the DNA damage signal by recruiting more substrates to facilitate
DNA repair. Ku70, initially described as an auto-antigen in the
blood of patients with systemic lupus erythematosus, forms
heterodimers with Ku80 (27) and
functions in DNA damage repair via the non-homologous end joining
(NHEJ)-mediated signaling pathway (28). NHEJ is also required for antigen
receptor gene rearrangements and the development of T and B cells
in the vertebrate immune system (29). TP53 is a major downstream effector
in the DNA damage signaling cascade, and the activation of TP53 is
required for DNA damage-induced cell cycle arrest, as well as
apoptosis if the DSBs are too severe to be repaired (30). Our previous study demonstrated that
neither the mRNA nor protein expression levels of TP53 are altered
in response to iodine-131 exposure (31). In the present study, the mRNA
expression levels of ATM and TP53 were found to be sustained in
patients with hyperthyroidism and the control group, whereas the
mRNA expression levels of Ku70 increased markedly in the tissue
samples of the patients with hyperthyroidism. However, the mRNA
expression levels of the three targets were not significantly
different between the early-stage hyperthyroidism and
non-early-stage hyperthyroidism groups. However, the specific
increase in the mRNA expression of Ku70 in patients, irrespective
of iodine-131 treatment, indicated that Ku70 is likely a potential
risk factor for hyperthyroidism.
Previous studies demonstrated that EGFR is required
for thyroid growth as one of the membrane fractions of thyroid
cells in normal and neoplastic tissues of various organs (32–34).
EGFR increases the proliferation of cultured dog thyroid cells, and
enhances the DNA synthesis in cultured porcine thyroid cells
(35). In addition, significantly
increased expression levels of EGFR have been reported in malignant
thyroid tissue samples (36). The
present study demonstrated that the mRNA expression levels of EGFR
were significantly increased in patients with hyperthyroidism,
which is concordant with the observations described by Marti et
al (37), in which that
nuclear expression of EGFR was enhanced in tissue samples of
patients with Graves disease and goiter, compared with normal
thyroid tissue samples. This indicates that the EGFR-dependent
regulation of thyroid cell proliferation under pathological
conditions may be associated with hyperthyroidism. However, the
mRNA expression levels of EGFR remained unchanged following
treatment with iodine-131.
NF-κB is involved in the signaling pathway of immune
and inflammatory responses (38).
Nandakumar et al (39)
demonstrated that NF-κB is activated patients with hyperthyroidism.
Vinayagamoorthi et al (40)
also demonstrated that the NF-κB signaling pathway is activated in
lymphocytes in an L-thyroxin-treated hyperthyroid rat model. The
present study demonstrated that the mRNA expression levels of NF-κB
were significantly lower in patients with hyperthyroidism, compared
with control subjects, which is concordant with the results of a
previous study by Kumar et al (41), who reported that triiodothyronine
treatment activated NF-κB, however, protein expression levels of
NF-κB were downregulated in response to persistent exposure (10
days) to triiodothyronine. Several studies have also demonstrated
that NF-κB negatively regulates apoptosis by upregulating the
expression of anti-apoptotic Bcl-2 (42–44);
and Bcl-2 is involved in the selection and maintenance of
long-lived memory T cells (45).
Bcl-2 protein expression levels are decreased in hyperthyroid rats
(46) and in the lymphocytes of
patients with hyperthyroidism (47). The results of the present study
also demonstrated that the mRNA expression of Bcl-2 was
significantly lower in patients with hyperthyroidism, compared with
healthy subjects, and this downregulation was positively correlated
with a decrease in the expression of NF-κB, determined using the
simple regression analysis. These data suggested that NF-κB and
Bcl-2-mediated apoptosis may be involved in the onset of
hyperthyroidism through disorder of immune responses. However, the
mRNA expression levels of Bcl-2, but not NF-κB, further decreased
in patients with iodine-131 therapy-induced early-stage
hypothyroidism. In addition to the significant decrease in the
expression of Bcl-2, higher mRNA expression levels of Egr-1 were
detected in the tissue samples of patients with early-stage
hypothyroidism, compared with the non-early-stage hypothyroidism
group. Bcl-2 and Egr-1 are anti-apoptotic and pro-apoptotic genes,
respectively, and the opposing changes in mRNA expression levels of
the two genes in the early-stage hypothyroidism group suggested
that dysregulation of apoptosis is a significant causative factor
in hypothyroidism. Following stepwise-selected multivariate
regression analysis of the seven gene targets, only Bcl-2 and Egr-1
exhibited characteristics as independent prognostic factors in
early-stage hypothyroidism.
