Introduction
The increasing application of cardiopulmonary bypass
(CPB) technology has improved cardiovascular surgeries and
significantly increased the survival rate of patients (1). Despite the advantages of CPB, it
causes damage to other body parts. Although numerous studies have
explored means of reducing the damage of CPB to the body, which led
to a significant improvement of the survival rate of patients and a
reduction in the incidence of post-operative complications with
regard to other organs, the incidence rate of neurological
complications has remained relatively constant; for example, the
incidence rates of stroke and encephalopathy following CPB are 2–5
and 10–30%, respectively (2). The
time of hospitalization of patients following CPB is increased in
order to minimize the risk of brain damage and other complications,
which leads to the consumption of medical resources and impedes the
development of cardiovascular surgery.
The mechanisms of CPB-associated brain injury
include cerebral artery embolization, hypoperfusion, cerebral
metabolic disorders, inflammatory damage and ischemia/re-perfusion
injury (1,3). Among these, inflammatory damage is
one of the hotspots of research regarding CPB-induced
complications. Due to the combination of the patients'
immune-associated stress response and damage from surgical
equipment and procedures during CPB, activation and release of
cytokines as an initiation of the 'inflammatory cascade' leads to
systemic inflammatory response syndrome and brain injury.
Therefore, mitigating inflammatory damage is an important aspect of
cerebral protection during CPB. Bowen et al (4) found that the prevention of the
inflammatory reaction not only alleviates inflammation-associated
damage but also induces the body's tolerance to ischemic brain
injury.
Pradillo et al (5) and Broad et al (6) found that pre-excited Toll-like
receptors (TLRs) can not only prevent the inflammatory reaction,
but also decrease the area of cerebral ischemic infarct and
increase the tolerance to brain injury. The TLR family (7) is a group of recognition receptors of
the immune response, which are highly expressed in the central
nervous system. TLR3 has a particularly important role within the
TLR family (8); first of all, TLR3
is highly expressed in astrocytes; furthermore, there are two
immune pathways downstream of the TLR family, including TLR2, 4, 8
and 9 (9,10), which proceed via the MyD88 pathway,
while only TLR3 solely relies on the TIR-domain-containing
adapter-inducing interferon-β (TRIF)-dependent pathway. Pan et
al (11) proved that
pre-excited TLR3 can increase the tolerance of brain tissue to
ischemic injury and reduce the inflammatory reaction, while
promoting the production of anti-inflammatory cytokines and
neuroprotective mediators and reducing ischemic brain injury.
The implementation of neuroprotective strategies in
the perioperative period can significantly reduce neurological
complications after CPB (12–14).
Apart from the inhibition of inflammation-induced damage, existing
neuro-protective measures include the improvement of CPB devices
and surgical techniques as well as ischemic pre-conditioning and
the application of protective drugs (15–17).
Protective drugs can be divided into classes including narcotics,
suppressors of the inflammation reaction and neuroprotective
agents. Anesthetic-induced pre-conditioning (APC) (18) and ischemic preconditioning (IPC)
(19) have exactly the same
mechanism of action, namely the non-noxious
stimuli-adaptation/noxious stimuli-tolerance. The neuroprotective
effects of sevoflurane amongst anesthetic drugs is one of the
current research hotspots (20,21).
As the inhalation of anesthetics is widely used in
clinical cardiovascular surgery, the safety of sevoflurane
(22) has been confirmed. Studies
have confirmed that sevoflurane has a protective effect against
damage to the nervous system (22,23).
Due to these properties, sevoflurane is suitable for patients
undergoing cardiovascular surgery, as it can not only be applied
for normal anesthesia but also meets the requirements of patients
with poor cardiac function, large hemodynamic fluctuations and high
demand for circulation stability, in addition to protecting the
nervous system during CPB (24).
Although the protective effects of sevoflurane on
the brain have been extensively studied, the exact underlying
mechanism has never been fully elucidated. Wang et al
(25) confirmed that sevoflurane
can upregulate the expression of inflammatory genes at the
transcriptional and translational level, repress the expression of
adhesion molecules and pro-inflammatory factors, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, inhibit the
infiltration of neutrophils and macrophages and alleviate
inflammatory damage to the nervous system
As these mechanisms are similar to those of the
protective effects of TLR3 on the brain, the present study
investigated whether sevoflurane protects the brain from
CPB-induced inflammatory damage by activating the TLR3 signaling
pathway. For this, a rat model of CPB, which closely resembles
clinical CPB in humans (26), was
used to study the influence of 2.4% sevoflurane pre-conditioning on
CPB-induced brain injury as well as the involvement of the TLR3
signaling pathway.
Materials and methods
Experimental devices
The following devices were used: Anesthesia
respirator (Draeger, Lübeck, Germany), TCI-III dual-channel
target-controlled infusion pump (Wuhan Changfeng Medical Appliance
Co., Ltd, Wuhan, China), patient monitor (Datex-Ohmeda, Helsinki,
Finland), NSP5100-type activated clotting time analyzer (Medtronic
Inc., Minneapolis, MN, USA), BT00-300 M constant current
peristaltic pump (Baoding Longer Precision Pump Co., Ltd., Baoding,
China), rat membrane oxygenator (Guangdong Kewei Medical Instrument
Co. Ltd., Guangdong, China), 856-type blood gas analyzer (Bayer,
Whippany, NJ, USA), high-speed refrigerated centrifuge (Hunan
Xiangyi Laboratory Instrument Development Co., Ltd., Hunan, China),
small animal ventilator (Jiangxi Province Bentley Anesthesia
Breathing Equipment Co., Ltd.) and disposable intravenous trocar
(16, 18, 20, 22, 23 and 24 G; BD Biosciences, Franklin Lakes, NJ,
USA).
Drugs and reagents
The following drugs and reagents were used in the
present study: Sevoflurane (Shanghai Hengrui Medicine Co., Ltd,
Shanghai, China), 10% chloral hydrate (Qingdao Yulong Algae Co.,
Ltd., Qingdao, China) 5% sodium bicarbonate (Jiang Su Runyang
Pharmaceutical Co., Ltd., Jiangsu, China), 20% mannitol (Baxter
Healthcare Trading Co, Ltd., Shanghai, China), 6% hydroxyethyl
starch (Beijing Fresenius-Kabi Pharmaceutical Co., Ltd., Beijing,
China), heparin sodium (Tianjin Pharmaceutical Group Co., Ltd.,
Tianjin, China), soda-lime (England Molecular Products Limited Co.,
Ltd., Harlow, UK), 10% formalin (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China), Ponceau S (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China), rat TLR3 (cat. no.
1487-TR-050), TRIF (cat. no. ab101232), IL-6 (cat. no. D6050),
interferon (IFN)-β (cat. no. 41410-1), S100-β (cat. no. DY5578)
ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA), terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
positive test kit (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China).
Experimental animals and grouping
A total of 64 adult male Sprague Dawley rats (age,
12 weeks), weighing 350–450 g were provided by the Shenyang
Military Region General Hospital Laboratory Animal Center
(Shenyang, China; lot no. 20120002). The rats were maintained in a
temperature (25°C) and humidity (50%) controlled environment, under
a 12 h light/dark cycle. The rats were sacrificed by cervical
dislocation. The animal experiments were approved by the Animal
Experiments Ethics Committee of the General Hospital of Shenyang
Military Region (Shenyang, China). Animals were fasted with free
access to water for 12 h prior to surgery.