In conclusion, the results of the present study
demonstrated that the mRNA expression levels of Ku70 and EGFR were
markedly increased, whereas those of NF-κB and Bcl-2 were decreased
in the tissue samples of patients with hyperthyroidism. The mRNA
expression levels of NF-κB changed marginally, whereas those of
Bcl-2 decreased further in the early-stage hypothyroidism group in
response to iodine-131 treatment. In addition, the expression
levels of Bcl-2 and Egr-1 were altered in an opposing manner in the
early-hypothyroidism group, compared with the non-early-stage
hypothyroidism group and hyperthyroidism group. Stepwise-selected
multivariate regression analysis indicated that Bcl-2 and Egr-1 may
serve as prognostic markers of early-stage hypothyroidism. However,
the molecular mechanism underlying the association between changes
in mRNA expression and the initiation of
hyperthyroidism/hypothyroidism requires further investigation.
Acknowledgments
The authors of the present study would like to thank
Professor Bingyin Shi and the staff of the Nuclear Medicine
Department of The First Affiliated Hospital of Xi'an Jiaotong
University College of Medicine (Xi'an, China) for their support.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81172598).
References
|
1
|
Bahn Chair RS, Burch HB, Cooper DS, Garber
JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM,
Rivkees SA, et al American Thyroid Association; American
Association of Clinical Endocrinologists: Hyperthyroidism and other
causes of thyrotoxicosis: management guidelines of the American
Thyroid Association and American Association of Clinical
Endocrinologists. Thyroid. 21:593–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vanderpump MP, Tunbridge WM, French JM,
Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H,
Tunbridge F, et al: The incidence of thyroid disorders in the
community: A twenty-year follow-up of the Whickham survey. Clin
Endocrinol (Oxf). 43:55–68. 1995. View Article : Google Scholar
|
|
3
|
Klein I and Ojamaa K: Thyroid hormone and
the cardiovascular system. N Engl J Med. 344:501–509. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Effraimidis G and Wiersinga WM: Mechanisms
in endocrinology: Autoimmune thyroid disease: Old and new players.
Eur J Endocrinol. 170:R241–R252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solomon B, Glinoer D, Lagasse R and
Wartofsky L: Current trends in the management of Graves' disease. J
Clin Endocrinol Metab. 70:1518–1524. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wartofsky L, Glinoer D, Solomon B,
Nagataki S, Lagasse R, Nagayama Y and Izumi M: Differences and
similarities in the diagnosis and treatment of Graves' disease in
Europe, Japan and the united states. Thyroid. 1:129–135. 1991.
View Article : Google Scholar
|
|
7
|
Kraft O: Hypothyroidism and radioiodine
therapy. Cancer Biother Radiopharm. 22:261–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berg GE, Michanek AM, Holmberg EC and Fink
M: Iodine-131 treatment of hyperthyroidism: Significance of
effective half-life measurements. J Nucl Med. 37:228–232.
1996.PubMed/NCBI
|
|
9
|
Catargi B, Leprat F, Guyot M, Valli N,
Ducassou D and Tabarin A: Optimized radioiodine therapy of Graves'
disease: Analysis of the delivered dose and of other possible
factors affecting outcome. Eur J Endocrinol. 141:117–121. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Jong JA, Verkooijen HM, Valk GD,
Zelissen PM and de Keizer B: High failure rates after 131I therapy
in graves hyperthyroidism patients with large thyroid volumes, high
iodine uptake and high iodine turnover. Clin Nucl Med. 38:401–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anai S, Shiverick K, Medrano T, Nakamura
K, Goodison S, Brown BD and Rosser CJ: Downregulation of BCL-2
induces downregulation of carbonicanhydrase IX, vascular
endothelial growth factor and pAkt and induces radiation
sensitization. Urology. 70:832–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider J, Illig T, Rosenberger A,
Bickeböller H and Wichmann HE: Detection of atm gene mutations in
young lung cancer patients: A population-based control study. Arch
Med Res. 39:226–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiyozuka M, Akimoto T, Fukutome M, Motegi
A and Mitsuhashi N: Radiation-induced dimer formation of EGFR:
Implications for the radiosensitizing effect of cetuximab.