The animals were randomly divided into three groups:
The sham operation group (H group; n=8), the CPB group (C group;
n=24) and the sevoflurane pre-conditioning group (S group;
n=32).
Model preparation and treatment
Rats were prepared for CPB by intraperitoneal
injection with 10% chloral hydrate (300 mg/kg) for anesthesia.
Photopic oral intubation was performed using a 16 G intravenous
catheter, and animals were mechanically ventilated with a small
animal ventilator (settings: Frequency, 60 beats/min; tidal volume,
3 ml/kg; inspiratory to expiratory ratio, 1:1.5) connected to a
monitor to observe the heart rate, oxygen saturation and rectal
temperature of the rats.
Hair was removed at the puncture site, followed by
disinfection, skin incision as well as isolation, exposure and
puncture of the vascular vein. Left femoral vein catheterization
(23G) was performed to open the fluid path, which was and connected
to a micro infusion pump. The left femoral artery was catheterized
(24G) and connected to the monitor for real-time monitoring of
blood pressure. The right internal jugular vein was catheterized
into the right atrium using a homemade long-short drainage needle
(16G) in order to drain blood for CPB. The right femoral artery was
catheterized for fixed CPB perfusion. Between the two puncture
sites with the drainage tube, a homemade blood storage device, a
constant peristaltic pump (Baoding Longer Precision Pump Co.,
Ltd.), silicone tubing (internal diameter, 4 mm) and a rat membrane
oxygenator (Guangdong Kewei Medical Instrument Co. Ltd.) were
installed to establish the CPB circuit. The left femoral vein was
selected as the systemic heparin site in the rats, and heparin
sodium (300 IU/kg) was injected when the activated clotting time
reached 400–500 sec. Rats were pre-charged without blood to
establish the without-blood pre-charge and the non-arrest charge
CPB model in rats.
CPB was then performed using the membrane oxygenator
to immediately supply oxygen. The low-flow CPB velocity was 35
ml/kg/min, which was later increased to 100–120 ml/kg/min at
full-flow bypass. To prevent the formation of air embolism, 1–2 ml
of blood was retained in the blood storage device whenever
possible. The mean arterial pressure (MAP) was maintained at >60
mmHg, the pH was kept at 7.35–7.45, the partial CO2
pressure (PaCO2) was kept at at 35–45 mmHg and
hematocrit (Hct) was maintained at >0.25. Rats were provided
with epinephrine hydrochloride (2–20 µg/100 g; Wuhan Grand
Pharmaceutical Group Co., Ltd., Wuhan, China) and fluids
intraoperatively in order to maintain a stable circulation.
Treatment of experimental groups
In the H group, intubation and mechanical
ventilation was performed in the right femoral artery only and the
right internal jugular vein was catheterized without bypass. In the
C group, the CPB model was established as described above. On the S
group, 2.4% sevoflurane pre-conditioning was performed for 1 h
prior to establishing the CPB model.
In groups C and S, CPB was performed for 1 h. During
sevoflurane pre-conditioning, soda lime was placed under the gauze.
For pre-conditioning, a box with two outlet holes was used - one
hole to connect to the anesthesia ventilator and the other one to
connect to the gas collection of the monitor to real-time monitor
the concentration of oxygen and sevoflurane. The temperature inside
the box was maintained at 35–37°C, the sevoflurane concentration
was adjusted to 2.4% and rats were pre-conditioned for 1 h.
Specimen collection and processing
Arterial and venous blood samples were collected at
the following time-points: Prior to CPB (T0), CPB for 30
min (T1), CPB for 1 h (T2), 1 h after CPB
(T3), 2 h after CPB (T4) and 3 h after CPB
(T5). In addition, at T0, T1,
T3 and T5, eight rats from the S group and C
group were sacrificed and their brain tissue was collected.
Brain tissue
Rats were infused with 250–400 ml saline, and the
systemic circulation system was flushed and perfused. The skull was
opened, the whole brain was removed, placed on an ice sheet and
divided into two halves by a median sagittal line. The hippocampus
was isolated from the two halves and then stored at −80°C (left)
and in 10% neutral formalin (right).
Venous blood serum and arterial blood
gas
Venous blood samples were collected by
centrifugation (10,000 × g; 10 min), and serum was stored in a
cryogenic refrigerator (−80°C). Blood samples from the left femoral
artery were subjected to blood gas analysis (0.3 ml per run). An
equivalent volume of 6% hydroxyethyl starch was intravenously
injected after sampling.
Determination of S100-β, IL-6, IFN-β in
the rat serum by ELISA assay
The serum levels of S100-β, IL-6, IFN-β were
determined according to the manufacturer's instructions of the
ELISA kits. The optical density was measured at 450 nm using a
microplate reader (ELX800; Bio-Tek Instruments, Inc., Winooski, VT,
USA) and analyzed using Gen5 software (Biotek Instruments,
Inc.).
Determination of TLR3 and TRIF in the rat
hippocampus by ELISA
The hippocampus was weighed and ground with 10
volumes of phosphate-buffered saline (PBS), followed by
centrifugation (10,000 × g, 15 min) and collection of the
supernatant. The protein levels of TLR3 and TRIF were detected by
ELISA according to the manufacturer's instructions.
Western blot analysis of protein levels
of TLR3 in the rat hippocampus
After weighing and grinding the hippocampus,
radioimmunoprecipitation assay buffer (Beijing Soledad Bao
Biological Technology Co., Ltd., Beijing, China) was added (1
ml/100 mg tissue) and then homogenized (IKA T10;
IKA®-Werke GmbH & Co. KG, Staufen, Germany) and
centrifuged (4°C, 12,000 × g, 15 min). The supernatant was
collected and protein concentrations were determined using a
Bicinchoninic Acid kit (Beijing Soledad Bao Biological Technology
Co., Ltd.). Loading buffer was then added to the protein samples.
The protein (80 µg) was separated by 8% SDS-PAGE at 100 V
for 10–20 min. The voltage was increased to 200 V when the
indicator entered the separation gel, and electrophoresis was
continued for another 30–40 min. The blot was then
electrophoretically transferred onto a nitrocellulose membrane and
blocked with 5% non-fat milk in PBS with Tween 20 (PBST). The
membrane was incubated with the rabbit anti-TLR3 polyclonal
antibody (1:600 dilution; cat. no. PL031444R; Beijing Biosynthesis
Biological Technology Co., Ltd., Beijing, China) and goat
polyclonal β-actin antibody (1:500 dilution; cat. no. ab8229) at
4°C overnight, washed with TBST five times and then incubated with
the horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (Alexa Fluor® 488; cat. no. ab175735;
1:5,000; Beijing Biosynthesis Biological Technology Co., Ltd.). The
membrane was visualized using an Enhanced Chemiluminescence kit
(Beijing Biosynthesis Biological Technology Co., Ltd.). Images were
analyzed using Quantity One version 4.62 software (Bio-Rad
Laboratories, Hercules, CA, USA), and expression of the target
protein was normalized to β-actin.