Anticancer Res. 33:4337–4346. 2013.PubMed/NCBI
|
|
14
|
Akimoto T, Hunter NR, Buchmiller L, Mason
K, Ang KK and Milas L: Inverse relationship between epidermal
growth factor receptor expression and radiocurability of murine
carcinomas. Clin Cancer Res. 5:2884–2890. 1999.PubMed/NCBI
|
|
15
|
Vera-Badillo FE, Al-Mubarak M, Templeton
AJ and Amir E: Benefit and harms of new anti-cancer drugs. Curr
Oncol Rep. 15:270–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmed MM, Venkatasubbarao K, Fruitwala SM,
Muthukkumar S, Wood DP Jr, Sells SF, Mohiuddin M and Rangnekar VM:
EGR-1 induction is required for maximal radiosensitivity in A375-C6
melanoma cells. J Biol Chem. 271:29231–29237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zagurovskaya M, Shareef MM, Das A, Reeves
A, Gupta S, Sudol M, Bedford MT, Prichard J, Mohiuddin M and Ahmed
MM: EGR-1 forms a complex with YAP-1 and upregulates Bax expression
in irradiated prostate carcinoma cells. Oncogene. 28:1121–1131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marinelli LD, Quimby EH and Hine GJ:
Dosage determination with radioactive isotopes. Nucleonics.
2:561948.PubMed/NCBI
|
|
19
|
Zhang W, Gao R, Yu Y, Guo K, Hou P, Yu M,
Liu Y and Yang A: Iodine-131 induces apoptosis in HTori-3 human
thyrocyte cell line and G2/M phase arrest in a p53-independent
pathway. Mol Med Rep. 11:3148–3154. 2015.
|
|
20
|
Erem C, Kandemir N, Hacihasanoglu A, Ersöz
HO, Ukinc K and Kocak M: Radioiodine treatment of hyperthyroidism:
prognostic factors affecting outcome. Endocrine. 25:55–60. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaidya B, Williams GR, Abraham P and
Pearce SH: Radioiodine treatment for benign thyroid disorders:
results of a nationwide survey of UK endocrinologists. Clin
Endocrinol (Oxf). 68:814–820. 2008. View Article : Google Scholar
|
|
22
|
Nygarrd B, Hegedüs L, Gervil M, Hjalgrim
H, Hansen BM, Søe-Jensen P and Hansen JM: Influence of compensated
radioiodine therapy on thyroid volume and incidence of
hypothyroidism in Graves' disease. J Intern Med. 238:491–497. 1995.
View Article : Google Scholar
|
|
23
|
Sridama V, McCormick M, Kaplan EL, Fauchet
R and DeGroot LJ: Long-term follow-up study of compensated low-dose
13lI therapy for Graves' disease. N Engl J Med. 311:426–432. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farkasova T, Gurska S, Witkovsky V and
Gabelova A: Significance of amino acid substitution variants of DNA
repair genes in radiosusceptibility of cervical cancer patients; a
pilot study. Neoplasma. 55:330–337. 2008.PubMed/NCBI
|
|
25
|
Krempler A, Deckbar D, Jeggo PA and
Löbrich M: An imperfect G2M checkpoint contributes to chromosome
instability following irradiation of S and G2 phase cells. Cell
Cycle. 6:1682–1686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darzynkiewicz Z, Zhao H, Halicka HD, Rybak
P, Dobrucki J and Wlodkowic D: DNA damage signaling assessed in
individual cells in relation to the cell cycle phase and induction
of apoptosis. Crit Rev Clin Lab Sci. 49:199–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mimori T, Akizuki M, Yamagata H, Inada S,
Yoshida S and Homma M: Characterization of a high molecular weight
acidic nuclear protein recognized by autoantibodies in sera from
patients with polymyositis-scleroderma overlap. J Clin Invest.
68:611–620. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dobbs TA, Tainer JA and Lees-Miller SP: A
structural model for regulation of NHEJ by DNA-PKcs
autophosphorylation. DNA Repair (Amst). 9:1307–1314. 2010.
View Article : Google Scholar
|
|
29
|
Helmink BA and Sleckman BP: The response
to and repair of RAG-mediated DNA double-strand breaks. Annu Rev
Immunol. 30:175–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Otsuka K and Ochiya T: Genetic networks
lead and follow tumor development: microRNA regulation of cell
cycle and apoptosis in the p53 pathways. Biomed Res Int.