In situ detection of apoptosis of
hippocampal neurons by TUNEL assay
Paraffin (Sinopharm Chemical Reagent Co., Ltd.)
sections of hippocampal tissue were de-paraffinized and
re-hydrated, digested with proteinase K solution for 15 min and
washed four times with water. Sections were then incubated with
H2O2-PBS buffer at room temperature for 15
min and washed with PBS three times for 5 min each. Samples were
incubated with PBS containing TUNEL stain (KGI Nanjing
Biotechnology Development Co., Ltd., Nanjing, China) for 60 min and
with stop buffer for 30 min, followed by washing with PBS. Sections
were incubated with horseradish peroxidase for 30 min, washed with
PBS and then incubated with
diaminobenzidine-H2O2 for 3 min. Following
washing with PBS, samples were stained with methyl green for 10
min, and washed with distilled water and n-butanol three times each
for, 1 min. Samples were dehydrated with xylene (Sinopharm Chemical
Reagent Co., Ltd.) three times, dried and mounted. Normal neuronal
nuclei were identified by a blue stain by hematoxylin (Sinopharm
Chemical Reagent Co., Ltd.), defining them as negative for
apoptosis. Damaged neuronal nuclei were identified by a brown
stain, which indicated DNA breakage, defining them as positive for
apoptosis. Fixed slices from each group were randomly selected from
which five fields of view were randomly selected to capture images
at high-power magnification (×400). The average integrated optical
density values of damaged neurons were determined with a
microscopic image analysis system (BX41; Olympus Corporation,
Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 statistical software (International Business Machines, Armonk,
NY, USA). Values are expressed as the mean ± standard deviation.
Comparisons between groups were performed using one-way analysis of
variance, and comparisons within a group were analyzed with
repeated measures analysis of variance. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Blood gas analysis and clinical
parameters during CPB
The MAP, heart rate (HR), Hct, rectal temperature,
pH value, PaCO2, PaO2, base excess (BE) value
and K+ at the indicated times in the three groups of
rats show no statistically significant differences (P>0.05). The
MAP and HR were significantly decreased during CPB (compared to
T0; P<0.05), while after CPB, a gradual recovery was
shown (compared to T0; P>0.05). The Hct value was
significantly decreased during CPB (P<0.05), while the pH, Hct,
PaCO2, PaO2, BE and K+ levels
exhibited no statistically significant differences at different
time-point within the groups (compared to T0;
P>0.05), Table I.
 | Table IClinical parameters and blood gas
analysis at various time-points. |
Table I
Clinical parameters and blood gas
analysis at various time-points.
| Project | T0 | T1 | T2 | T3 | T4 | T5 |
|---|
| MAP (mmHg) | 105.81±11.67 | 79.46±7.21a | 73.66±5.28a | 96.54±6.11 | 93.25±8.04 | 103.74±8.23 |
| HR (/min) | 309.70±13.84 |
289.49±12.02a | 290.50±9.74a | 300.03±11.34 | 303.69±9.86 | 311.35±9.24 |
| RT (°C) | 36.96±0.33 | 34.42±0.26 | 35.22±0.33 | 36.61±0.32 | 36.85±0.27 | 36.87±0.41 |
| pH | 7.40±0.03 | 7.37±0.02 | 7.37±0.02 | 7.38±0.02 | 7.39±0.03 | 7.39±0.03 |
| PaCO2
(mmHg) | 38.34±2.63 | 39.27±3.22 | 39.32±4.01 | 40.22±2.79 | 39.64±2.51 | 40.75±3.03 |
| PaO2
(mmHg) | 322.28±21.35 | 309.52±14.39 | 307.47±19.38 | 301.52±10.83 | 311.29±17.85 | 309.02±15.63 |
| BE (mmol/l) | −1.3±0.53 | −2.5±0.63 | −2.3±0.37 | −2.2±0.45 | −1.3±0.25 | −2.5±0.34 |
| Hct | 38.68±3.48 | 29.83±2.66a | 30.13±2.19a | 34.62±3.07a | 33.24±2.75a | 32.56±2.81a |
| K+
(mmol/l) | 4.27±0.39 | 4.30±0.38 | 4.25±0.82 | 4.17±0.40 | 4.31±0.42 | 4.33±0.37 |
Sevoflurane-pre-conditioning attenuates
CPB-induced increases in serum levels of S100-β and IL-6
The serum concentration of S100-β and IL-6 in the C
group was significantly increased during and after CPB at
T1–T5 (P<0.05). Of note, the S100-β and
IL-6 concentration in the S group was significantly lower than that
in the C group (P<0.05) indicating that pre-treatment with
sevofluorane attenuated the CPB-induced increases in S100-β and
IL-6 (Tables II and III; Figs.
1 and 2).
 | Figure 1Concentration of S100-β in the
experimental groups at various time-points. Values are expressed as
the mean ± standard deviation. #P<0.05 compared with
T0; *P<0.05 compared to H group;
Δ0P<0.05 compared with S group. H, sham group; C, CPB
group; S, sevoflurane-pre-conditioned group; T0, prior
to CPB; T1, CPB for 30 min; T2, CPB for 1 h;
T3, 1 h after 1-h CPB; T4, 2 h after 1-h CPB;
T5, 3 h after 1-h CPB; CPB, cardiopulmonary bypass. |
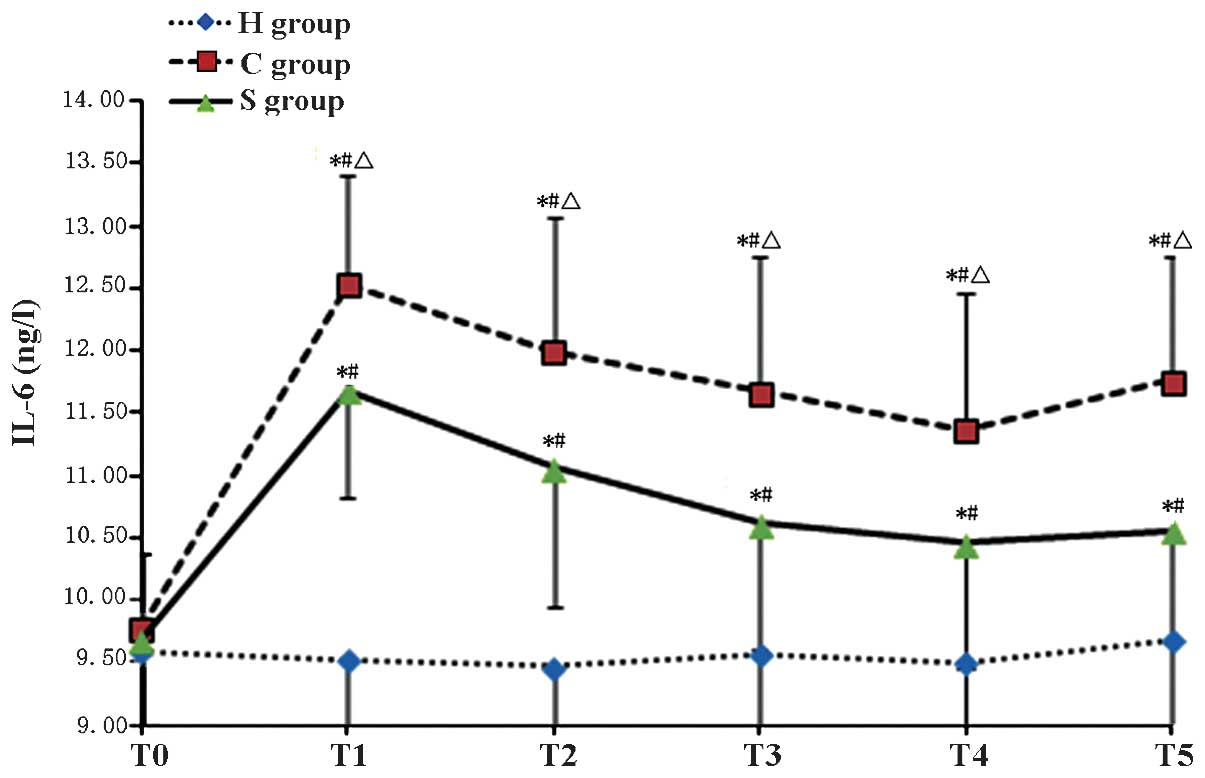 | Figure 2Serum levels of IL-6 in the
experimental groups at various time-points. Values are expressed as
the mean ± standard deviation. #P<0.05 compared with
T0; *P<0.05 compared to H group;
ΔP<0.05 compared with H group. H, sham group; C, CPB
group; S, sevoflurane-pre-conditioned group; T0, prior
to CPB; T1, CPB for 30 min; T2, CPB for 1 h;
T3, 1 h after 1-h CPB; T4, 2 h after 1-h CPB;
T5, 3 h after 1-h CPB; CPB, cardiopulmonary bypass; IL,
interleukin. |
 | Table IIS100-β levels in the experimental
groups at various time-points. |
Table II
S100-β levels in the experimental
groups at various time-points.
| Group | T0 | T1 | T2 | T3 | T4 | T5 |
|---|
| H group | 7.21±0.76 | 7.39±0.80 | 7.14±0.78 | 7.19±0.80 | 7.32±0.79 | 7.10±0.79 |
| C group | 7.58±0.68 | 15.79±1.33a,b | 15.12±1.34a,b | 14.21±1.32a,b | 15.21±1.41a,b | 15.79±1.43a,b |
| S group | 7.19±0.82 | 14.28±1.32a–c | 13.12±1.34a–c | 11.35±1.43a–c | 12.12±1.41a–c | 13.53±1.42a–c |
 | Table IIISerum levels of IL-6 in the
experimental groups at various time-points. |
Table III
Serum levels of IL-6 in the
experimental groups at various time-points.
| Group | T0 | T1 | T2 | T3 | T4 | T5 |
|---|
| H group | 9.60±0.85 | 9.53±0.76 | 9.47±0.85 | 9.57±0.72 | 9.51±0.71 | 9.79±0.68 |
| C group | 9.77±0.61 | 12.53±1.07a,b | 11.99±1.09a,b | 11.67±1.10a,b | 11.37±0.99a,b | 11.76±1.08a,b |
| S group | 9.69±0.80 | 11.68±1.20a–c | 10.06±1.12a–c | 10.62±1.00a–c | 10.58±0.91a–c | 10.16±0.94a–c |
Sevoflurane-pre-conditioning aggravates
CPB-induced increases in serum levels of IFN-β as well as
hippocampal levels of TLR3 and TRIF
The serum concentration of IFN-β as well as
hippocampal levels of TLR3 and TRIF in the C group were
significantly increased during and after CPB at
T1–T5 (P<0.05). Of note, IFN-β, TLR3 and
TRIF levels in the S group were significantly increased compared
with those in the C group (P<0.05), indicating that
pre-treatment with sevofluorane aggravated the CPB-induced
increases IFN-β, TLR3 and TRIF (Tables IV–Table VVI; Figs.
3Figure 4Figure 5Figure 6–7)
 | Figure 3Concentration of IFN-β in the
experimental groups at various time-points. Values are expressed as
the mean ± standard deviation. #P<0.05 compared with
T0; *P<0.05 compared to H group;
ΔP<0.05 compared with C group. H, sham group; C, CPB
group; S, sevoflurane-pre-conditioned group; T0, prior
to CPB; T1, CPB for 30 min; T2, CPB for 1 h;
T3, 1 h after 1-h CPB; T4, 2 h after 1-h CPB;
T5, 3 h after 1-h CPB; CPB, cardiopulmonary bypass; IFN,
interferon. |
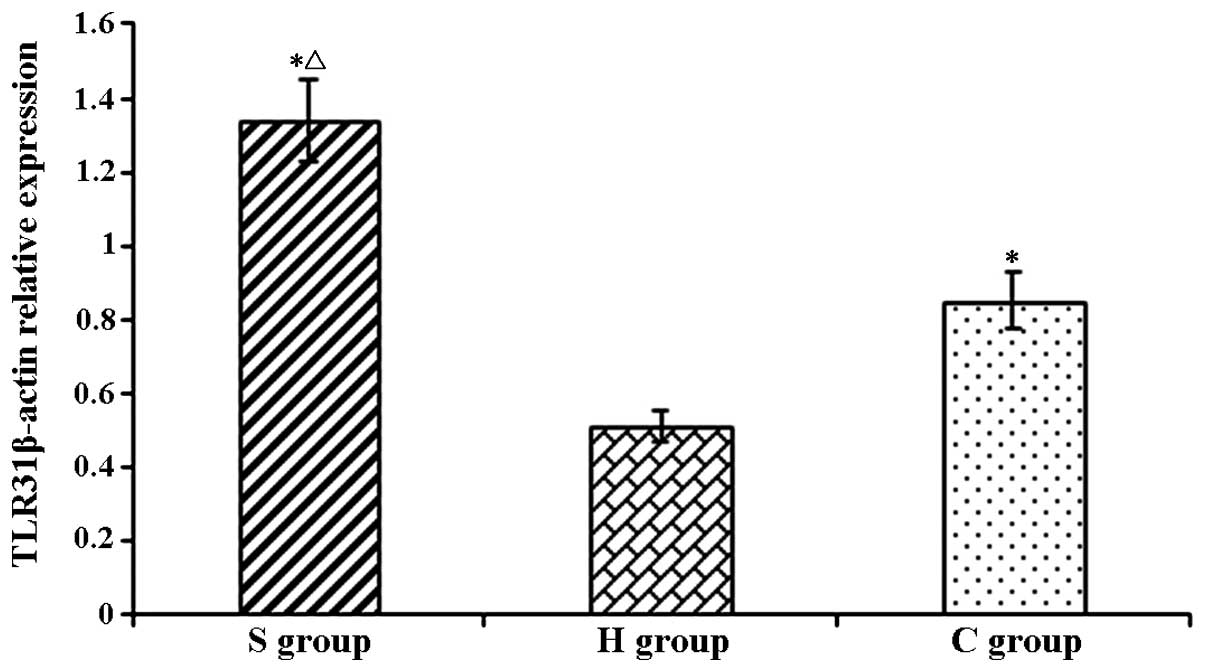
 | Figure 4TLR3 protein levels in rat hippocampi
in the experimental groups at various time-points. Values are
expressed as the mean ± standard deviation. #P<0.05 compared
with T0; *P<0.05 compared to H group;
ΔP<0.05 compared with C group. H, sham group; C, CPB
group; S, sevoflurane-pre-conditioned group; T0, prior
to CPB; T1, CPB for 30 min; T2, CPB for 1 h;
T3, 1 h after 1-h CPB; T4, 2 h after 1-h CPB;
T5, 3 h after 1-h CPB; CPB, cardiopulmonary bypass; TLR,
Toll-like receptor. |
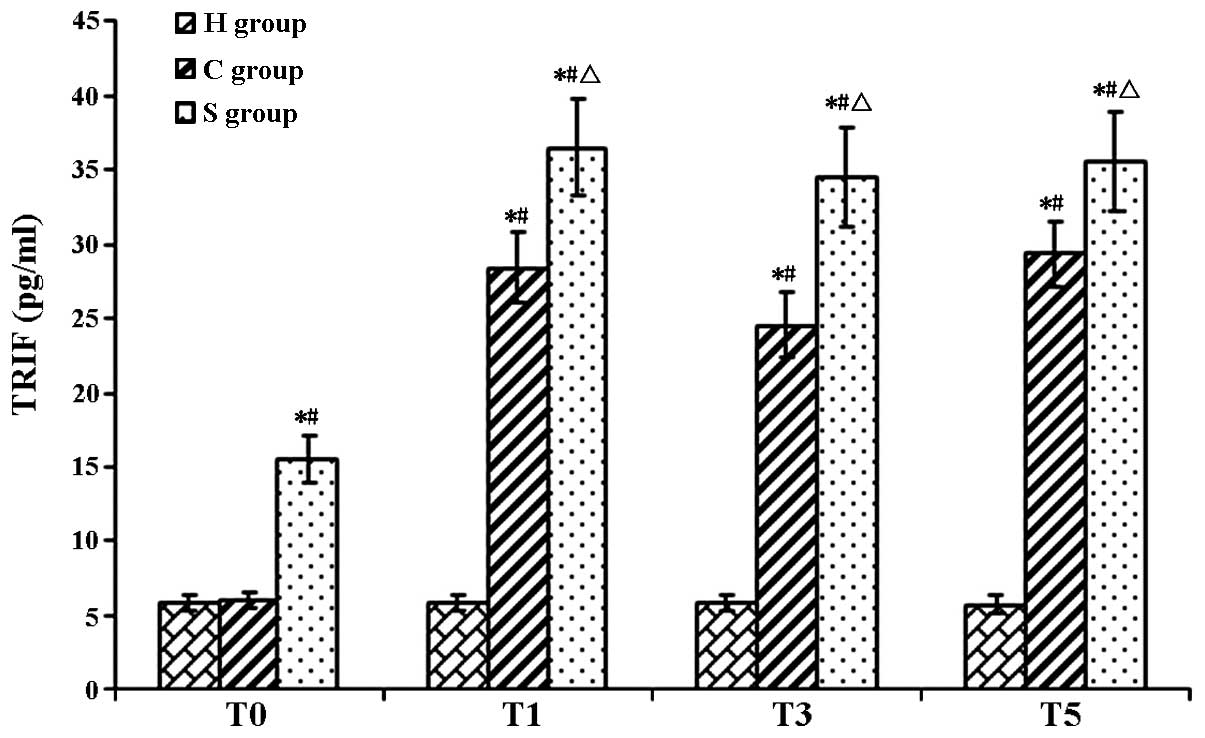 | Figure 5TRIF protein levels in rat hippocampi
in the experimental groups at various time-points. Values are
expressed as the mean ± standard deviation. #P<0.05 compared
with T0; *P<0.05 compared to H group;
ΔP<0.05 compared with H group. H, sham group; C, CPB
group; S, sevoflurane-pre-conditioned group; T0, prior
to CPB; T1, CPB for 30 min; T2, CPB for 1 h;
T3, 1 h after 1-h CPB; T4, 2 h after 1-h CPB;
T5, 3 h after 1-h CPB; CPB, cardiopulmonary bypass;
TRIF, TIR-domain-containing adapter-inducing interferon-β. |
 | Table IVSerum levels of IFN-β in the
experimental groups at various time-points. |