2014:7497242014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weixiao Z, Rui G, Yan Y, Kun G, Peng H,
Yan L and Mingqi Y: Iodine-131 induces apoptosis in the HTori 3
cell line and G2/M arrest in a p53-independent pathway. Mol Med
Rep. 11:3148–3154. 2015.
|
|
32
|
Kasai K, Kuroda H, Hashigami Y, Ishikawa
M, Nakamura T and Shimoda SI: Specific epidermal growth factor
receptors on porcine and human thyroid membranes. Horm Metab Res.
17:592–594. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanamori A, Abe Y, Yajima Y, Manabe Y and
Ito K: Epidermal growth factor receptors in plasma membranes of
normal and diseased human thyroid glands. J Clin Endocrinol Metab.
68:899–903. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saller B, Stapfer G, Bein B, Hoermann R,
Spelsberg F and Mann K: Increased binding capacity of receptors for
the epidermal growth factor in benign thyroid nodules and thyroid
malignancies. Clin Investig. 71:898–902. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Westermark K, Karlsson FA and Westermark
B: Epidermal growth factor modulates thyroid growth and function in
culture. Endocrinology. 112:1680–1686. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizukami Y, Nonomura A, Michigishi T,
Yokoyama K, Noguchi M, Hashimoto T, Nakamura S and Matsubara F:
Immunohistochemical demonstration of epidermal growth-factor
receptors in normal, benign and malignant thyroid tissues. Int J
Oncol. 1:331–335. 1992.PubMed/NCBI
|
|
37
|
Marti U, Ruchti C, Kämpf J, Thomas GA,
Williams ED, Peter HJ, Gerber H and Bürgi U: Nuclear localization
of epidermal growth factor and epidermal growth factor receptors in
human thyroid tissues. Thyroid. 11:137–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aggarwal BB, Takada Y, Shishodia S,
Gutierrez AM, Oommen OV, Ichikawa H, Baba Y and Kumar A: Nuclear
transcription factor NF-kappa B: Role in biology and medicine.
Indian J Exp Biol. 42:341–353. 2004.PubMed/NCBI
|
|
39
|
Nandakumar DN, Koner BC, Vinayagamoorthi
R, Nanda N, Negi VS, Goswami K, Bobby Z and Hamide A: Activation of
NF-kappaB in lymphocytes and increase in serum immunoglobulin in
hyperthyroidism: Possible role of oxidative stress. Immunobiology.
213:409–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vinayagamoorthi R, Koner BC, Kavitha S,
Nandakumar DN, Padma Priya P and Goswami K: Potentiation of humoral
immune response and activation of NF-kappaB pathway in lymphocytes
in experimentally induced hyperthyroid rats. Cell Immunol.
238:56–60. 2005. View Article : Google Scholar
|
|
41
|
Kumar A, Sinha RA, Tiwari M, Singh R, Koji
T, Manhas N, Rastogi L, Pal L, Shrivastava A, Sahu RP and Godbole
MM: Hyperthyroidism induces apoptosis in rat liver through
activation of death receptor-mediated pathways. J Hepatol.
46:888–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen F, Castranova V and Shi X: New
insights into the role of nuclear factor-kappaB in cell growth
regulation. Am J Pathol. 159:387–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mattson MP and Camandola S: NF-κB in
neuronal plasticity and neurodegenerative disorders. J Clin Invest.
107:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen C, Edelstein LC and Gelinas C: The
Rel/NF-kappaB family directly activates expression of the apoptosis
inhibitor Bcl-x(L). Mol Cell Biol. 20:2687–2695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
46
|
Klatka M, Grywalska E, Polak A and
Roliński J: Impact of treatment with methimazole on the Bcl-2
expression in CD8+ peripheral blood lymphocytes in children with
Graves' disease. Ann Agric Environ Med. 20:884–888. 2013.PubMed/NCBI
|
|
47
|
Giriş M, Erbil Y, Depboylu B, Mete O,
Türkoğlu U, Abbasoğlu SD and Uysal M: Heme oxygenase-1 prevents
hyperthyroidism induced hepatic damage via an antioxidant and
antiapoptotic pathway. J Surg Res. 164:266–275. 2010. View Article : Google Scholar
|