Table IV
Serum levels of IFN-β in the
experimental groups at various time-points.
| Group | T0 | T1 | T2 | T3 | T4 | T5 |
|---|
| H group | 156.55±13.82 | 160.02±13.68 | 156.45±14.59 | 160.02±13.98 | 152.20±14.87 | 157.59±13.59 |
| C group | 156.41±14.06 |
221.26±18.66a,b |
202.29±17.29a,b |
188.79±16.84a,b |
198.69±17.13a,b |
190.95±16.39a,b |
| S group | 160.71±14.03 |
252.26±19.74a–c |
248.66±18.38a–c |
232.45±18.06a–c |
235.98±18.45a–c |
236.55±18.58a–c |
 | Table VTLR3 expression levels rat hippocampi
in the experimental groups at various time-points. |
Table V
TLR3 expression levels rat hippocampi
in the experimental groups at various time-points.
| Group | T0 | T1 | T3 | T5 |
|---|
| H group | 5.01±0.53 | 5.21±0.53 | 4.58±0.48 | 5.11±0.45 |
| C group | 5.08±0.51 | 7.14±0.67a,b | 7.61±0.69a,b | 7.38±0.55a,b |
| S group | 6.35±0.60a,c | 8.73±0.72a–c | 8.60±0.80a–c | 8.54±0.78a–c |
 | Table VITRIF expression levels in rat
hippocampi in the experimental groups at various time-points. |
Table VI
TRIF expression levels in rat
hippocampi in the experimental groups at various time-points.
| Group | T0 | T1 | T3 | T5 |
|---|
| H group | 5.84±0.46 | 5.66±0.48 | 5.24±0.54 | 5.76±0.55 |
| C group | 6.04±0.11 | 28.42±0.22a,b | 24.59±0.34a,b | 29.40±0.31a,b |
| S group | 25.48±0.29a,c | 36.49±0.33a–c | 34.53±0.25a–c | 35.53±0.27a–c |
Sevoflurane-pre-conditioning reduces
CPB-induced apoptosis of hippocampal neurons
The results of the TUNEL assay showed that the
number of apoptotic hippocampal neuron cells was extremely low.
However, in the C and S groups, the number of apoptotic hippocampal
neurons was significantly higher than that in the H group
(P<0.05). Of note, the number of apoptotic cells was
significantly reduced in the S group as compared to that in the C
group, indicating that sevoflurane had a protective effect on
hippocampal neurons during and following CPB (Figs. 8 and 9).
Discussion
Impact of sevoflurane pre-conditioning on
brain injury during CPB
Sevoflurane is commonly used as an anesthetic in the
clinic at a concentration of 1 minimum alveolar concentration (MAC)
(27,28), and 2.4% sevoflurane is equivalent
to 1 MAC sevoflurane in rats. Hu et al (29) found that after pre-conditioning
with 2.4% sevoflurane for 1 h, ischemia-re-perfusion-induced brain
injury was reduced. The rat middle cerebral artery occlusion model
is used in current studies on brain injury, but sevoflurane has
rarely been used in rat models of CPB. To better simulate clinical
CPB and referring to preliminary studies by our group, 2.4%
sevoflurane pre-conditioning of rats for 1 h was selected prior to
establishing the CPB model.
S100-β is a sensitive and neuron-specific protein,
which is lowly expressed in normal brain tissue. Only when brain
tissue is damaged, cell and blood-brain barrier permeability
increase, S100-β is activated and then immediately expressed,
released into the cerebrospinal fluid and passes through the
blood-brain barrier; therefore, S100-β levels in the peripheral
blood effectively reflect the early extent of brain damage
(30).
Accordingly, in studies on CPB-induced brain injury,
S100-β protein is utilized as a specific marker of early brain
injury (31,32). Singh et al (33) examined the effects of sevoflurane
and isoflurane anesthesia as well as total intravenous anesthesia
on patients undergoing coronary artery bypass grafting and found
that the protein levels of S100-β increased rapidly after CPB. Of
note, sevoflurane was able to reduce the serum levels of S100-β in
the patients. In the present study, the levels of S100-β were close
to normal prior to CPB. During the CPB, S100-β levels in the H
group remained low, indicating that simple puncture did not cause
any significant brain damage. Compared with that in the H group,
the S100-β concentration in the C and S groups significantly
increased during CPB and remained at elevated levels after CPB,
indicating that CPB caused brain injury in rats.
In the present study, TUNEL staining was performed
to detect cell apoptosis. Compared to that in the H group, cell
apoptosis was significantly increased in the C and S groups. Of
note, sevoflurane pre-conditioning significantly reduced S100-β
levels and the number of apoptotic hippocampal neurons at each
time-point compared with those in the C group. These results
suggested that CPB caused brain damage and apoptosis of hippocampal
neurons, while sevoflurane pre-conditioning had a protective effect
as indicated by decreased levels of serum S100-β and apoptotic
hippocampal neurons.
Sevoflurane pre-conditioning alleviates
brain damage through reducing the CPB-induced inflammatory
response
Various factors during CPB can cause the release of
a large number of lymphocytes and other pro-inflammatory cytokines
as well as the development of systemic inflammatory response
syndrome (34). These factors
include non-physiological perfusion or reduced local oxygen supply,
which can be caused by insufficient blood supply, resulting in
great damage to the brain. Recent studies have shown that
inflammation is an important cause of cognitive dysfunction after
CPB (35,36); therefore, the focus of the present
study was on how to positively and effectively reduce
inflammation-induced damage during CPB.
IPC can enhance the brain's tolerance to traumatic
effects of ischemia and hypoxia through pre-experimental cerebral
ischemia (37). The inflammatory
reaction is an important cause of brain damage and inflammation may
synergistically aggravate hypoxic-ischemic brain damage (38). A study showed that prevention of
inflammation can enhance the protective effect of IPC against
cerebral ischemia, as IPC alleviates the extent of ischemia by
decreasing the expression of pro-inflammatory genes and reducing
neutrophil and macrophage infiltration in the ischemic area of
infiltration (4).
In a study on patients with coronary artery bypass
and valve replacement, Ramlawi et al (39) found that in the early
post-operative stage, serum levels of IL-1 are significantly
increased in patients with cognitive decline, and that the amount
of cognitive decline is IL-1 concentration-dependent.
Ashraf et al (40) found that during CPB, S100-β protein
levels positively correlated with IL-6 expression levels, while
expression of IL-6 promoted the protein levels of S100-β as well as
the participation of inflammatory mediators and exacerbated brain
damage.
Vila et al (41) showed that IL-6 is closely
associated with the area of acute cerebral infarction. The serum
concentration of IL-6 was in parallel with the size of the cerebral
infarction area, an independent risk factor of brain damage. The
possible mechanisms is the promotion of aggregation and adhesion of
inflammatory cells, partial infarction of brain tissue, activation
of matrix metalloproteinases, destruction of the blood-brain
barrier, stimulation of platelet activity, promotion of fibrinogen
production and reduction of cerebral perfusion (42–45).
Bedirli et al (46) found that sevoflurane
pre-conditioning reduced ischemia-reperfusion-induced levels of
inflammatory cytokines (TNF-α and IL-1β), thus reducing the
inflammatory reaction caused by brain damage and thereby protecting
the brain.
The present study found that the concentration of
IL-6 in the CPB group was significantly higher than that in Group H
during CPB and largely remained at a high level, indicating that
inflammation occurs during CPB. Compared with those in the C group,
the levels of IL-6 in the S group were significantly lower at each
time-point. These results suggested that sevoflurane
pre-conditioning alleviated the inflammatory response during CPB
and exerted a protective effect on the brain through inhibiting the
expression of the inflammatory cytokine IL-6.
The TLR3 signaling pathway is involved in
the protective effect of sevoflurane on the brain during CPB
Previous studies found that pre-excited TLRs
suppress brain inflammation and thus alleviate the effects of brain
injury (7,8). TLRs are innate immunity receptors
widely expressed in the central nervous system, and TLR1-9 are
expressed in primary cultured human microglia cells. The TLR family
is involved in two signaling pathways: The MyD88-dependent pathway
and the TRIF-dependent pathway. TLRs (except TLR3) increase the
synthesis and release of pro-inflammatory cytokines by combining
with the downstream protein MyD88 via the MyD88-dependent pathway.
In the TRIF-dependent pathway, TLR3 and TLR4 are involved in type I
interferon signaling to increase the expression of
anti-inflammatory cytokines such as IFN-β.
The TLR3 pathway is the only solely TRIF-dependent
pathway amongst all TLR pathways. TLR3 is expressed throughout the
central nervous system and is highly expressed in astrocytes; TLR3
is particularly sensitive to cell damage (47). TLRs are involved in the brain
damage response to ischemia caused by inflammation through which
they are activated to alleviate inflammation, and are
pre-stimulated to provide a powerful neuroprotective effect
(48). Pan et al (11) stimulated the expression of TLR3 by
using the TLR3 agonist polyinosinic polycytidylic acid, which
inhibited the release of pro-inflammatory cytokines as well as the
expression of nuclear factor (NF)-κB and IL-6, and mitigated nerve
cell damage.
In the present study, the TLR3 levels in the C group
were significantly increased with the onset of CPB as compared with
those in the H group, indicating CPB activated the expression n of
TLR3 expression. Compared to those in the CPB group, the expression
levels of TLR3 were significantly elevated in the S group even at
T0 prior to CPB, which may be due to sevoflurane-induced
pre-activated expression of the TLR3. During and after CPB, the
levels of TLR3 in the S group were significantly higher than those
in the C group, indicating that sevoflurane pre-conditioning
up-regulated the expression of TLR3, thereby exerting a protective
effect on the brain and reducing brain damage in rats caused by
CPB.
TLR3 signaling pathways associated with
the neuroprotective effect of sevoflurane pre-conditioning
When the body is subjected to external pathogen
invasion, TLR3 combines with the pathogen through the
TRIF-dependent pathway, activating the expression of downstream
anti-inflammatory cytokines, including IFN-β, inhibiting the
expression of pro-inflammatory cytokines, such as IL-6, and
alleviating inflammation. TLR3 signaling is transduced exclusively
through the TRIF pathway; thus, activating the expression of TLR3
may help to restore the balance between pro-inflammatory and
anti-inflammatory cytokines following CPB, resulting in
neuroprotection.
Previous studies suggested that pre-treatment with
lipo-polysaccharide (LPS) or de-regulation of TRIF expression
through IPC, ultimately increasing the release of anti-inflammatory
cytokines, inhibits the release of pro-inflammatory cytokines
mediated by NF-κB, thus protecting the brain (6,8) Nhu
et al (49) found that LPS
pre-treatment can upregulate TLR3 expression, thereby increasing
the expression of downstream proteins of the TRIF pathway.
In the present study, it was observed that TLR3,
TRIF and IFN-β expression levels were elevated in the C group,
showing the same tendency as S100-β protein. After sevoflurane
preconditioning, the expression levels of TLR3 and TRIF, IFN-β were
further increased, while the expression of the pro-inflammatory
cytokine IL-6 and the brain damage marker S100-β was decreased. The
TLR3 signaling pathway may be pre-activated by sevoflurane,
increasing the expression levels of TLR3 and its downstream
signaling molecule TRIF prior to as well as during CPB to exert
their protective effects against brain damage.
The present study generated a rat model of CPB and
observed that sevoflurane pre-conditioning had a protective role on
the brain to reduce CPB-induced damage. This protective role may be
associated with the TLR3 signaling pathway, providing a novel
target to prevent brain damage and improve the outcomes of clinical
CPB. The findings of the present study can be concluded as follows:
i) Sevoflurane pre-treatment has a protective effect against
CPB-induced brain injury; ii) sevoflurane pre-treatment alleviates
cerebral injury caused by a CPB-induced inflammatory reaction,
through affecting the TLR3 signaling pathway; and iii) TLR3
signaling pathways involved in the neuroprotective effects of
sevoflurane include the pre-activation of TLR3, up-regulation of
the downstream TRIF, inhibition of the expression of
pro-inflammatory cytokines IL-6 and upregulation of the
anti-inflammatory factor IFN-β.
Further in-depth studies are required to explore the
downstream mechanisms of TLR3-associated brain protection by
sevoflurane. In addition, it should be attempted to discover the
role of the each TLR and their cross-protective mechanisms in order
to ultimately provide a theoretical support for the effects of
cardiovascular surgeries on the brain as well as strategies to
prevent brain damage.
Acknowledgments
The present study was supported by the Liaoning
Natural Science Foundation (grant no. 20102244).
References
|
1
|
Lombard FW and Mathew JP: Neurocognitive
dysfunction following cardiac surgery. Semin Cardiothorac Vasc
Anesth. 14:102–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McKhann GM, Grega MA, Borowicz LM Jr,
Baumgartner WA and Selnes OA: Stroke and encephalopathy after
cardiac surgery. An update Stroke. 37:562–571. 2006. View Article : Google Scholar
|
|
3
|
Mei B: Study of brain injury and brain
protection during cardiopulmonary bypass progress. Zhong Guo Ti Wai
Xun Huan Za Zhi. 8:125–127. 2010.In Chinese.
|
|
4
|
Bowen KK, Naylor M and Vemuganti R:
Prevention of inflammation is a mechanism of
preconditioning-induced neuro-protection against focal cerebral
ischemia. Neurochem Int. 49:127–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pradillo JM, Fernández-López D,
García-Yébenes I, Sobrado M, Hurtado O, Moro MA and Lizasoain I:
Toll-like receptor 4 is involved in neuroprotection afforded by
ischemic preconditioning. J Neurochem. 109:287–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Broad A, Kirby JA and Jones DE; Applied
Immunology and Transplantation Research Group: Toll-like receptor
interactions: Tolerance of MyD88-dependent cytokines but
enhancement of MyD88-independent interferon-beta production.
Immunology. 120:103–111. 2007. View Article : Google Scholar
|
|
7
|
Marsh B, Stevens SL, Packard AE, Gopalan
B, Hunter B, Leung PY, Harrington CA and Stenzel-Poore MP: Systemic
lipopolysaccharide protects the brain from ischemic injury by
reprogramming the response of the brain to stroke: A critical role
for IRF3. J Neurosci. 29:9839–9849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh BJ and Stenzel-Poore MP: Toll-like
receptors: Novel pharmacological targets for the treatment of
neurological diseases. Curr Opin Pharmacol. 8:8–13. 2008.
View Article : Google Scholar
|
|
9
|
De Miranda J, Yaddanapudi K, Hornig M,
Villar G, Serge R and Lipkin WI: Induction of Toll-like receptor
3-mediated immunity during gestation inhibits cortical neurogenesis
and causes behavioral disturbances. MBio. 1:e00176–10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bsibsi M, Persoon-Deen C, Verwer RW,
Meeuwsen S, Ravid R and Van Noort JM: Toll-like receptor 3 on adult
human astrocytes triggers production of neuroprotective mediators.
Glia. 53:688–695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan LN, Zhu W, Li C, Xu XL, Guo LJ and Lu
Q: Toll-like receptor 3 agonist Poly I:C protects against simulated
cerebral ischemia in vitro and in vivo. Acta Pharmacol Sin.
33:1246–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djaiani G, Fedorko L, Borger MA, Green R,
Carroll J, Marcon M and Karski J: Continuous-flow cell saver
reduces cognitive decline in elderly patients after coronary bypass
surgery. Circulation. 116:1888–1895. 2007. View Article : Google Scholar
|
|
13
|
Yan YG, Wang DJ, Wu Z, Li QZ and Zhou Q:
Relevant factors for severe neurological complications after
coronary artery bypass graft. Zhong Guo Zu Zhi Gong Cheng Yan Jiu.
14:1174–1178. 2010.In Chinese.
|
|
14
|
Anttila V, Hagino I, Iwata Y, Mettler BA,
Lidov HG, Zurakowski D and Jonas RA: Aprotinin improves cerebral
protection: Evidence from a survival porcine model. J Thorac
Cardiovasc Surg. 132:948–953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elefteriades JA: What is the best method
for brain protection in surgery of the aortic arch? Straight DHCA.
Cardiol Clin. 28:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nelson DP, Andropoulos DB and Fraser CD
Jr: Perioperative neuroprotective strategies. Semin Thorac
Cardiovasc Surg Pediatr Card Surg Annu. 11:49–56. 2008. View Article : Google Scholar
|
|
17
|
Head BP and Patel P: Anesthetics and brain
protection. Curr Opin Anesthesiol. 20:395–399. 2007. View Article : Google Scholar
|
|
18
|
Xiong L, Zheng Y, Wu M, Hou L, Zhu Z,
Zhang X and Lu Z: Preconditioning with isoflurane produces
dose-dependent neuro-protection via activation of adenosine
triphosphate-regulated potassium channels after focal cerebral
ischemia in rats. Anesth Analg. 96:233–237. 2003.
|
|
19
|
Kitagawa K, Matsumoto M, Tagaya M, Hata R,
Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K and Mikoshiba K:
'Ischemic tolerance' phenomenon found in the brain. Brain Res.
528:21–24. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Q, Dong H, Deng J, Wang Q, Ye R, Li
X, Hu S, Dong H and Xiong L: Sevoflurane preconditioning induces
neuroprotection through reactive oxygen species-mediated
up-regulation of antioxidant enzymes in rats. Anesth Analg.
112:931–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Lei B, Popp S, Meng F, Cottrell JE
and Kass IS: Sevoflurane immediate preconditioning alters hypoxic
membrane potential changes in rat hippocampal slices and improves
recovery of CA1 pyramidal cells after hypoxia and global cerebral
ischemia. Neuroscience. 145:1097–1107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Payne RS, Akca O, Roewer N, Schurr A and
Kehl F: Sevoflurane-induced preconditioning protects against
cerebral ischemic neuronal damage in rats. Brain Res. 1034:147–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HP, Jeong EJ, Kim MH, Hwang JW, Lim
YJ, Min SW, Kim CS and Jeon YT: Effects of sevoflurane on neuronal
cell damage after severe cerebral ischemia in rats. Korean J
Anesthesiol. 61:327–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dabrowski W, Rzecki Z, Czajkowski M, Pilat
J, Wacinski P, Kotlinska E, Sztanke M, Sztanke K, Stazka K and
Pasternak K: Volatile anesthetics reduce biochemical markers of
brain injury and brain magnesium disorders in patients undergoing
coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth.
26:395–402. 2012. View Article : Google Scholar
|
|
25
|
Wang H, Lu S, Yu Q, Liang W, Gao H, Li P,
Gan Y, Chen J and Gao Y: Sevoflurane preconditioning confers
neuroprotection via anti-inflammatory effect. Front Biosci (Elite
Ed). 3:604–615. 2011. View
Article : Google Scholar
|
|
26
|
Song DD, Gao GJ, Sun YJ, Zhang TZ, Zhou J,
Zhang Y and Yao Q: Rats without blood prefilled established model
of cardiopulmonary bypass with cardiac arrest. Zhong Guo Ti Wai Xun
Huan Za Zhi. 4:232–235. 2009.In Chinese.
|
|
27
|
Obal D, Preckel B, Scharbatke H,
Müllenheim J, Höterkes F, Thämer V and Schlack W: One MAC of
sevoflurane provides protection against reperfusion injury in the
rat heart in vivo. Br J Anaesth. 87:905–911. 2001. View Article : Google Scholar
|
|
28
|
Payne RS, Akca O, Roewer N, Schurr A and
Kehl F: Sevoflurane-induced preconditioning protects against
cerebral ischemic neuronal damage in rats. Brain Res. 1034:147–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Zhang Y, Li W, Liu J and Li Y:
Preconditioning with sevoflurane ameliorates spatial learning and
memory deficit after focal cerebral ischemia-reperfusion in rats.
Int J Dev Neurosci. 31:328–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Groom RC, Quinn RD, Lennon P, Welch J,
Kramer RS, Ross CS, Beaulieu PA, Brown JR, Malenka DJ, O'Connor GT,
et al: Microemboli from cardiopulmonary bypass are associated with
a serum marker of brain injury. J Extra Corpor Technol. 42:40–44.
2010.PubMed/NCBI
|
|
31
|
Herrmann M, Ebert AD, Galazky I,
Wunderlich MT, Kunz WS and Huth C: Neurobehavioral outcome
prediction after cardiac surgery role of neurobiochemical markers
of damage to neuronal and glial brain tissue. Stroke. 31:645–650.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aly WW, Abdul-Rahman SA, El Said SMS, et
al: S100B and delirium in the geriatric acute care setting. Adv
Aging Res. 3:1–5. 2014. View Article : Google Scholar
|
|
33
|
Singh SP, Kapoor PM, Chowdhury U and Kiran
U: Comparison of S100β levels and their correlation with
hemodynamic indices in patients undergoing coronary artery bypass
grafting with three different anesthetic techniques. Ann Card
Anaesth. 14:197–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nikolakopoulou Z, Smith M, Hector LR, et
al: S100A12 as a biomarker for neutrophil mediated inflammation in
patients undergoing cardiac surgery necessitating cardiopulmonary
bypass. Thorax. 68:141. 2013. View Article : Google Scholar
|
|
35
|
Song J, Park J, Kim JY, Kim JD, Kang WS,
Muhammad HB, Kwon MY, Kim SH, Yoon TG, Kim TY and Chung JW: Effect
of ulinastatin on perioperative function and systemic inflammatory
reaction during cardiac suregry: A randomized double-blinded study.
Korean J Anesthesiol. 64:334–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li W, Wu X, Yan F, Liu J, Tang Y, Ma K and
Li S: Effects of pulmonary artery perfusion with urinary trypsin
inhibitor as a lung protective strategy under hypothermic low-flow
cardiopulmonary bypass in an infant piglet model. Perfusion.
29:434–442. 2014. View Article : Google Scholar
|
|
37
|
Liu XQ, Sheng R and Qin ZH: The
neuroprotective mechanism of brain ischemic preconditioning. Acta
Pharmacol Sin. 30:1071–1080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lakhan SE, Kirchgessner A and Hofer M:
Inflammatory mechanisms in ischemic stroke: Therapeutic approaches.
J Transl Med. 7:972009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramlawi B, Rudolph JL, Mieno S, Feng J,
Boodhwani M, Khabbaz K, Levkoff SE, Marcantonio ER, Bianchi C and
Sellke FW: C-Reactive protein and inflammatory response associated
to neurocognitive decline following cardiac surgery. Surgery.
140:221–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ashraf S, Bhattacharya K, Tian Y and
Watterson K: Cytokine and S100B levels in paediatric patients
undergoing corrective cardiac surgery with or without total
circulatory arrest. Eur J Cardiothorac Surg. 16:32–37. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vila N, Castillo J, Dávalos A and Chamorro
A: Proinflammatory cytokines and early neurological worsening in
ischemic stroke. Stroke. 31:2325–2329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Selzner N, Selzner M, Odermatt B, Tian Y,
Van Rooijen N and Clavien PA: ICAM-1 triggers liver regeneration
through leukocyte recruitment and Kupffer cell-dependent release of
TNF-alpha/IL-6 in mice. Gastroenterology. 124:692–700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu ZM, Yang QD, Liu YH, Huang XS and
Zhang N: Serum IL-6 in patients with cerebral infarction and
SICAM-1 and its clinical significance. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 29:326–329. 2004.In Chinese.
|
|
44
|
Reinsfelt B, Ricksten SE, Zetterberg H,
Blennow K, Fredén-Lindqvist J and Westerlind A: Cerebrospinal fluid
markers of brain injury, inflammation and blood-brain barrier
dysfunction in cardiac surgery. Ann Thorac Surg. 94:549–555. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee SH, Kim BJ, Kim YB, Chung PW, Moon HS,
Suh BC, Yoon WT, Jin DK, Park YS, Lee YT and Park KY: IL-1β
induction and IL-6 suppression are associated with aggravated
neuronal damage in a lipopolysaccharide-pretreated kainic
acid-induced rat pup seizure model. Neuroimmunomodulation.
19:319–325. 2012. View Article : Google Scholar
|
|
46
|
Bedirli N, Bagriacik EU, Emmez H, Yilmaz
G, Unal Y and Ozkose Z: Sevoflurane and isoflurane preconditioning
provides neuroprotection by inhibition of apoptosis-related mRNA
expression in a rat model of focal cerebral ischemia. J Neurosurg
Anesthesiol. 24:336–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barreto G, White RE, Ouyang Y, Xu L and
Giffard RG: Astrocytes: Targets for neuroprotection in stroke. Cent
Nerv Syst Agents Med Chem. 11:164–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rosenzweig HL, Lessov NS, Henshall DC,
Minami M, Simon RP and Stenzel-Poore MP: Endotoxin preconditioning
prevents cellular inflammatory response during ischemic
neuroprotection in mice. Stroke. 35:2576–2581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nhu QM, Cuesta N and Vogel SN:
Transcriptional regulation of lipopolysaccharide (LPS) -induced
toll-like receptor (TLR) expression in murine macrophages: Role of
interferon regulatory factors 1 (IRF-1) and 2 (IRF-2). J Endotoxin
Res. 12:285–295. 2006. View Article : Google Scholar
|